Abstract
Obturator nerve block has been commonly used for pain management to prevent involuntary reflex of the adductor thigh muscles. One of several options for this block is chemical neurolysis. Neurolysis is done with chemical agents. Chemical agents used in the neurolysis of the obturator nerve have been alcohol, phenol, and botulinum toxin. In the current case, a patient with spasticity of the adductor thigh muscle due to cervical cord injury had obturator nerve neurolysis done with botulinum toxin type B (BoNT-B). Most of the previous studies have used BoNT-A with only a few reports that have used BoNT-B. BoNT-B has several advantages and disadvantages over BoNT-A. Thus, we report herein a patient who successfully received obturator nerve neurolysis using BoNT-B to treat adductor thigh muscle spasm.
Diseases that are known to cause spasticity of the thigh adductor muscle include stroke, spinal cord injury, brain injury, and multiple sclerosis, and the major mechanism is damage of the upper motor neuron. The purpose of lower limb spasticity management is to reduce pain by reducing muscular overactivity and advancing the articular range of motion and thereby improving the quality of life [1]. The treatment of lower limb spasticity is divided into internal medical treatment and neurolysis. Antispastic medication includes benzodiazepines, dantrolene, tizanidine, and baclofen. However, long-term use of these drugs has been reported to cause side effects such as hepatotoxicity, sedation, and dizziness [2]. The agents for chemical neurolysis include phenol, alcohol, and botulinum toxin (BoNT). Phenol and alcohol causes a nerve block by denaturing proteins, resulting in neural tissue damage. On the other hand, BoNT causes a nerve block by repressing the secretion of acetylcholine at the presynaptic nerve ending [3]. BoNT has the advantage of reducing spasticity and achieving nerve block without any tissue damage by injecting it directly into a muscle or the part near the nerve. Among the seven types of BoNT, Types A (BoNT-A) and Type B (BoNT-B) are used clinically. BoNT-A has been used for the reduction of spasticity in previous studies [1,4], but BoNT-B has not. We were able to successful reduce lower limb spasticity through obturator nerve block neurolysis using BoNT-B in a patient with lower limb spasticity due to cervical cord injury. Therefore, we report our case with a review of the literature.
A 36-year old man, 165 cm and 61.1 kg in height and weight, experienced cervical cord injury (C3-C4) due to a motorbike accident in December 2009 resulting in secondary quadriplegia. The patient did not have any specific findings in the medical history. The patient underwent posterior laminectomy and fusion of the C3-5 level because of the instability of the cervical spine in March 2010. The patient was sent to a pain clinic at our hospital in June 2010 because of severe lower limb spasticity due to secondary quadriplegia. At that time, the spasticity index was 1/1 at the elbow, 3/3 at the hip joint, 3/3 at the knee, and 1+/1+ at the ankle according to the modified Ashworth scale [5]. The lower limb spasticity of the patient was exacerbated by the supine position and minimal irritation. Treatment for rehabilitation was hard to do because of the involuntary flexion. In particular, an operation was needed because of a severe coccyx sore but it could not be done because the patient was not able to take the prone position due to the spasticity. The department of rehabilitation had been injecting dantrolene 200 mg and baclofen 50 mg per day for five months to reduce spasticity, which was similar to the maximum dose of each drug but there was no particular improvement in the spasticity.
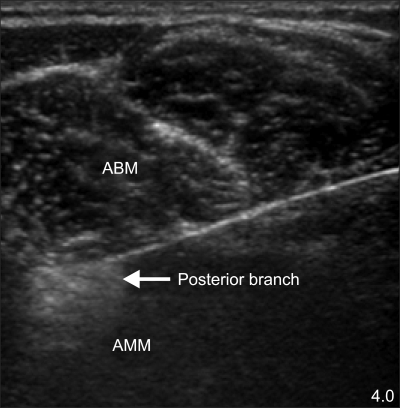
We decided to perform chemodenervation of the obturator nerve using BoNT-B (MYOBLOC™, Solstice neurosciences, USA) to reduce the lower limb spasticity of the patient. A sufficient explanation was provided to the patient about the operation and its complications and written consent was received from him. There were no abnormal findings in the preoperative blood test, urinalysis, and electrocardiography (ECG). During the operation, we monitored the blood pressure, ECG, and pulse oxygen saturation. The patient took the supine position, but the involuntary flexion was so severe because of the lower limb spasticity that he opened the hip joint as much as possible with the help of an assistant and fixed both lower limbs while bending the knee joints. The operative region was coated with Betadine and coveted with a sterile covering. Then, the pubic tubercle and anterior superior iliac spine were identified. On the line connecting the two points, the