INTRODUCTION
The concept of preemptive analgesia to reduce the magnitude and duration of postoperative pain was first introduced in 1983 by Woolf [1] who showed evidence for a central component of post-injury pain hypersensitivity in experimental studies. Subsequently, several experimented studies demonstrated that various anti-nociceptive techniques applied before injuries were more effective in reducing the post-injury central sensitization phenomena compared to administration after injury.
Although early reviews of clinical findings were mostly negative [2,3] there is still a widespread belief in the efficacy of preemptive analgesia among clinicians.
Since ketamine is a well known general anesthetic and short acting intra operative analgesic that acts on nicotinic and muscarinic receptors [4], in this study, we evaluated whether preemptive use of ketamine decreases post operative pain in patients undergoing appendectomies.
Go to : 
MATERIALS AND METHODS
In a double blind, randomized clinical trial, 80 adult male patients who were undergoing an operation for acute appendicitis were included in this study. The study was approved by the local ethical committee. After obtaining informed written consent, patients were randomly assigned to two groups (ketamine and control). Patients were excluded if they had a history of cardiovascular disease, hypertension (as evaluated by cardiovascular internist), increased intracranial pressure, epilepsy, cerebrovascular accident (as evaluated by one neurologist), psychiatric disorders, and drug abuse.
To examine the preemptive effect of ketamine, a randomized controlled trial was done from April 2010 to March 2011. A double - blind technique was used in which the surgeon and research physician who was responsible for data collection were unaware of the allocation of the study participants. The authors randomly assigned patients into a case group and a control group (patients with an even identical number were assigned to the ketamine group and those with an odd number to the control group).
In operating room, patients in ketamine group, received 0.5 mg/kg of ketamine IV, 10 minutes before surgical incision by specialist nurse. In the control group, 0.5 mg/kg of normal saline was injected. All patients were premedicated with midazolam 0.05 mg/kg IV before anesthesia to avoid the probable side effects of ketamine. Patients were operated on under general anesthesia with a Mcburney incision and the appendectomy was done. General anesthesia was induced with thiopental (6 mg/kg) and atracurium (0.5 mg/kg). For maintenance, isoflurane (0.5-1%), 50% N2O, and 50% O2 were used.
Postoperatively, if patients asked for analgesia, 1 mg/kg of pethidine was administered intravenously for adequate analgesia. Pain intensity was assessed at time 0 (immediately after arousal) and 4, 12, and 24 hours postoperatively by a physician who was unaware of the allocation of the study's participants. Time 0 was the time of complete consciousness. Pain was scored using the 10 point visual analogue scale (VAS; 0 = no pain, 10 = worst pain imaginable).
Other than the VAS score, the interval time for the first request of analgesia and the number of times pethidine was injected in the first 24 hours was recorded. Patients also were checked for side effects such as delusions and delirium. Data were presented as the mean ± SD for quantitative variables. The Mann Whitney test was used to compare VAS scores, interval time of the first request of analgesia, and total amount of analgesia in the first 24 hours. Statistical analysis was done with SPSS 11.5 (Chicago, SPSS Inc). A P less than 0.05 was considered statistically significant.
Go to : 
RESULTS
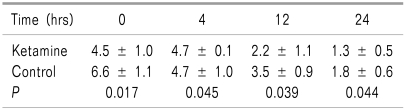
Eighty patients (40 in the ketamine group and 40 in the control group) were enrolled in this study. The mean age of the patients was 25.3 ± 11.4 years (rang 16-24 years). The duration of the surgery was 33.2 ± 10.3 minutes. The duration of the anesthesia was 47.2 ± 11.2 minutes. Table 1 shows the comparison of the basic data in the two groups. There were no significant differences in these variables between the groups. VAS scores are presented in Table 2. For all of the evaluated times, the VAS score was significantly lower in the ketamine group than that of the control group. The interval time for the first analgesic request was 23.1 ± 6.7 minutes in the ketamine group and 18.1 ± 7.3 minutes in the control group (P = 0.02). In the ketamine group, 42.5% of the patients did not need analgesics postoperatively. The total number of pethidine injections in first 24 hours postoperatively was 0.6 ± 0.6 in the ketamine group and 2.0 ± 0.8 in the control group (P = 0.032). There were no drug side effects in the ketamine group.
Go to : 
DISCUSSION
Surgical procedures almost invariably cause tissue damage resulting in pain. The impact of inadequate pain relief is well known and it can result in delayed mobilization and related complications as well as psychological distress and anxiety. The main finding of this study shows that preemptive intravenous low dose ketamine decreased postoperative pain in patients undergoing appendectomies. Ketamine is a well known general anesthetics and short acting intraoperative analgesic in use for almost 4 decades [5]. It is well-known that high doses of ketamine act as an intravenous anesthetic, and low doses of ketamine act as an analgesic agent [6,7].
Some studies have reported the recent discovery of the N-methyl-D-aspartate (NMDA) receptor [8], which seems to play a role in pain transmission, and according to other studies [9], ketamine binds to these receptors with a nonselective antagonism reducing hyperalgesia. Ketamine acts on nicotinic [10,11] and muscarinic receptors; it blocks sodium channels in the peripheral and human central nervous system and interacts with opioid receptors, µ, δ, and κ, and with calcium channels [12]. Ketamine also acts as a non-competitive antagonist at the phencyclidine receptor site in the NMDA receptor complex channel [13,14]. The role of NMDA receptors in the processing of nociceptive input is antagonized by low-doses of ketamine, which induces a noncompetitive blockade [15-18]; this raises the possibility that ketamine can become "trapped" in the receptor channel until the channel reopens after agonist activation. Many clinical trials have been done to evaluate ketamine administration for postoperative pain management; the oral [19], rectal [20], and intranasal [21] routes of administration for ketamine have been evaluated to provide premedication for general anesthesia [19] or sedation [22], but the analgesic effects of these routes of administration during the postoperative period have not been well determined. Some authors [21,22] evaluated the subcutaneous administration of this general intravenous anesthetic; it has been shown that low doses (1.7 µg/kg per min) subcutaneous.
Ketamine administered after major abdominal surgery did not produce adverse effects and provided postoperative analgesia equivalent to a subcutaneous morphine infusion of 2 mg/h. The rationale for preemptive analgesia in the management of postoperative pain has been reported by many authors [23,24]; since ketamine is an NMDA-receptor (involved in the mechanisms of hyperalgesia) antagonist, it is hypothesized to prevent or reverse central sensitization and thus, to reduce postoperative pain. In carrying out our study and analyzing the literature, we had to answer some clinical questions, and the first was "Can ketamine be administered as a postoperative analgesic?" According to the literature and to the results of this study, we can answer this question as "yes." Preemptive ketamine for the control of postoperative pain is currently in use even if its use is controversial; it use has consensus and dissension; different doses and routes of administration (intravenous or epidural) are suggested, as well as different adequate perioperative times of administration (at the induction of anesthesia or at the awakening). In this study, VAS measurements showed that ketamine provided good analgesia. Moreover, some patients who received preemptive ketamine did not require any postoperative analgesia within the first 24 hours of the operation. In our investigation, there was a statistical difference between the two groups in the total dose of analgesic consumption postoperatively and in the time interval to request the first analgesic.
In conclusion, a low dose of intravenously administered ketamine had a preemptive effect in reducing pain after appendectomies.
Go to : 




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download