Abstract
Botulinum toxin has been used for the treatment of many clinical disorders by producing temporary skeletal muscle relaxation. In pain management, botulinum toxin has demonstrated an analgesic effect by reducing muscular hyperactivity, but recent studies suggest this neurotoxin could have direct analgesic mechanisms different from its neuromuscular actions. At the moment, botulinum toxin is widely investigated and used in many painful diseases such as myofascial syndrome, headaches, arthritis, and neuropathic pain. Further studies are needed to understand the exact analgesic mechanisms, efficacy and complications of botulinum toxin in chronic pain disorders.
Go to : 
Botulinum toxin (BTX) is a pre-synaptic neuromuscular blocking agent that triggers chemical denervation by temporarily suppressing secretion of acetylcholine at motor nerve endings; therefore, BTX injections are useful for diseases with increased involuntary muscle activity or tension [1].
Justinus Kerner first reported on a case of death from BTX found in smoked sausage (botulus in Latin) in 1817, but the possibility for medical use was suggested much later [2]. After Alan Scott first used BTX-A for the treatment of strabismus, BTX injections have been used in many diseases, and its uses are continually expanding [2]. This review will focus mainly on the use of BTX in pain disorders.
BTX, a strong bacterial toxin, is created from fermentation of clostridium botulinum. BTX is a 300-900 kilodalton (kDa) protein complex, and the structure and total molecular weight is different depending on the type of bacteria and serotype. This protein complex is stable in acid but is decomposed in a physiological state to yield 150 kDa of neurotoxin composed of two chains. Eight different serotypes (A, B, C1, C2, D, E, F, G) are known, and 7 of these (not C2) are neurotoxins [3]. All types suppress secretion of acetylcholine at nerve endings, but there are differences in intracellular protein, mode of action, and effectiveness. From these, type A has been the most researched, and studies about the effects of other serotypes are on the increase. BTX is a neurotoxin that irreversibly combines with the pre-synaptic membrane of motor nerve endings, and this unique combination selectively acts on cholinergic synapses to suppress the secretion of acetylcholine. However, it does not affect nerve conduction or the production and storage of acetylcholine. Neurotoxins enter muscle nerve endings through pre-synaptic receptors via endocytosis and then are separated into two polypeptide chains by a protease. The separation rate of the chains is different depending on serotype: BTX-A separates 90-95%, whereas BTX-B separates only about 70% [3]. Heavy chains (100 kDa) internalize the toxin in the cell, while light chains (50 kDa) move to the cytosol through the vesicular membrane and specifically sever SNARE (Soluble NSF Attachment Protein Receptors) proteins involved with exocytosis of synaptic vesicles. The destruction of SNARE composites interferes with attachment of synaptic vesicles on the cell membrane, which cuts off the convergence of vesicles, and finally leads to suppression of acetylcholine secretion and induction of chemical denervation to paralyze muscle fiber [3]. The final effect of all serotype toxins appears to be suppression of acetylcholine secretion; however, each serotype destroys different areas of the SNARE protein, so action and potency are diverse [2].
The clinical effects of BTX appear to be weakness or paralysis of capacity proportional skeletal muscles. When a appropriate amount of BTX is injected into the muscle, partial chemical denervation is induced to reduce muscle contraction without complete paralysis and secretion of glandular tissues. The initial action of BTX on the muscle is dysfunction of alpha motor neurons that stimulate muscular fiber, and it also affects the gamma motor neurons distributed in the muscle spindles to reduce afferent feedback and weaken myotonus [4].
Go to : 
Skeletal muscle strength generally weakens 2-5 days after BTX injection, minimizes within 2 weeks and then recovers. This weakening effect continues from 6 weeks to 6 months (median 3-4 months), and the injection dose influences the degree and period of denervation. Muscle atrophy and change in muscular fiber appear during the period when the effect is strong, and the effect gradually weakens after 2-3 months. Recovery from local paralysis is explained in two ways: neuronal budding and regeneration of SNARE complexes [3,4].
Go to : 
Clinically, BTX is used locally, but it is known to distribute in the blood through systemic absorption. Because of less potent BTX coming into the market and high-dose BTX being used often, the danger of antibody formation that neutralizes the effect of BTX has increased. When antibodies are formed, the effect and duration of BTX injection decreases. In the case of BTX-A in dystonia, antibody formation rate is 1-5% in standard dosage [2].
Since BTX-B proteins do not combine with the areas with which BTX-A proteins combine, BTX-A antibodies do not interfere BTX-B [5], and treatments using different serotypes of BTX have been developed for patients with activated immune systems. However, BTX-B has a lower chain separation rate, which means lower potency and higher dosages, leading to increased immunogenic potential through more exposure of proteins. Therefore, more clinical research in this area is needed. Compared to BTX-B, BOTOX™ is 40-70 times and DYSPORT™ is 10-20 times stronger. However, there is no accurate data on conversion factors between BTX-B and BTX-A, so it is difficult to compare efficacy, side effects and cost.
