MATERIALS AND METHODS
This retrospective study analyzed characteristics of women with a history of labor and delivery who were diagnosed with RAD from six hospitals within the Partners Healthcare System in Massachusetts, USA. The review board of the Research Patient Data Registry approved the study protocol (#2018P003056).
Medical records of women with a diagnosis of RAD, defined by the International Classification of Diseases codes (Ninth Revision Clinical Modification ICD-9-CM and Tenth Revision ICD-10-CM), from January 1, 2009 to December 31, 2018 from six hospitals of the Partners Healthcare System were collected. Women who delivered at these hospitals with delivery notes available were included. Data including demographic information, diagnosis (
Table 1), health history, delivery notes, and ultrasound (when available) were extracted.
Table 1
Diagnostic codes in ICD-9-CM and ICD-10-CM used for research patient data registry search
|
Diagnosis |
ICD-9-CM |
ICD-10-CM |
|
RAD |
728.84 |
M62.08 |
|
Hernia |
Diaphragmatic |
553.3 |
K44.9 |
|
Ventral |
553.2 |
k46.9; k43.9 |
|
Groin |
550.9 |
K40.90; K40.20 |
|
Incisional |
553.21 |
K43.2; K43.0; K40.2 |
|
Umbilical |
552.1 |
K42.0; K42.9 |
|
Low back pain |
724.2 |
M54.5 |
|
Incontinence |
788.33; 788.34; 788.35; 788.37; 788.39 |
N39.46 |
|
Pelvic and perineal pain |
456.5 |
M25.551; M25.552; M79.651; M79.652; R10.2 |
|
Superficial fascial lesions |
728.89; 728.79; 756.89 |
S39.011A; S39.012A |
|
Fascial lesions in low back pain |
728.89; 728.79; 756.89 |
S39.012A |
|
Depressive disorder |
296.20; 296.30; 296.32; 296.33; 296.35; 296.36; 296.50; 296.80; 296.89; 300.4; 309.1; 309.0; 311; V79.0 |
F31.30; 31.31; F31.32; F32.0; F32.1; F32.2; F32.3; F32.4; F32.5; F32.89; F32.9; F33.0; F33.1; F33.2; F33.40; F33.41; F33.42; F33.9; F43.21; F43.23; FZ13.31 |
|
Major depressive disorder |
296.2; 296.20; 296.30; 296.31; 296.32; 296.33; 296.35; 296.36 |
F32.0; F32.1; F32.2; F32.3; F32.4; F32.5; F32.9; F33.0; F33.1; F33.2; F33.40; F33.41; F33.42; F33.9 |

The demographic and health history variables included in this study were age at onset of RAD, age of labor and delivery, body mass index (BMI), race or ethnicity, smoking state, and alcohol use. Only records from first labor and delivery were analyzed. Apart from LBP and PP, other concomitant conditions that were identified included hernia, mixed incontinence, depressive disorder (DD), and strain of muscle, fascia, and tendon (SMFT) (See
Table 1 for ICD codes used in database search). In this study, there was a focus on the trends of cumulative composition ratio for conditions, including RAD, umbilical hernia, LBP, PP, DD, and SMFT and the timing relationship between them and the first delivery. The timing relationship between LBP/PP and RAD were also analyzed. The cumulative composition ratio of disease was defined as the percentage of patients who were diagnosed with the disease in the past or current year, accounting for all patients. The time interval was set as 2-years, centered on labor and delivery.
SPSS Statistics version 20 (IBM Corp., Armonk, NY) was used to perform all statistical analyses. Data was summarized as frequencies and percentages or mean and standard deviation. Pearson’s and continuity correction chi-square tests and Fisher’s exact test were used for counting data. The Mann–Whitney U nonparametric test was used for continuous variables. Linear-by-Linear association chi-square tests were used to test the difference between the cumulative composition ratio of conditions. Wilcoxon’s W nonparametric test was used to test the difference of age at onset between groups. Univariate and multivariate binary logistic regression analysis models were used to identify the independent risk factors, using LBP and PP as the dependent variables, respectively. All variables on univariate analysis were used to identify the independent risk factors of LBP and PP as well. The odds ratio (OR) and 95% confidence interval (CI) were estimated from multivariate regression analysis. A forward stepwise regression analysis (α entry = 0.10, α removal = 0.10) was used to reduce confounding bias. A two-tailed P value less than 0.05 was considered statistically significant for all tests. The risk ratio formula was used to calculate the relative risk.
Go to :

RESULTS
Of the 866 women with RAD, 417 women underwent labor delivery. Data from the 317 (76.0%) women who had complete delivery operative notes were analyzed. Of the 317 women, 218 (68.8%) had one or more CD history. Age at onset of RAD in the non-CD group (without CD exposure) was earlier than those in CD group (
P = 0.017) (
Fig. 1). Women who underwent CD demonstrated 4.5 times greater risk of RAD than those who had no CD exposure. Most CDs were performed with Pfannenstiel skin-incision (98.2%), low transverse uterine incision (93.1%), and suture closure method (80.7%). Epidural anesthesia accounted for 73.7% of all anesthesia in non-CD group, whereas spinal anesthesia accounted for 57.3% in CD group (
Table 2).