locations of the femoral artery and femoral vein were found using an ultrasound linear probe (6-13 MHz, MicroMAXX™, Sonosite, USA). Moving the linear probe medially and distally, we first checked the pectineus muscle and then the adductor longus as well as the adductor brevis. Later, we identified the interfascial layer in between the two adductors and then the anterior branch of the obturator nerve. We identified the posterior branch of the obturator nerve by moving the linear probe to the inner side since it exists at the interfascial layer between the adductor brevis and the adductor magnus. Local anesthesia was done with 1% lidocaine and a 22-G needle was inserted to the interfascial layer where the anterior branch of the obturator nerve is. After checking the hydrodissection by injecting 1 ml of 2% lidocaine, 5 ml of 2% lidocaine was injected slowly (Fig. 1). In the same manner, the needle was inserted to the interfascial layer between the adductor brevis and the adductor magnus where the posterior branch of the obturator nerve is to do a diagnostic block (Fig. 2). The nerve block was also done to the anterior and posterior branches of the obturator nerve on the opposite side one by one in the same manner. While the patient was in the supine position for 30 minutes, we observed if lower limb spasticity was reduced and if other complications took place. After 30 minutes, the spasticity index was reduced from 3/3 to 1+/1+ without any complications. We decided to do the chemodenervation of the obturator nerve using BoNT-B and approached it in the same manner through the marked region from the diagnostic block. BoNT-B 1,250 units to each branch and 2,500 units to each of the both obturator nerves were injected. The total dose was 5,000 units. We verified that there were no complications by catamnesis for 30 minutes after we finished the procedure. The follow-up observation of three months after the procedure showed that the spasticity of the hip joint was maintained at 1+/1+, and the involuntary reflex was reduced greatly.
As in this case, lower limb spasticity due to quadriplegia increases muscle tone and stretch reflex and thereby, results in problems such as causing discomfort in patients, interfering with rehabilitation treatment, and lowering perineal hygiene [1]. Thus, obturator nerve blocks are done to relax the hip adductor muscles involved in lower limb spasticity. Generally, a diagnostic nerve block is followed by chemodenervation using alcohol and phenol in order to extend the period of the obturator nerve block. BoNT is used with two methods: direct injection of BoNT into the adductor muscles causing lower limb spasticity and obturator nerve block. There has been a report that the reduction of spasticity was achieved by directly injecting BoNT-A into the adductor muscles, but there has been no report on obturator nerve block using BoNT to our knowledge.
BoNT has been used to treat various diseases since Scott [6] treated a patient with strabismus by injecting it to the affected muscles. Since BoNT acts through the inhibition of acetylcholine secretion, it can affect all regions where acetylcholine is secreted including the neuromuscular junction, preganglionic neuron, and parasympathetic post ganglionic neuron. For instance, BoNT can be used to treat cervical dystonia and hemifacial spasm, which are caused by hyperactivity of the muscles, and to treat hyperhidrosis, which is caused by excessive activation of sweat glands innervated by the sympathetic nerve [7,8]. Recently, BoNT has been used to treat various diseases including complex regional pain syndrome and overactive bladder and to perform sympathetic nerve blocks [9,10]. BoNT has been classified into seven types: types A, B, C, D, E, F, and G depending on the type of antigen. Among them, only types A and B are used clinically [3]. Both BoNT-A and BoNT-B seem to both inhibit acetylcholine secretion, but their mechanisms for inhibition and target proteins are different. BoNT-A binds to SNAP-25 (synaptosomal-associated protein 25), which is one of the subunits of SNARE (soluble N-ethylmaleimide-sensitive-factor attachment protein receptor) that causes fusion of the presynaptic membrane and synaptic vesicles and thus, cleaves the synaptic vesicles so that they cannot fuse. However, BoNT-B inhibits acetylcholine secretion by cleaving the synaptobrevin involved in the exocytosis of acetylcholine [11]. The different target proteins affects the duration of action of BoNT-A and BoNT-B. According to a study that compared the duration of action of BoNT-A and BoNT-B in the treatment of cervical dystonia, the duration of action of BoNT-B was 12.1 weeks and that of BoNT-A 14 weeks (P = 