INTRODUCTION
MATERIALS AND METHODS
1. Materials and surgery
2. Pain-related behavior testing
3. Histopathology
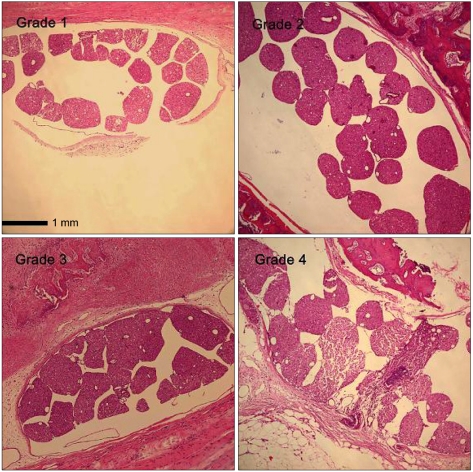 | Fig. 1The grade of cauda equina clumping on the light microscopic findings at L5 or L6 level at the 21st day after laminectomy in ×10 scale (hematoxylin and eosin staining). In grade 1, most rootlets are distributed separately in the subarachnoid space. In grade 2, more than half of the rootlets are grouped to one another. In grade 3, all nerve rootlets are grouped or adhered to form a lump. In grade 4, severe peridural adhesion can be seen. |
RESULTS
1. Pain-related behavior testing
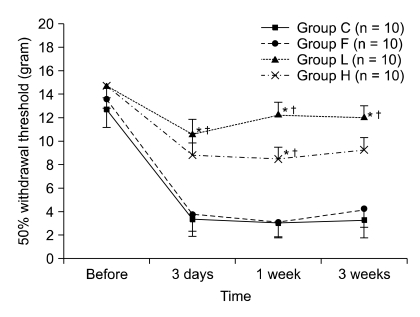 | Fig. 2The changes of 50% withdrawal threshold before laminectomy and 3 days, 1 week, and 3 weeks after laminectomy. Values are mean ± SE. Group C: laminectomy only, group L: laminectomy and low molecular weight hyaluronic acid application, group H: laminectomy and high molecular weight hyaluronic acid application, and group F: laminectomy and fat interposition. In groups C and F, 50% withdrawal thresholds are decreased significantly 3 days after laminectomy and maintained thereafter. Overall the mechanical allodynia in groups C and F are more significant than that of other groups from 3 days after laminectomy. *P < 0.05 vs. group C and †P < 0.05 vs. group F. |
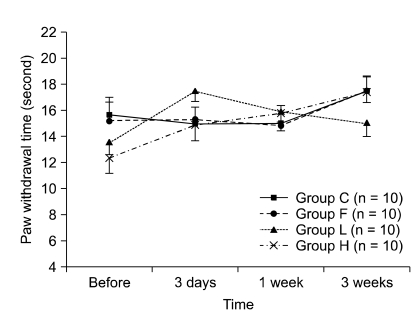 | Fig. 3The changes of paw withdrawal time before laminectomy and 3 days, 1 week, and 3 weeks after laminectomy. Values are mean ± SE. Group C: laminectomy only, group L: laminectomy and low molecular weight hyaluronic acid application, group H: laminectomy and high molecular weight hyaluronic acid application, and group F: laminectomy and fat interposition. There was no significant difference in thermal hyperalgesia among groups. |
2. Histopathology
Table 1
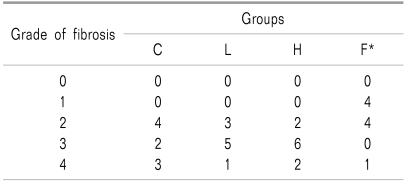
Values are numbers of rats. Group C: laminectomy only, Group L: laminectomy and low molecular weight hyaluronic acid application, Group H: laminectomy and and high molecular weight hyaluronic acid application, Group F: laminectomy and fat interposition. The grade of thickness of peridural fibrosis: Grade 0: less than 1 mm, Grade 1: 1-2 mm, Grade 2: 2-3 mm, Grade 3: 3-4 mm, and Grade 4: more than 4 mm. *P < 0.05 vs. corresponding data of other groups.
Table 2
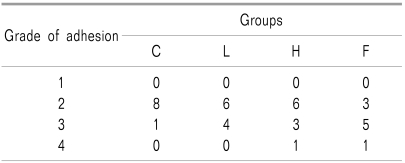
Values are numbers of rats. Group C: laminectomy only, group L: laminectomy and low molecular weight hyaluronic acidapplication, group H: laminectomy and and high molecular weight hyaluronic acid application, and group F: laminectomy and fat interposition. The grade of cauda equina adhesion: grade 1: most rootlets are distributed separately in the subarachnoid space, grade 2: more than half of the rootlets are grouped to one another, grade 3: all nerve rootlets are grouped or adhered to form a lump, and group 4: lumped adhesion of all nerve rootles are adhered to the dura and the extraduralscar tissue.
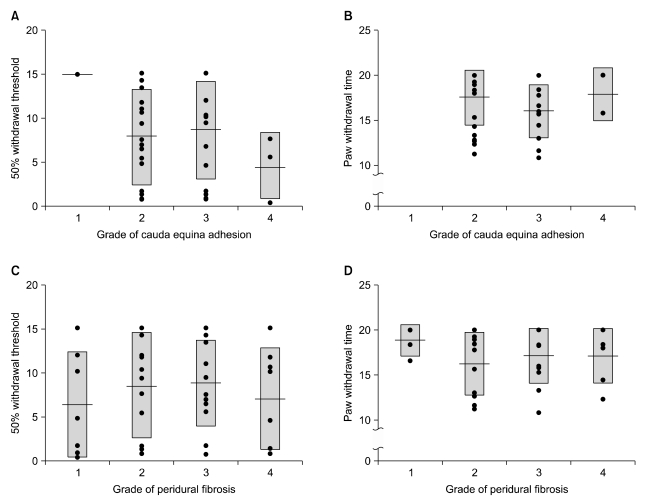 | Fig. 4The correlations between pain behaviors and histopathologic findings. (A) Correlations between 50% withdrawal threshold and grade of cauda equina adhesion. (B) Correlations between paw withdrawal time and grade of cauda equina adhesion. (C) Correlations between 50% withdrawal threshold and grade of eridural fibrosis. (D) Correlations between paw withdrawal time and grade of peridural fibrosis. Boxes are mean ± SD and center lines are means. It is supposed that the more the fibrotic change or cauda equina adhesion, the less the 50% withdrawal threshold or paw withdrawal time. However, there are no significant correlations between pain behaviors and histopathological findings. |
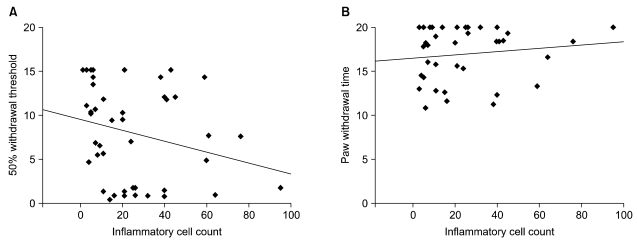 | Fig. 5The correlations between pain behaviors and inflammatory cells count. It is supposed that the more the inflammatory change, the less the 50% withdrawal threshold or paw withdrawal time. However, there are no significant correlations between pain behaviors and inflammatory cell count. In graph (A) y0 = 9.5 and slope = -0.062 and in graph (B) y0 = 16.5 and slope = 0.019. y0 means y-intersect where x value is 0. |




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download