In treating patients with dystonia, BTX-B showed higher frequency of dry mouth and visual impairment than BTX-A [6], and although this is an undesirable complication, it is an interesting phenomenon because it means that BTX-B has higher affinity to autonomic nerve fibers.
Go to : 
The method, dosage, and frequency of BTX injection should depend on patient circumstances. For deep muscles, it helps to conduct BTX injection under radiograph, electromyography and electrical stimulation. Dosage should be decided on the basis of symptom(s), muscle type, weight, previous injection reaction, and accompanying diseases. It is recommended to use the minimum amount needed to achieve the desired effect while minimizing adverse effects. Side effects such as pain in the injected area, bruises and muscular weakness are most common, while fatigue, fever, dry mouth, and ptosis can also appear 1-2 weeks after injection. Rarely, an allergic reaction can be triggered and injection in areas near the neck and mouth can cause dysphagia. Headache, lethargy, and muscle pain can appear when an excessive dosage is used, but all side effects are temporary and reversible [7].
BTX should not be used when there is an infection in the injection site, allergy to the medication, myasthenia gravis, or Eaton Lambert syndrome. BTX injection is also prohibited in children less than 12 years of age and pregnant and nursing mothers. Caution is needed for patients with peripheral motor neuron disease, neuromuscular conduction disease, and those taking drugs that can interfere with neuromuscular conduction [7].
Go to : 
BTX weakens muscles with acute pain and ceases the repetition cycle of muscle spasm and pain, to maintain pain alleviation and allow physical activities that help long-term recovery.
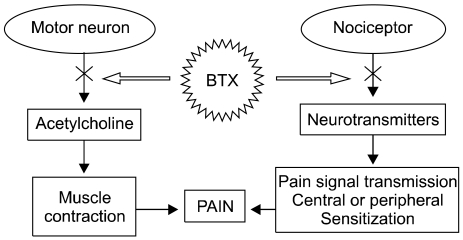
The exact mode of action of BTX for pain relief has not been revealed completely, but it may be multifactorial (Fig. 1). Reduction of dystonia and muscle spasm is considered to be the effect of acetylcholine secretion, but in recent animal testing, other analgesic effects of BTX have been suggested [8]. It has been suggested that BTX suppresses the secretion of neurotransmitters directly decreasing peripheral sensitization and indirectly decreasing central sensitization. However, there is still some debate [4,8].
Go to : 
The specificity of BTX on cholinergic nerves is due to specific acceptors in the nerve terminal membrane, but it has been revealed experimentally that BTX suppresses other neurotransmitters such as norepinephrine in the same mode of action it destroys SNARE complexes [2]. However, many nerve cells do not have specific acceptors for BTX; in addition, it is absorbed only through pinocytotic pathways and is therefore not highly effective. As a result, BTX is useful for neuromuscular diseases because of its high affinity to cholinergic synapses.
However, several exceptions to BTX's acceptor specificity have been noted. For example, BTX-A affects secretion of substance P in nerves cultured from the dorsal root ganglion of mouse embryos and decreases the secretion of calcitonin gene-related peptide (CGRP) in cultures of neurons from the trigeminal ganglion. Furthermore, subcutaneous injection of BTX-A showed decreased inflammation from stimulus, secretion of glutamate in the nociceptor's axon, and activity of spinal dorsal horn neurons in mice [2]. These results suggest that BTX-A directly suppresses nociceptors through suppressing the secretion of neurotransmitters that act on nerve conduction and peripheral and central sensitizations. Therefore, BTX is a strong suppressant of acetylcholine as well as providing pain relief and anti-inflammatory effects by suppressing secretion of other neurotransmitters and neuropeptides [9].
More research is needed to explain the suppressant effect of BTX on pain receptors, but BTX's mode of action can be considered as follows: 1) normalization of muscular hyperactivity, 2) normalization of excessive muscle spindle activity, 3) retrograde neuronal flow to the CNS, 4) suppression of neuropeptide secretion in nociceptors in the central and peripheral nervous systems [10].
Go to : 
BTX is used in the treatment of strabismus, blepharospasm, hemifacial spasm, adductor spasmodic dysphonia, bruxism, mandibular dystonia, cervix dystonia, local or segmental dystonia, hypercontractility of the internal anal sphincter, detrusor dyssynergy, spasticity, and stuttering [1,2]. As the safety of BTX has been shown, it is also used in beauty treatments, hyperhidrosis, sialorrhea and neuropathic pain. The efficacy of BTX in these diseases can be explained by the effect on cholinergic nerve conduction.
BTX has been used for 20 years in the treatment of diseases with accelerated muscle contraction and tension. Experimental research of BTX in new domains is being conducted, especially in the treatment of primary headache and cervical, scapular and lumbar myofascial pain syndrome (MFPS) [1,2].
