INTRODUCTION
MATERIALS AND METHODS
1. Animals
2. Induction of NP
3. Experimental groups and administration of drugs
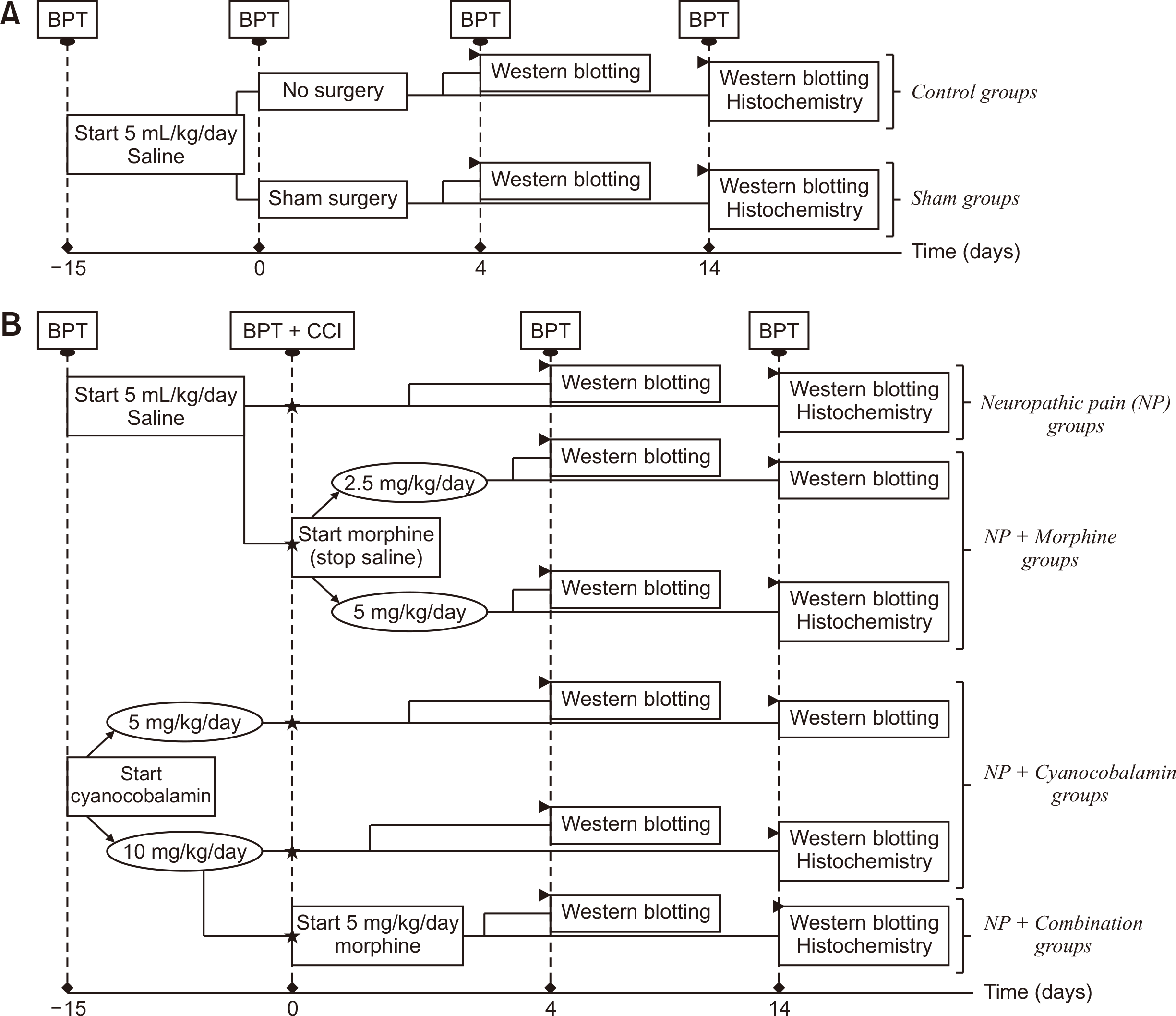 | Fig. 1Flowchart showing the experimental design of the present study. (A) Experimental groups without CCI and (B) experimental groups with CCI/neuropathic pain. Asterisk (⋆) represent CCI. Arrowhead (►) indicates the sacrification and the subsequent process. Notes: 1) The pain thresholds of all animals, including rat groups sacrificed on the 14th postoperative day were measured on the 4th postoperative day and these data were also used to assess pain threshold change on the 4th postoperative day. 2) Spinal cord tissues used in Western Blotting and Fluorescence Immunohistochemistry studies were obtained from separate animals. CCI: chronic construction ınjury of sciatic nerve, BPT: behavioral pain tests. |
4. Behavioral pain tests
5. Western blot analysis in the lumbar spinal cord
6. Fluorescence immunohistochemistry
7. Statistical analysis
RESULTS
1. Assessment of behavioral pain tests
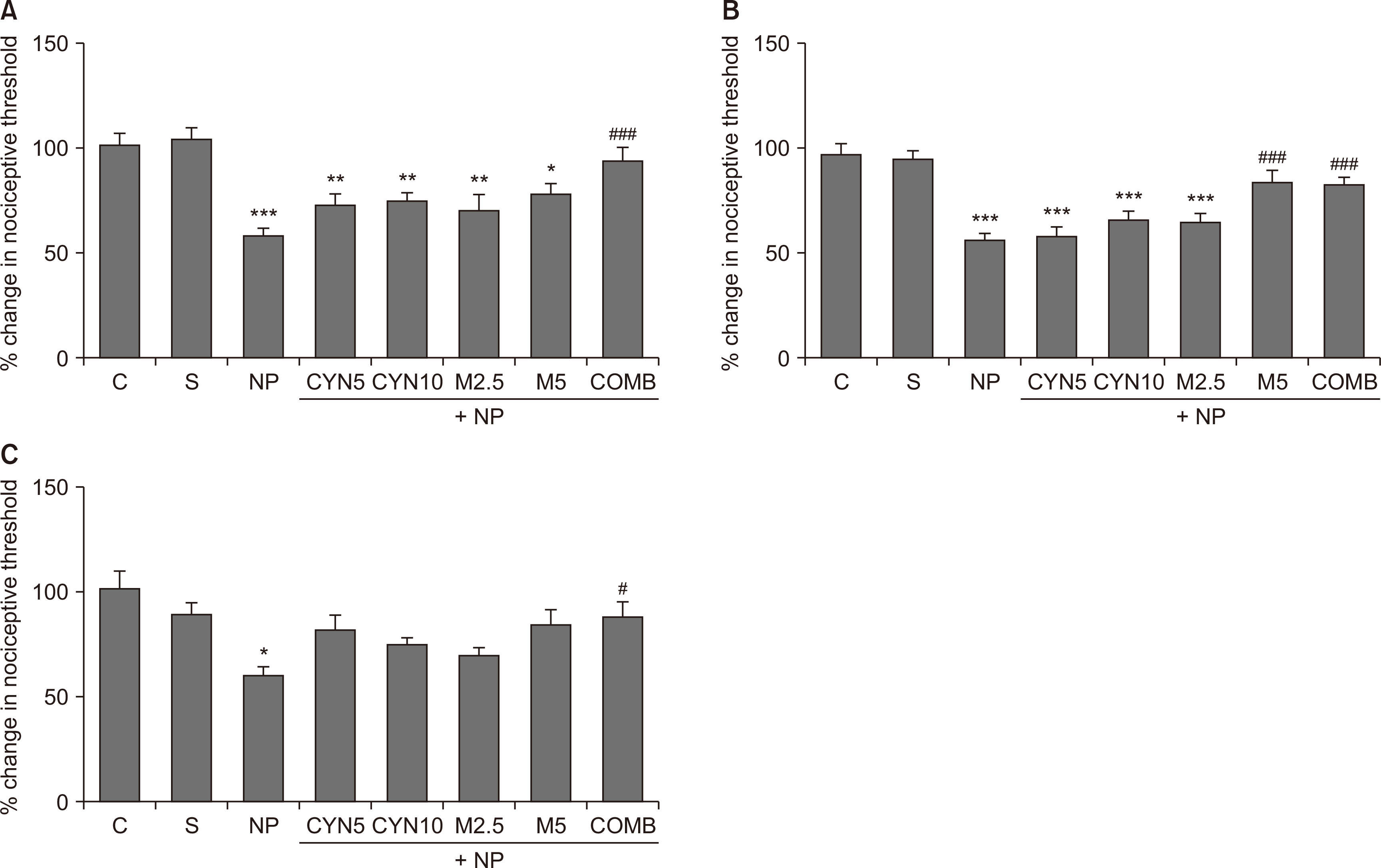 | Fig. 2Nociceptive thresholds of lesioned paws were expressed on the 4th postoperative day as the percentage change in the threshold, calculated