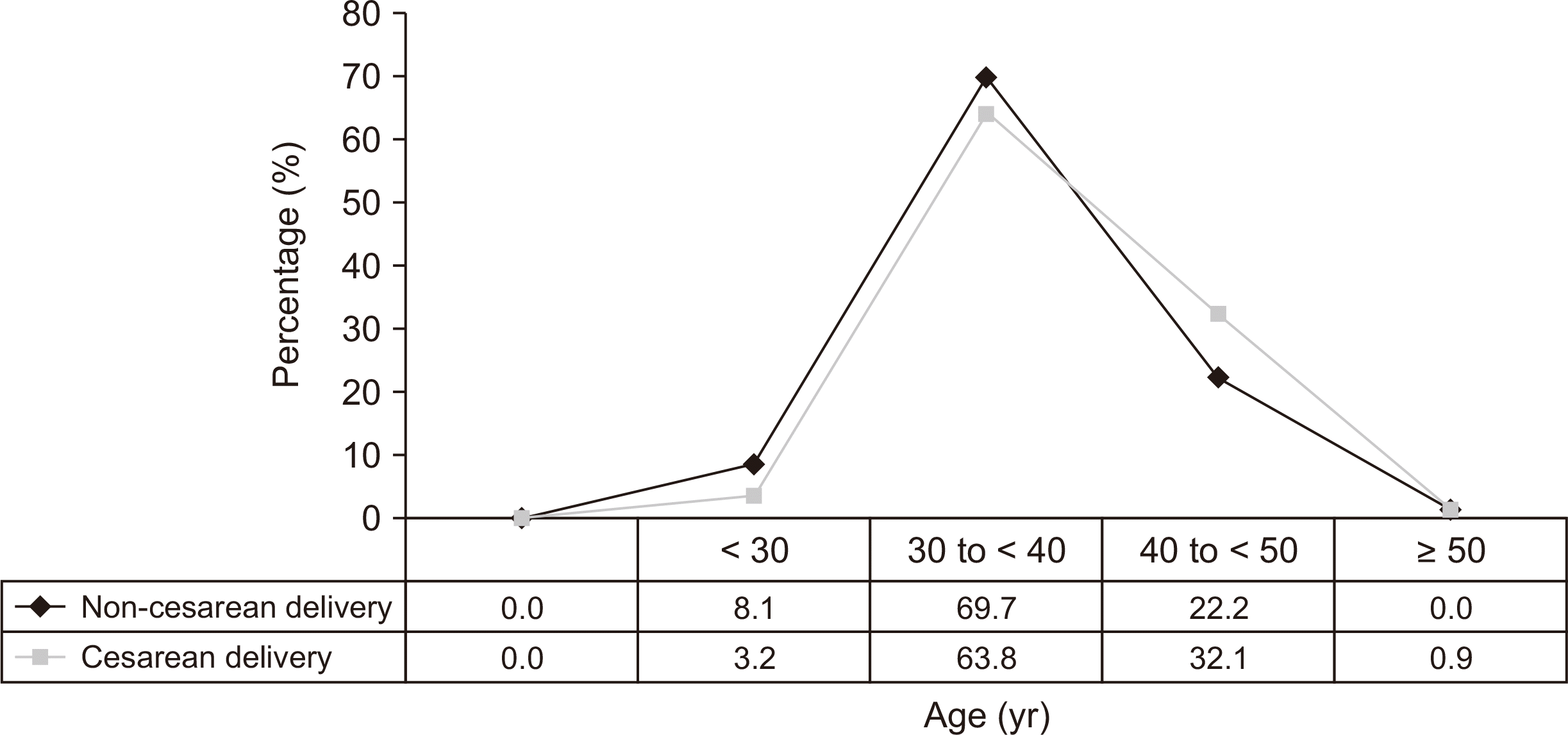 | Fig. 1The comparison of onset age of rectus abdominis diastasis between non-cesarean delivery and cesarean delivery group. 
|
Table 2
Patients demographics and characteristics
|
Characteristics |
Non-CD group (n = 99) |
CD group (n = 218) |
Total (n = 317) |
P value |
|
Demographic characteristic |
|
Age of first delivery (yr) |
33.2 ± 4.3 |
35.5 ± 4.4 |
34.8 ± 4.5 |
< 0.001a
|
|
Age at onset of RAD (yr) |
35.9 ± 4.7 |
37.5 ± 4.6 |
37.0 ± 4.7 |
0.004a
|
|
Race or ethnic group |
|
Black/African |
3 (3.0) |
12 (5.5) |
15 (4.7) |
0.868 |
|
Asian |
7 (7.1) |
12 (5.5) |
19 (6.0) |
|
|
Hispanic |
4 (4.0) |
10 (4.6) |
14 (4.4) |
|
|
White |
80 (80.8) |
172 (78.9) |
252 (79.5) |
|
|
Others |
5 (5.1) |
12 (5.5) |
17 (5.4) |
|
|
Body mass index (kg/m2) |
|
Mean |
26.0 ± 4.6 |
28.3 ± 5.9 |
27.6 ± 5.6 |
0.001a
|
|
18.0 to < 25 |
45 (45.5) |
68 (31.2) |
112 (35.3) |
0.028b
|
|
25 to < 30 |
35 (35.4) |
83 (38.1) |
118 (37.2) |
|
|
30 to < 40 |
18 (18.2) |
58 (26.6) |
76 (24.0) |
|
|
≥ 40 |
1 (1.0) |
10 (4.6) |
11 (3.5) |
|
|
Social history |
|
Smoking state |
9 (9.1) |
28 (12.8) |
37 (11.7) |
0.335 |
|
Alcohol use |
48 (48.5) |
84 (38.5) |
132 (41.6) |
0.096 |
|
Depressive disorder |
20 (20.2) |
53 (24.3) |
73 (23.0) |
0.421 |
|
Concomitant conditions |
|
Pelvic pain |
21 (21.2) |
48 (22.0) |
69 (21.8) |
0.872 |
|
Low back pain |
20 (20.2) |
58 (26.6) |
78 (24.6) |
0.220 |
|
Strain of muscle, fascial and tendon |
2 (2.0) |
17 (7.8) |
19 (6.0) |
0.080c
|
|
Mixed incontinence |
5 (5.1) |
8 (3.7) |
13 (4.1) |
0.566 |
|
Hernias |
31 (31.3) |
68 (31.2) |
99 (31.2) |
0.983 |
|
Diaphragmatic hernia |
1 (1.0) |
5 (2.3) |
6 (1.9) |
0.740c
|
|
Ventral hernia |
13 (13.1) |
38 (17.4) |
51 (16.1) |
0.334 |
|
Groin hernia |
5 (5.1) |
3 (1.4) |
8 (2.5) |
0.122c
|
|
Incisional hernia |
0 |
8 (3.7) |
8 (2.5) |
0.061d
|
|
Umbilical hernia |
26 (26.3) |
46 (21.1) |
72 (22.7) |
0.309 |
|
Adhesions in abdominal and/or pelvic cavity |
|
Minimal |
- |
8 (3.7) |
- |
- |
|
Moderate |
- |
16 (7.3) |
- |
- |
|
Dense |
- |
16 (7.3) |
- |
- |
|
Not reported |
99 (100.0) |
178 (81.7) |
- |
- |
|
Characteristics of first delivery procedure |
|
Skin-incision type |
|
Pfannenstiel |
- |
214 (98.2) |
- |
- |
|
Vertical |
- |