0.033), indicating that BoNT-B had a shorter duration of action than that of BoNT-A. Another drawback of BoNT-B is that the incidence of dry mouth and dysphagia are not rare and the incidence rate is higher than that of BoNT-A [12]. However, it has been reported that the use of BoNT-B was never stopped because of complications since most of them were mild. BoNT-B has several advantages over BoNT-A. First, BoNT-B is effective for managing patients with Type A-resistance. BoNT is often injected repeatedly. One report has stated that 5-10% of patients showed resistance when BoNT-A was repeatedly injected over a short interval of two months [13]. It is also known that treatment with BoNT-B is effective without showing any particular resistant among such patients [14]. Second, BoNT-B is more stable than BoNT-A. BoNT-A is produced in a powder formulation to avoid lyophilization whereas BoNT-B is produced in a liquid formulation. BoNT-B is stored at room temperature or in a refrigerator (2-25℃). In addition, BoNT-B is stable in the sense that the potency does not decrease over time even after initially opening the container. However, BoNT-A should be kept in a freezer (-5℃) or refrigerator and must be used within four hours after diluting it with 0.9% saline solution under refrigeration [15]. In other words, the liquid form of BoNT-B can be used for injection until supplies run out, while the reconstituted lyophilized powder of BoNT-A in 0.9% saline solution has to be used within four hours after dilution. Thus, injection treatment should be carefully planned when using BoNT-A.
The greatest advantage of chemodenervation with BoNT compared with alcohol and phenol is that the damage to the surrounding tissues can be minimized. Alcohol and phenol cause nerve blocks by denaturing proteins and inadvertent dysesthesia or chemical neuritis may accompany the treatment [16]. These may be due to abnormal reactions following permanent protein denaturation or complications caused by the diffusion of the chemical agents to the surrounding tissues. However, BoNT does not have such complications because it just inhibits acetylcholine secretion and does not cause tissue denaturation. In this case report, BoNT was injected to the surrounding regions of the obturator nerve to achieve reduced spasticity of the thigh adductors, which is different from previous case reports where BoNT was directly injected into the muscles. One animal study on the mechanism of BoNT reported that the injected BoNT permeates to the nerve sheath and inhibits acetylcholine secretion at the neuromuscular junctions [17]. This means that BoNT injection into a region around a nerve can also have the effect of reducing spasticity, which is the same as direct BoNT injection into the muscles. BoNT-B may be more useful for intramuscular injection and nerve block than BoNT-A since BoNT-B diffuses to the surrounding tissues to a lesser degree than that of BoNT-A as reported by [18]. However, this property may be a drawback of BoNT when compared with with alcohol or phenol. For example, one study showed that it took about 91 days to recover the function of the neuromuscular junction after the injection of BoNT was [19]. This period of time is almost the same as the duration of action of BoNT, which is 2-3 months [3]. Since the duration of action is shorter than the mean duration of action of alcohol and phenol, the nerve block has to be done repeatedly, which is expensive. However, this may be compensated by BoNT-A since it was reported to be superior in terms of cost-effectiveness in cases where an anti-spastic drug was first injected into patients with lower limb spasticity compared to cases where a reduction in spasticity was achieved with BoNT-A [20]. With respect to the BoNT-B dosage, one study reported that no significant complications were found when an initial dosage of 2,500-10,000 units was given to patients with cervical dystonia [18]. In our case for both obturator nerve blocks, we used 2,500 units each for the right and left sides. We thought it appropriate not to increase the initial dosage greatly unless the patient had undergone BoNT treatment in advance. BoNT-B and BoNT-A both have pros and cons. It is important to select the more appropriate one depending on the case. Further studies are need so that BoNT-B may be used more widely in chemodenervation.
References
1. O'Brien CF. Treatment of spasticity with botulinum toxin. Clin J Pain. 2002; 18(6 Suppl):S182–S190. PMID: 12569967.
2. Chou R, Peterson K, Helfand M. Comparative efficacy and safety of skeletal muscle relaxants for spasticity and musculoskeletal conditions: a systematic review. J Pain Symptom Manage. 2004; 28:140–175. PMID: 15276195.