As the effect of common treatment for MFPS is in many cases unsatisfactory, the treatment is needed for a long time. In addition, as painkillers are used long-term, this ineffective treatment is costly and complications such as gastrointestinal and renal toxicity can be a problem. On the other hand, simple BTX injections accompanied by physiotherapy can be effective for 1-3 months without these complications. However, fatigue, muscle pain, and headaches can appear as complications for a few days [11-13].
Göbel et al. [14] verified the usefulness and safety of BTX in MFPS through a comparative study that observed pain where BTX was injected in trigger points on the neck or shoulder. There have also been reports that BTX was effective for a patient with temporomandibular joint dysfunction [15] and a patient with fasciitis in the foot [16].
Foster et al. [17] verified long-term effectiveness of BTX in patients with chronic back pain through a double-blind randomized study. 60% of the BTX group injected with 40-50 units in 5 areas in the muscle next to the spine showed a significant decrease in pain, and the pain relief was maintained for 3-4 months. However, one study on BTX's efficacy for chronic back pain was problematic because it was conducted on only a few patients with varying pathophysiology [18].
Generally, BTX-A shows a consistent effectiveness for migraines [19,20], and the treatment effect appears usually 3 months after injection. Hypotonia, neck stiffness and neck pain are the most common complications at about 3% [20]. BTX-A has a preventative effect on migraines and works well in the treatment of post-whiplash and cervicogenic headaches. However, there are also reports that BTX-A does not have an effect on the degree of pain, number or duration of episodic migraines [21].
The treatment mechanism of BTX for migraines is uncertain, but it has been suggested that it is due to muscle relaxation and resultant decrease in pressure on the trigeminal nerve. The fact that many patients with migraines have hypertrophic corrugator muscles that pressure the trigeminal nerve and temporal region supports this theory [22]. Another suggested treatment mechanism is associated with neurotransmitters through influence on the peripheral and central nervous systems.
There are reports that BTX is effective on chronic daily headaches. For tension headaches, tension relaxation was anticipated due to the contraction of cephalic muscle. However, in many clinical tests, BTX was found to be ineffective or less effective on tension headaches in comparison with migraine. The ineffectiveness is believed to result from the weaker central sensitization compared to the case of migraines.
There is a report that BTX decreased the duration and degree of pain and increased joint motion range in post-whiplash headaches and muscle pain [23].
BTX-A is known to combine with nociceptors in the C fiber to suppress secretion of pain transmitters, such as substance P, CGRP, and glutamate, which leads to a decrease in pain transmission and peripheral sensitization. This result implies BTX can be used in the clinical treatment of various arthralgias because BTX joint injection affects efferent nerve function (secretion of neurotransmitters in the sensory nerves) [24].
There have been studies of patients with knee osteoarthritis or pain after knee artificial joint surgery that observed improvement in pain and function. However, further research is necessary on possible complications such as aggravation of infection and pain, effect on muscle strength and sensory nerves, and neuropathic joint degeneration [24].
In chronic genitourinary pain syndrome, BTX-A has been associated with a decrease in muscle tension and direct pain relief [25]. There are also studies showing that pain and function improved in vulvodynia, vaginismus, levator ani convulsion, chronic prostatitis, and interstitial cystitis when BTX was injected [25].
Secretion of pro-inflammatory agents (cytokines, adenosine, bradykinin, serotonin, prostaglandins) sensitizes neurotransmission and increases pain sensation by creating a temporary neuropathic state. The theory that BTX can treat neuropathic pain by suppressing the secretion of neurotransmitters to affect pain transmission, peripheral and central sensitization needs to be verified further [2].
Patients with complex regional pain syndrome in the areas near the neck, below the larynx, or near the scapula usually accompany MFPS in the same area, and depending on muscle size, 25-50 IU of BTX-A can be injected in trigger points. There are reports of improvement in pain and function after injecting BTX-A or -B for patients with neuropathy after spinal cord injury, neuralgia after herpes zoster, trigeminal neuralgia, and pain after amputation of upper or lower limbs [26].
Go to : 
BTX can be safely used in the treatment of chronic pain where complications from drug treatment are a concern. The initial cost of BTX treatment can be expensive, but it has the advantage that there are less complications and hospitalization is unnecessary or of short duration. Furthermore, treatment effect can be maintained for 3-4 months, while supplementary medications are reduced.
Further research is necessary on the exact treatment mechanism of BTX for chronic pain and its role in multifactorial treatment. Future research should include expanding the domain of treatable diseases, comparing injection intervals, formation of antibodies, cost, and complications such as muscle weakness.
Go to : 
References
1. Lew MF. Review of the FDA-approved uses of botulinum toxins, including data suggesting efficacy in pain reduction. Clin J Pain. 2002; 18(6 Suppl):S142–S146. PMID: 12569961.