4 (1.8) |
- |
- |
|
Closure method |
|
Staples |
- |
42 (19.3) |
- |
- |
|
Suture |
- |
176 (80.7) |
- |
- |
|
Uterine incision |
|
Low transverse |
- |
203 (93.1) |
- |
- |
|
Other |
- |
15 (6.9) |
- |
- |
|
Anesthesia type |
|
Spinal |
2 (2.0) |
125 (57.3) |
127 (40.1) |
- |
|
Epidural |
73 (73.7) |
62 (28.5) |
135 (42.6) |
- |
|
Combined spinal epidural |
- |
21 (9.6) |
21 (6.6) |
- |
|
General |
- |
10 (4.6) |
10 (3.2) |
- |
|
Local |
9 (9.1) |
- |
9 (2.8) |
- |
|
None |
15 (15.2) |
- |
15 (4.7) |
- |

The age of first delivery, onset age of RAD, and BMI in CD group was significantly higher than that in non-CD group (35.5 years old
vs. 33.2 years old,
P < 0.001; 37.5 years old
vs. 35.9 years old,
P = 0.004; 28.3 kg/m
2 vs. 26.0 kg/m
2,
P = 0.001; respectively). The distribution of the onset age of RAD in the CD group was significantly different from that in non-CD group as well (
P for trend = 0.017) (
Fig. 1). One hundred thirty-two cases (41.6%) in this population were alcohol users. Adhesions in the abdominal and/or pelvic cavity were noted in 40 subjects (17.3%) who underwent CD procedures (
Table 2). In this population, the rates of concomitant LBP, DD, umbilical hernia, PP, and SMFT were 24.6%, 23.0%, 22.7%, 21.8%, and 6.0%, respectively (
Table 2). The prevalence of LBP, PP, mixed incontinence, hernias, DD, and SMFT in the non-CD group did not differ from that in the CD group (20.2%
vs. 26.6%,
P = 0.220; 21.2%
vs. 22.0%,
P = 0.872; 5.1%
vs. 3.7%,
P = 0.566; 31.2%
vs. 31.2%,
P = 0.983; 20.2%
vs. 24.3%,
P = 0.421; 2.0%
vs. 7.8%,
P = 0.080; respectively) (
Table 2).
The cumulative composition ratio of LBP in every age stage of the period from 8 years pre-first delivery to 8 years post-first delivery was significantly higher than in the other five conditions (RAD, umbilical hernia, PP, DD, and SMFT) (
P for trend < 0.001) (
Fig. 2). Before the first delivery, the proportion of the patients with LBP was 57.7% (45/78), which was higher than other concomitant condition (
P < 0.001), and the rate increased rapidly up to 83.3% within 2 years after first delivery (
P = 0.001) (
Fig. 3). In addition, after first delivery, 43 of 69 subjects (62.3%) had PP compared to 20 of 69 (29.0%) before first delivery (
P < 0.001) (
Fig. 2).