3. Dressler D, Adib Saberi F. Botulinum toxin: mechanisms of action. Eur Neurol. 2005; 53:3–9. PMID: 15650306.

4. Calderón-González R, Calderón-Sepúlveda RF. Clinical treatment (non surgical) of spasticity in cerebral palsy. Rev Neurol. 2002; 34:1–6. PMID: 11988886.
5. Ashworth B. Preliminary trial of carisoprodol in multiple sclerosis. Practitioner. 1964; 192:540–542. PMID: 14143329.
6. Scott AB. Botulinum toxin injection into extraocular muscles as an alternative to strabismus surgery. Ophthalmology. 1980; 87:1044–1049. PMID: 7243198.

7. Truong DD, Stenner A, Reichel G. Current clinical applications of botulinum toxin. Curr Pharm Des. 2009; 15:3671–3680. PMID: 19925419.

8. Grunfeld A, Murray CA, Solish N. Botulinum toxin for hyperhidrosis: a review. Am J Clin Dermatol. 2009; 10:87–102. PMID: 19222249.
9. Kharkar S, Ambady P, Yedatore V, Schwartzman RJ. Intramuscular botulinum toxin A (BtxA) in complex regional pain syndrome. Pain Physician. 2011; 14:311–316. PMID: 21587336.
10. Lim SJ, Park HJ, Lee SH, Moon DE. Ganglion impar block with botulinum toxin type a for chronic perineal pain -a case report-. Korean J Pain. 2010; 23:65–69. PMID: 20552077.

11. Aoki KR. Pharmacology and immunology of botulinum toxin type A. Clin Dermatol. 2003; 21:476–480. PMID: 14759579.

12. Comella CL, Jankovic J, Shannon KM, Tsui J, Swenson M, Leurgans S, et al. Comparison of botulinum toxin serotypes A and B for the treatment of cervical dystonia. Neurology. 2005; 65:1423–1429. PMID: 16275831.

13. Greene P, Fahn S, Diamond B. Development of resistance to botulinum toxin type A in patients with torticollis. Mov Disord. 1994; 9:213–217. PMID: 8196686.

14. Factor SA, Molho ES, Evans S, Feustel PJ. Efficacy and safety of repeated doses of botulinum toxin type B in type A resistant and responsive cervical dystonia. Mov Disord. 2005; 20:1152–1160. PMID: 15954134.

15. Callaway JE. Botulinum toxin type B (Myobloc): pharmacology and biochemistry. Clin Dermatol. 2004; 22:23–28. PMID: 15158541.

16. Akkaya T, Unlu E, Alptekin A, Gumus HI, Umay E, Cakci A. Neurolytic phenol blockade of the obturator nerve for severe adductor spasticity. Acta Anaesthesiol Scand. 2010; 54:79–85. PMID: 19839948.

17. Al-Saleem FH, Ancharski DM, Ravichandran E, Joshi SG, Singh AK, Gong Y, et al. The role of systemic handling in the pathophysiologic actions of botulinum toxin. J Pharmacol Exp Ther. 2008; 326:856–863. PMID: 18539649.

18. Figgitt DP, Noble S. Botulinum toxin B: a review of its therapeutic potential in the management of cervical dystonia. Drugs. 2002; 62:705–722. PMID: 11893235.
19. Callaway JE, Arezzo JC, Grethlein AJ. Botulinum toxin type B: an overview of its biochemistry and preclinical pharmacology. Semin Cutan Med Surg. 2001; 20:127–136. PMID: 11474745.

20. Ward A, Roberts G, Warner J, Gillard S. Cost-effectiveness of botulinum toxin type a in the treatment of post-stroke spasticity. J Rehabil Med. 2005; 37:252–257. PMID: 16024483.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download