2. Colhado OC, Boeing M, Ortega LB. Botulinum toxin in pain treatment. Rev Bras Anestesiol. 2009; 59:366–381. PMID: 19488551.

3. Setler PE. Therapeutic use of botulinum toxins: background and history. Clin J Pain. 2002; 18(6 Suppl):S119–S124. PMID: 12569958.

4. Freund B, Schwartz M. Temporal relationship of muscle weakness and pain reduction in subjects treated with botulinum toxin A. J Pain. 2003; 4:159–165. PMID: 14622713.

5. Factor SA, Molho ES, Evans S, Feustel PJ. Efficacy and safety of repeated doses of botulinum toxin type B in type A resistant and responsive cervical dystonia. Mov Disord. 2005; 20:1152–1160. PMID: 15954134.

6. Comella CL, Jankovic J, Shannon KM, Tsui J, Swenson M, Leurgans S, et al. Comparison of botulinum toxin serotypes A and B for the treatment of cervical dystonia. Neurology. 2005; 65:1423–1429. PMID: 16275831.

7. Apostol C, Abdi S, Moeller-Bertram T, Smith HS, Argoff CE, Wallace M. Smith HS, editor. Botulinum toxins for the treatment of pain. Current therapy in pain. 2009. Philadelphia: Saunders;p. 489–498.

8. Arezzo JC. Possible mechanisms for the effects of botulinum toxin on pain. Clin J Pain. 2002; 18(6 Suppl):S125–S132. PMID: 12569959.