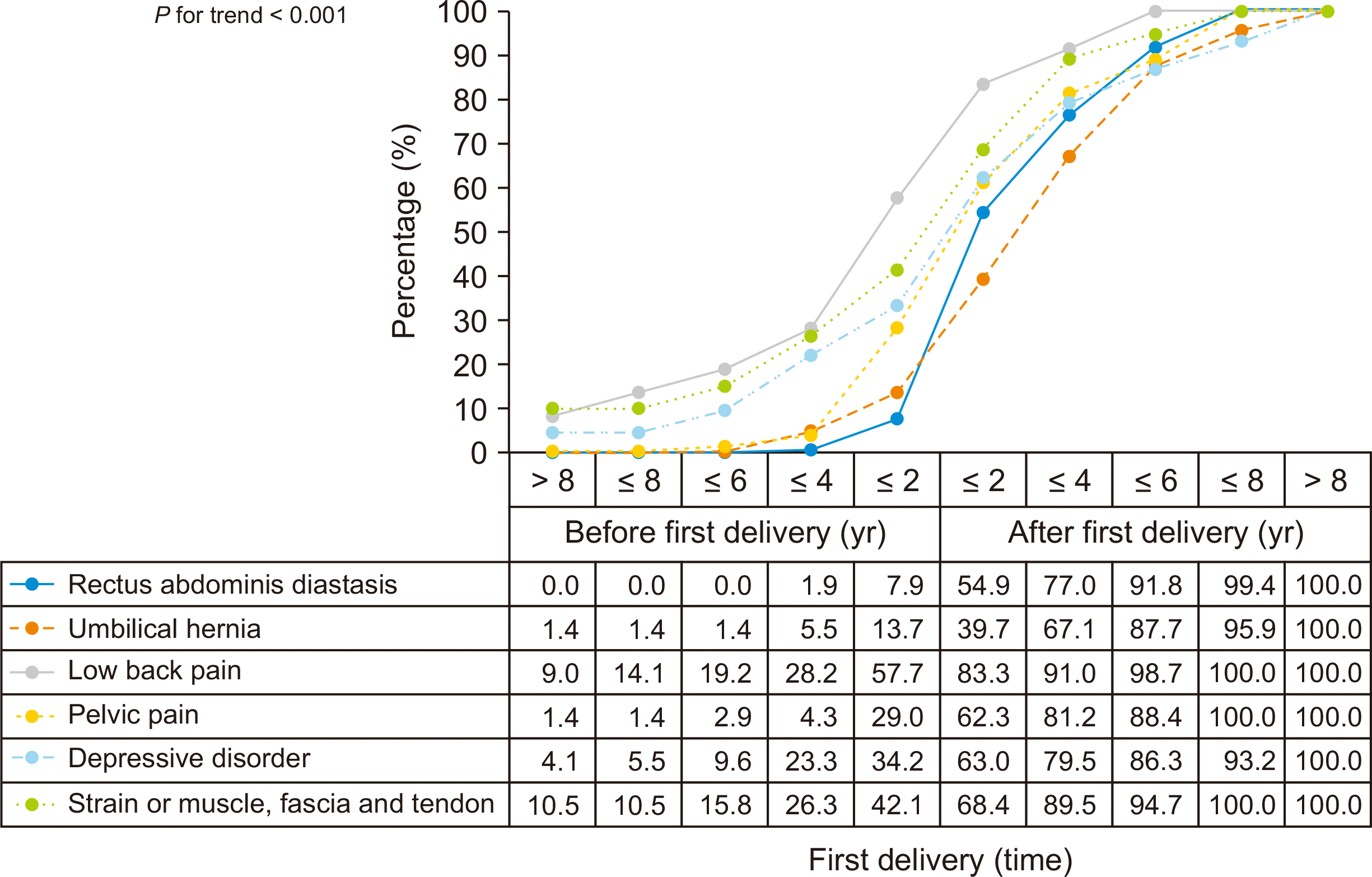 | Fig. 2The time relations between concomitant conditions and first delivery. 
|
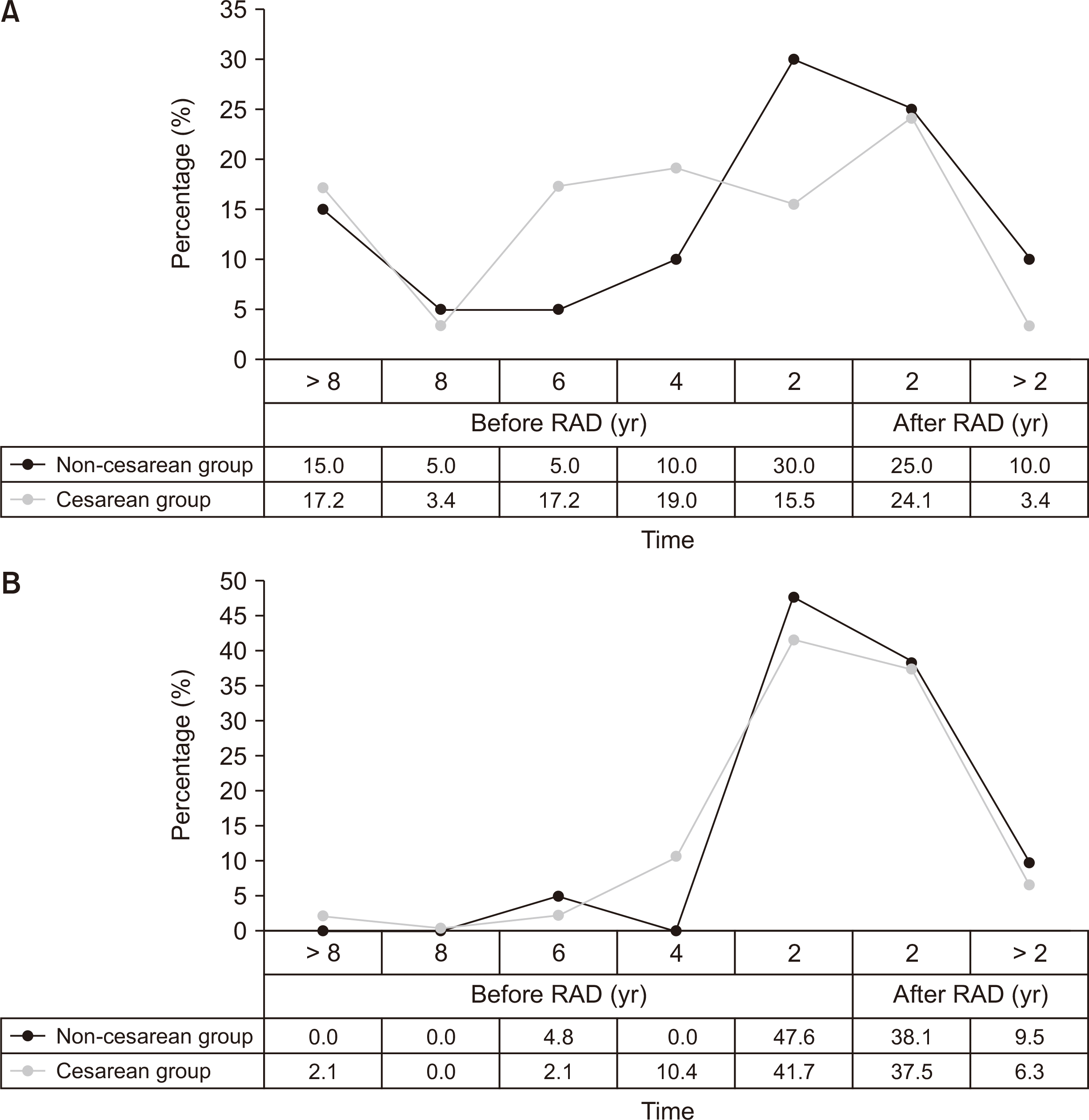 | Fig. 3The relation between low back pain (LBP)/pelvic pain (PP) and rectus abdominis diastasis (RAD). (A) Onset age of LBP related to RAD. (B) Onset age of PP related to RAD. 