9. Sycha T, Samal D, Chizh B, Lehr S, Gustorff B, Schnider P, et al. A lack of antinociceptive or antiinflammatory effect of botulinum toxin A in an inflammatory human pain model. Anesth Analg. 2006; 102:509–516. PMID: 16428552.

10. Göbel H, Heinze A, Heinze-Kuhn K, Austermann K. Botulinum toxin A in the treatment of headache syndromes and pericranial pain syndromes. Pain. 2001; 91:195–199. PMID: 11275374.

11. Kamanli A, Kaya A, Ardicoglu O, Ozgocmen S, Zengin FO, Bayik Y. Comparison of lidocaine injection, Botulinum toxin injection, and dry needling to trigger points in myofascial pain syndrome. Rheumatol Int. 2005; 25:604–611. PMID: 15372199.

12. Fishman LM, Konnoth C, Rozner B. Botulinum neurotoxin type B and physical therapy in the treatment of piriformis syndrome: a dose-finding study. Am J Phys Med Rehabil. 2004; 83:42–50. PMID: 14709974.

13. Lang AM. Botulinum toxin type B in piriformis syndrome. Am J Phys Med Rehabil. 2004; 83:198–202. PMID: 15043354.

14. Göbel H, Heinze A, Reichel G, Hefter H, Benecke R. Dysport myofascial pain study group. Efficacy and safety of a single botulinum type A toxin complex treatment (Dysport) for the relief of upper back myofascial pain syndrome: results from a randomized double-blind placebo-controlled multicentre study. Pain. 2006; 125:82–88. PMID: 16750294.

15. von Lindern JJ, Niederhagen B, Bergé S, Appel T. Type A botulinum toxin in the treatment of chronic facial pain associated with masticatory hyperactivity. J Oral Maxillofac Surg. 2003; 61:774–778. PMID: 12856249.

16. Babcock MS, Foster L, Pasquina P, Jabbari B. Treatment of pain attributed to plantar fasciitis with botulinum toxin a: a short-term, randomized, placebo-controlled, double-blind study. Am J Phys Med Rehabil. 2005; 84:649–654. PMID: 16141740.

17. Foster L, Clapp L, Erickson M, Jabbari B. Botulinum toxin A and chronic low back pain: a randomized, double-blind study. Neurology. 2001; 56:1290–1293. PMID: 11376175.

18. Difazio M, Jabbari B. A focused review of the use of botulinum toxins for low back pain. Clin J Pain. 2002; 18(6 Suppl):S155–S162. PMID: 12569963.

19. Silberstein S, Mathew N, Saper J, Jenkins S. Botulinum toxin type A as a migraine preventive treatment. For the BOTOX Migraine Clinical Research Group. Headache. 2000; 40:445–450. PMID: 10849039.

20. Silberstein SD, Göbel H, Jensen R, Elkind AH, Degryse R, Walcott JM, et al. Botulinum toxin type A in the prophylactic treatment of chronic tension-type headache: a multicentre, double-blind, randomized, placebo-controlled, parallel-group study. Cephalalgia. 2006; 26:790–800. PMID: 16776693.

21. Evers S, Vollmer-Haase J, Schwaag S, Rahmann A, Husstedt IW, Frese A. Botulinum toxin A in the prophylactic treatment of migraine--a randomized, double-blind, placebo-controlled study. Cephalalgia. 2004; 24:838–843. PMID: 15377314.

22. Smuts JA, Schultz D, Barnard A. Mechanism of action of botulinum toxin type A in migraine prevention: a pilot study. Headache. 2004; 44:801–805. PMID: 15330827.

23. Freund BJ, Schwartz M. Use of botulinum toxin in chronic whiplash-associated disorder. Clin J Pain. 2002; 18(6 Suppl):S163–S168. PMID: 12569964.

24. Mahowald ML, Krug HE, Singh JA, Dykstra D. Jankovic J, Albanese A, Atassi MZ, Dolly JO, Hallett M, Mayer NH, editors. Botulinum toxin for osteoarticular pain. Botulinum toxin. Therapeutic clinical practice and science. 2009. Philadelphia: Saunders;p. 295–306.

Go to : 




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download