|
Before RAD, 13 of 20 cases (65.0%) had LBP in the non-CD group compared with 42 of 58 (72.4%) in CD group (P = 0.531). There were no significant differences in the distribution of the age at onset of LBP between the CD group and non-CD groups (P for trend = 0.235). The rate of PP the in non-CD group was 52.4% compared to 56.3% in the CD group before RAD (P = 0.766). In the case of PP, 20 of 48 women (41.7%) first encountered pain within 2 years before having RAD in the CD group compared to 10 of 21 (47.6%) in the non-CD group (P = 0.646). The distribution of age at onset of PP in the CD group also did not differ from that in non-CD group (P for trend = 0.496).
For the women with RAD in this study, subjects with LBP had higher prevalence of DD (34.6%
vs. 19.3%,
P = 0.005), SMFT (15.4%
vs. 2.9%,
P < 0.001), and PP (34.6%
vs. 17.6%,
P = 0.002) than those without LBP (
Table 3). Univariate and multivariate regression analysis identified that women with DD, SMFT, and PP were more likely to have LBP (OR = 1.91, 95% CI 1.06 to 3.47,
P = 0.032; OR = 4.50, 95% CI 1.64 to 12.36,
P = 0.003; OR = 2.14, 95% CI 1.17 to 3.89,
P = 0.013; respectively) (
Table 4).
Table 3
Characteristics of postpartum rectus abdominis diastasis patients with and without low back pain
|
Variable |
Without low back pain (n = 239) |
With low back pain (n = 78) |
P value |
|
Demographic characteristics |
|
|
|
|
Age (yr) |
|
|
|
|
< 30 |
6 (2.5) |
1 (1.3) |
0.303a
|
|
30 to < 40 |
145 (60.7) |
41 (52.6) |
|
40 to < 50 |
86 (36.0) |
36 (46.2) |
|
50 to < 60 |
2 (0.8) |
0 |
|
Race or ethnic group |
|
|
|
|
Black/African |
10 (4.2) |
5 (6.4) |
0.788a
|
|
Asian |
13 (5.4) |
6 (7.7) |
|
Hispanic |
10 (4.2) |
4 (5.1) |
|
White |
194 (81.2) |
58 (74.4) |
|
Others |
12 (5.0) |
5 (6.4) |
|
Body mass index (kg/m2) |
|
|
|
|
18.5 to < 25 |
90 (37.7) |
22 (28.2) |
0.419a
|
|
25 to < 30 |
86 (36.0) |
32 (41.0) |
|
30 to < 40 |
56 (23.4) |
20 (25.6) |
|
≥ 40 |
7 (2.9) |
4 (5.1) |
|
Delivery mode |
|
|
|
|
Non-cesarean |
79 (33.1) |
20 (25.6) |
0.220 |
|
Cesarean |
160 (66.9) |
58 (74.4) |
|
Social history |
|
|
|
|
Smoking state |
27 (11.3) |
10 (12.8) |
0.716 |
|
Alcohol use |
103 (43.1) |
29 (37.2) |
0.357 |
|
Depressive disorder |
46 (19.2) |
27 (34.6) |
0.005 |
|
Concomitant conditions |
|
|
|
|
Pelvic pain |
42 (17.6) |
27 (34.6) |
0.002 |
|
Strain of muscle, fascial and tendon |
7 (2.9) |
12 (15.4) |
< 0.001 |
|
Adhesions |
30 (12.6) |
10 (12.8) |
0.951 |
|
Hernia status |
77 (32.2) |
22 (28.2) |
0.507 |
|
Hernia type |
|
|
|
|
Diaphragmatic hernia |
2 (0.8) |
4 (5.1) |
0.053b
|
|
Ventral hernia |
38 (15.9) |
13 (16.7) |
0.873 |
|
Groin hernia |
4 (1.7) |
4 (5.1) |
0.203b
|
|
Incisional hernia |
7 (2.9) |
1 (1.3) |
0.697b
|
|
Umbilical hernia |
57 (23.8) |
15 (19.2) |
0.398 |

Table 4
Univariate and multivariate binary regression analyses of underlying factors to low back pain in postpartum patients with rectus abdominis diastasis
|
Variable |
Univariate |
|
Multivariate |
|
P value |
OR (95% CI) |
P value |
OR (95% CI) |
|
Demographic characteristics |
|
|
|
|
|
|
Age (yr) |
|
|
|
|
|
|
< 30 |
|
1 |
|
0.405 |
NS |
|
30 to < 40 |
0.629 |
1.70 (0.20-14.50) |
|
|
|
|
40 to < 50 |
0.402 |
2.51 (0.29-21.62) |
|
|
|
|
50 to < 60 |
0.999 |
0 |
|
|
|
|
Race or ethnic group |
|
|
|
|
|
|
Black/African |
|
1 |
|
0.529 |
NS |
|
Asian |
0.914 |
0.92 (0.22-3.92) |
|
0.346 |
NS |
|
Hispanic |
0.782 |
0.80 (0.17-3.89) |
|
0.339 |
NS |
|
White |
0.365 |
0.60 (0.20-1.82) |
|
0.602 |
NS |
|
Others |
0.811 |
0.83 (0.19-3.72) |
|
0.808 |
NS |
|
Body mass index (kg/m2) |
|
|
|
|
|
|
18.5 to < 25 |
|
1 |
|
0.622 |
NS |
|
25 to < 30 |
0.183 |
1.52 (0.82-2.82) |
|
|
|
|
30 to < 40 |
0.282 |
1.49 (0.73-2.92) |
|
|
|
|
≥ 40 |
0.205 |
2.34 (0.63-8.70) |
|
|
|
|
Cesarean exposure |
0.221 |
1.43 (0.81-2.55) |
|
0.628 |
NS |
|
Social history |
|
|
|
|
|
|
Smoking state |
0.716 |
1.16 (0.53-2.51) |
|
0.568 |
NS |
|
Alcohol use |
0.358 |
0.78 (0.46-1.32) |
|
0.327 |
NS |
|
Depressive disorder |
0.006 |
2.22 (1.23-3.19) |
|
0.032 |
1.91 (1.06-3.47) |
|
Concomitant conditions |
|
|
|
|
|
|
Strain of muscle, fascial and tendon |
< 0.001 |
6.03 (2.28-15.92) |
|
0.003 |
4.50 (1.64-12.36) |
|
Adhesions |
0.951 |
1.03 (0.48-2.20) |
|
N/A |
N/A |
|
Pelvic pain |
0.002 |
2.48 (1.40-4.41) |
|
0.013 |
2.14 (1.17-3.89) |
|
Hernia status |
0.507 |
0.83 (0.47-1.45) |
|
0.575 |
NS |
|
Hernia type |
|
|
|
|
|
|
Diaphragmatic hernia |
0.034 |
6.41 (0.15-35.68) |
|
0.326 |
NS |
|
Ventral hernia |
0.873 |
1.06 (0.53-2.11) |
|
0.724 |
NS |
|
Groin hernia |
0.108 |
3.18 (0.78-13.01) |
|
0.065 |
NS |
|
Incisional hernia |
0.427 |
0.43 (0.05-3.51) |
|
0.182 |
NS |
|
Umbilical hernia |
0.399 |
0.76 (0.40-1.44) |
|
0.667 |
NS |

Women with PP in the present study had higher coexisting conditions of LBP (39.1%
vs. 20.6%,
P < 0.001) and SMFT (13.0%
vs. 4.0%,
P = 0.005) as well. Interestingly, the occurrence of umbilical hernia in the PP group was significantly lower than non-PP group (10.1%
vs. 26.2%,
P = 0.005) (
Table 5). Univariate and multivariate regression analysis also identified that women with LBP tended to have PP (OR = 2.44, 95% CI 1.36 to 4.40,
P = 0.003). Notably, these analyses implied that women with concomitant umbilical hernia were less likely to have PP (OR = 0.33, 95% CI 0.14 to 0.78,
P = 0.011). Additionally, subjects of Hispanic ethnicity in this sample had a higher risk of concomitant PP than others (OR = 3.75, 95% CI 1.21 to 11.63,
P = 0.022) (
Table 6).
Table 5
Characteristics of postpartum rectus abdominis diastasis patients with and without pelvic pain
|
Variable |
Without pelvic pain (n = 248) |
With pelvic pain (n = 69) |
P value |
|
Demographic characteristics |
|
|
|
|
Age (yr) |
|
|
|
|
< 30 |
3 (1.2) |
4 (5.8) |
0.034a
|
|
30 to < 40 |
154 (62.1) |
32 (46.4) |
|
|
40 to < 50 |
90 (36.3) |
32 (46.4) |
|
|
50 to < 60 |
1 (0.4) |
1 (1.4) |
|
|
Race or ethnic group |
|
|
|
|
Black/African |
10 (4.0) |
5 (7.2) |
0.036 |
|
Asian |
17 (6.9) |
2 (2.9) |
|
|
Hispanic |
7 (2.8) |
7 (10.1) |
|
|
White |
202 (81.5) |
50 (72.5) |
|
|
Others |
12 (4.8) |
5 (7.2) |
|
|
Body mass index (kg/m2) |
|
|
|
|
18.5 to < 25 |
90 (36.3) |
22 (31.9) |
0.673a
|
|
25 to < 30 |
92 (37.1) |
26 (37.7) |
|
|
30 to < 40 |
59 (23.8) |
17 (24.6) |
|
|
≥ 40 |
7 (2.8) |
4 (5.8) |
|
|
Delivery mode |
|
|
|
|
Non-cesarean |
78 (31.5) |
21 (30.4) |
0.872 |
|
Cesarean |
170 (68.5) |
48 (69.6) |
|
|
Social history |
|
|
|
|
Smoking state |
30 (12.1) |
7 (10.1) |
0.655 |
|
Alcohol abuse |
102 (41.1) |
30 (43.5) |
0.726 |
|
Depressive disorder |
53 (21.4) |
20 (29.0) |
0.184 |
|
Accompanying systems |
|
|
|
|
Strain of muscle, fascial and tendon |
10 (4.0) |
9 (13.0) |
0.005 |
|
Adhesions |
30 (12.1) |
10 (14.5) |
0.596 |
|
Low back pain |
51 (20.6) |
27 (39.1) |
< 0.001 |
|
Hernia status |
84 (33.9) |
15 (21.7) |
0.054 |
|
Hernia type |
|
|
|
|
Diaphragmatic hernia |
3 (1.2) |
3 (4.3) |
0.233b
|
|
Ventral hernia |
44 (17.7) |
7 (10.1) |
0.129 |
|
Groin hernia |
5 (2.0) |
3 (4.3) |
0.510b
|
|
Incisional hernia |
7 (2.8) |
1 (1.4) |
0.834b
|
|
Umbilical hernia |
65 (26.2) |
7 (10.1) |
0.005 |

Table 6
Univariate and multivariate binary regression analyses of underlying factors to pelvic pain in postpartum patients with rectus abdominis diastasis
|
Variable |
Univariate |
|
Multivariate |
|
P value |
OR (95% CI) |
P value |
OR (95% CI) |
|
Demographic characteristics |
|
|
|
|
|
|
Age (yr) |
|
|
|
|
|
|
< 30 |
|
1.00 |
|
0.304 |
NS |
|
30 to < 40 |
0.018 |
0.16 (0.03-0.73) |
|
|
|
|
40 to < 50 |
0.095 |
0.27 (0.06-1.26) |
|
|
|
|
50 to < 60 |
0.858 |
0.75 (0.03-17.51) |
|
|
|
|
Race or ethnic group |
|
|
|
|
|
|
Black/African |
|
1.00 |
|
0.126 |
NS |
|
Asian |
0.118 |
0.24 (0.04-1.45) |
|
0.200 |
NS |
|
Hispanic |
0.365 |
2.00 (0.45-8.96) |
|
0.022 |
3.75 (1.21-11.63) |
|
White |
0.217 |
0.50 (0.16-1.51) |
|
0.468 |
NS |
|
Others |
0.811 |
0.83 (0.19-3.72) |
|
0.237 |
NS |
|
Body mass index (kg/m2) |
|
|
|
|
|
|
18.5 to < 25 |
|
1.00 |
|
0.699 |
NS |
|
25 to < 30 |
0.656 |
1.16 (0.61-2.19) |
|
|
|
|
30 to < 40 |
0.651 |
1.18 (0.58-2.41) |
|
|
|
|
≥ 40 |
0.205 |
2.34 (0.63-8.70) |
|
|
|
|
Cesarean exposure |
0.872 |
1.05 (0.59-1.87) |
|
0.765 |
NS |
|
Social history |
|
|
|
|
|
|
Smoking state |
0.656 |
0.82 (0.34-1.96) |
|
0.575 |
NS |
|
Alcohol abuse |
0.726 |
1.10 (0.64-1.89) |
|
0.680 |
NS |
|
Depressive disorder |
0.186 |
1.50 (0.82-2.74) |
|
0.665 |
NS |
|
Concomitant conditions |
|
|
|
|
|
|
Strain of muscle, fascial and tendon |
0.008 |
3.57 (1.39-9.18) |
|
0.094 |
NS |
|
Adhesions |
0.597 |
1.23 (0.57-2.66) |
|
NA |
NA |
|
Low back pain |
0.002 |
2.48 (1.40-4.41) |
|
0.003 |
2.44 (1.36-4.40) |
|
Hernia status |
0.057 |
0.54 (0.29-1.02) |
|
0.451 |
NS |
|
Hernia type |
|
|
|
|
|
|
Diaphragmatic hernia |
0.113 |
3.71 (0.73-18.82) |
|
0.147 |
NS |
|
Ventral hernia |
0.134 |
0.52 (0.22-1.22) |
|
0.802 |
NS |
|
Groin hernia |
0.286 |
2.21 (0.52-9.48) |
|
0.162 |
NS |
|
Incisional hernia |
0.538 |
0.52 (0.06-4.26) |
|
0.594 |
NS |
|
Umbilical hernia |
0.007 |
0.32 (0.14-0.73) |
|
0.011 |
0.33 (0.14-0.78) |

Go to :

DISCUSSION
The principal findings of this retrospective cohort investigation of a multi-institutional sample of postpartum women with concomitant RAD are as follows. First, women who had a history of CD procedure exposure demonstrated 4.5 times greater risk of RAD than those women who delivered vaginally. Second, the onset age range of RAD in both the CD and non-CD group were concentrated between those 30 to 40 years old, even though the onset age in the non-CD group was significantly earlier than those who underwent CD. Third, the prevalence of LBP and PP among this population was high. Forth, the prevalence and characteristics of the pain in women with a history of non-CD did not differentiate from those with CD. Fifth, the age at onset of LBP/PP showed no difference between the CD and non-CD groups in women with RAD. Sixth, women with DD in the RAD population had approximately 2 times greater risk of LBP than those without it. Seventh, PP and SMFT are risk factors of the development of LBP in women with RAD. Eighth, LBP was found to be significantly associated with PP in women with RAD. Finally, Hispanic race was demonstrated to be a role in the development of PP in women with RAD.
LBP and PP were thought to be related to first labor and delivery in women with RAD. LBP and PP were thought to be related to pregnancy [
6]. Moreover, a 2012 review reported that the global prevalence of LBP among women was 8.7%, and several studies reported that the prevalence of LBP in women with RAD ranged from 24% to 69%, which is much higher than the global prevalence [
4,
7-
9]. The present study confirmed that LBP/PP was associated with labor and delivery in RAD patients, no matter if it was via VD or CD. In this study, over one quarter of LBP patients had the first onset during the period between 2 years pre- and post-first delivery, while one-third of the new onsets of PP arose during this period. The prevalence of LBP/PP in this study was less than thirty percent, which is lower than reported in previous studies (approximately half in the general pregnancy population) [
6,
10,
11]. This could be due to a different study method, as only individuals with diagnosed ICD codes were included.
The authors identified the following innovative findings. Firstly, the data demonstrated that the age at onset of LBP/PP showed no difference between the CD and non-CD groups in women with RAD. Secondly, it was found that the risk of RAD in women who underwent CD delivery was 4.5 times that of those who delivered vaginally. Thirdly, the age at onset of RAD in women who had undergone VD was significantly younger than those who underwent CD. Interestingly, the onset age range of both groups was concentrated between 30 and 40 years old, which confirmed that pregnancy and CD were important risk factors contributing to RAD [
12-
14]. The results of the present study also implied that the importance of CD in the development of RAD might be underestimated.
LBP interacted with DD in postpartum women with concomitant RAD. Several investigations have linked DD to chronic spinal pain [
15,
16]. Oon-Arom et al. [
16] found that patients with physical pain symptoms tended to express some subjective symptoms of DD. Schmaling and Nounou [
15] reported DD patients were more likely to develop chronic spinal pain than those participants without DD.
The present study also confirmed that women with DD in the RAD population had approximately a 2 times greater risk of LBP than those without it. The data demonstrated that the age at onset of LBP was significantly earlier than that of DD in postpartum women who had RAD. It is possible that LBP interacted with DD. Further study is warranted to discern which is the triggering factor.
PP and SMFT are risk factors of development of LBP in postpartum women with concomitant RAD. The authors demonstrated that women with PP had greater than 2 times the risk of development of LBP than those without PP. It was also worth noting that in the present study, women with SMFT had a 4.5 times greater risk of developing LBP than those without SMFT. SMFT is the most important manifestation of myofascial syndrome, being found to be the principal cause of pain [
17-
19]. In the present study, the authors hypothesize that, in the process of the development of chronic pain, the fascia plays a crucial role, since it is an uninterrupted, viscoelastic, continuous, collagen-containing tissue covering the entire body. Fascia interpenetrates and surrounds all organs, muscles, bones, and nerve fibers [
20,
21]. It has been reported that deep fascia might be closely related to non-specific LBP [
22,
23]. The present study confirmed that SMFT was strongly related to LBP in postpartum women with RAD, which also stressed the importance of fascia in LBP.
LBP and Hispanic race are risk factors in the development of PP in postpartum women with concomitant RAD. The prevalence of PP among women varies from 6% to 27% [
24,
25]. In this study, the prevalence of PP was over one-fifth among women with a history of delivery who had RAD. There was no significant difference in terms of age at onset of LBP/PP, regardless of delivery method. It was found that the prevalence of LBP in women with PP, with a rate of approximately 40%, was significantly higher than those without PP. With univariate and multivariate regression analyses, the authors identified LBP as a risk factor related to PP. This suggested a close relationship between LBP and PP. While the consensus on whether race is a risk factor for chronic pain has not been reached [
9,
26,
27], it was intriguing that the authors found that Hispanic women had approximately a 4-fold greater risk of developing PP, but not for developing LBP.
There are several clinical and research implications that follow this study. The prevalence of umbilical hernia RAD patients with PP was significantly lower than that of those without PP. Univariate and multivariate regression analysis also identified a negative association between pain and hernia. For clinicians and policy makers, the present study draws attention to women with RAD, as the authors revealed several common risk factors and potential complications. However, to the authors’ knowledge, there is no prior literature that reports a link between umbilical hernia and PP, and prospective studies are necessary to further elaborate this matter. Additionally, the present study was not tailored towards finding the precise mechanism of the development of LBP and PP in women with RAD, though it may lead to its exploration in future research proposals. Once additional research has been completed, the authors’ findings may be used in clinical settings.
Our study had several strengths and limitations. Ten years of data were included from multicenter information records, due to the retrospective nature of the study, many cases were excluded due to either no history of delivery or incomplete delivery operative notes, which may lead to sampling bias. The study focused on the association between LBP/PP and the first delivery, and the authors did not count the parity and plurality which might also interact with the pain, so these may have biased the results of this study.
It was reported that pain intensity was associated with RAD size and gestational trimester [
28]. However, the current ICD coding mechanisms do not offer details of the data points. Fortunately, we included comprehensive analysis of concomitant conditions, which provided us with the opportunity to not only identify findings similar to multidisciplinary collaboration, but also study the association across more than a 15-year longitudinal span.
In conclusion, this study revealed the linkages and relationships between rectal abdominis diastasis, PP, and LBP in coordination with each other and additional factors such as delivery and race. We found that women with a history of CD were more likely to develop RAD, while the age of onset of RAD in women who underwent VD was significantly younger than those who underwent CD. The authors also found that LBP/PP were related to delivery, and the age at onset of LBP/PP showed no difference between the CD and non-CD groups in women with RAD. There was no significant difference in the age distribution at onset of LBP/PP among RAD women with or without CD exposure. In addition, DD, SMFT, and PP were risk factors contributing to the developing of LBP. Race and LBP also played roles in the development of PP. This paper sheds light on the characteristics of these conditions and sets the stage for additional research in this field of study.
Go to :






 PDF
PDF Citation
Citation Print
Print





 XML Download
XML Download