using the following formula: % change in nociceptive threshold = (Postoperative paw withdrawal latency/Basal paw withdrawal latency) × 100. Mechanical hyperalgesia, tactile allodynia, and thermal hyperalgesia are shown by using (A) analgesia meter, (B) dynamic plantar esthesiometer, and (C) plantar test. Statistical analyzes of data obtained from behavioral pain tests were performed using one-way analysis of variance followed by posthoc Bonferonni test (n = 11-20). Data are presented as mean ± standard error of mean. (A) Analgesia meter. ***P < 0.001, **P = 0.008, **P = 0.006, **P = 0.003, *P = 0.018; NP, CYN5, CYN10, M2.5, and M5, respectively, vs. S group. ###P < 0.001, COMB vs. NP group. (B) Dynamic plantar esthesiometer. ***P < 0.001, ***P < 0.001, ***P < 0.001, ***P < 0.001; NP, CYN5, CYN10, and M2.5, respectively, vs. S group. ###P < 0.001, ###P < 0.001; M5 and COMB, respectively, vs. NP group. (C) Plantar test. *P = 0.016, NP vs. S group. #P = 0.030, COMB vs. NP group. C: control, S: Sham, NP: saline-injected neuropathic pain, CYN5 and CYN10: NP + 5 and 10 mg/kg/day cyanocobalamin, respectively, M2.5 and M5: NP + 2.5 and 5 mg/kg/day morphine, respectively, COMB: NP + 10 mg/kg/day cyanocobalamin + 5 mg/kg/day morphine. |
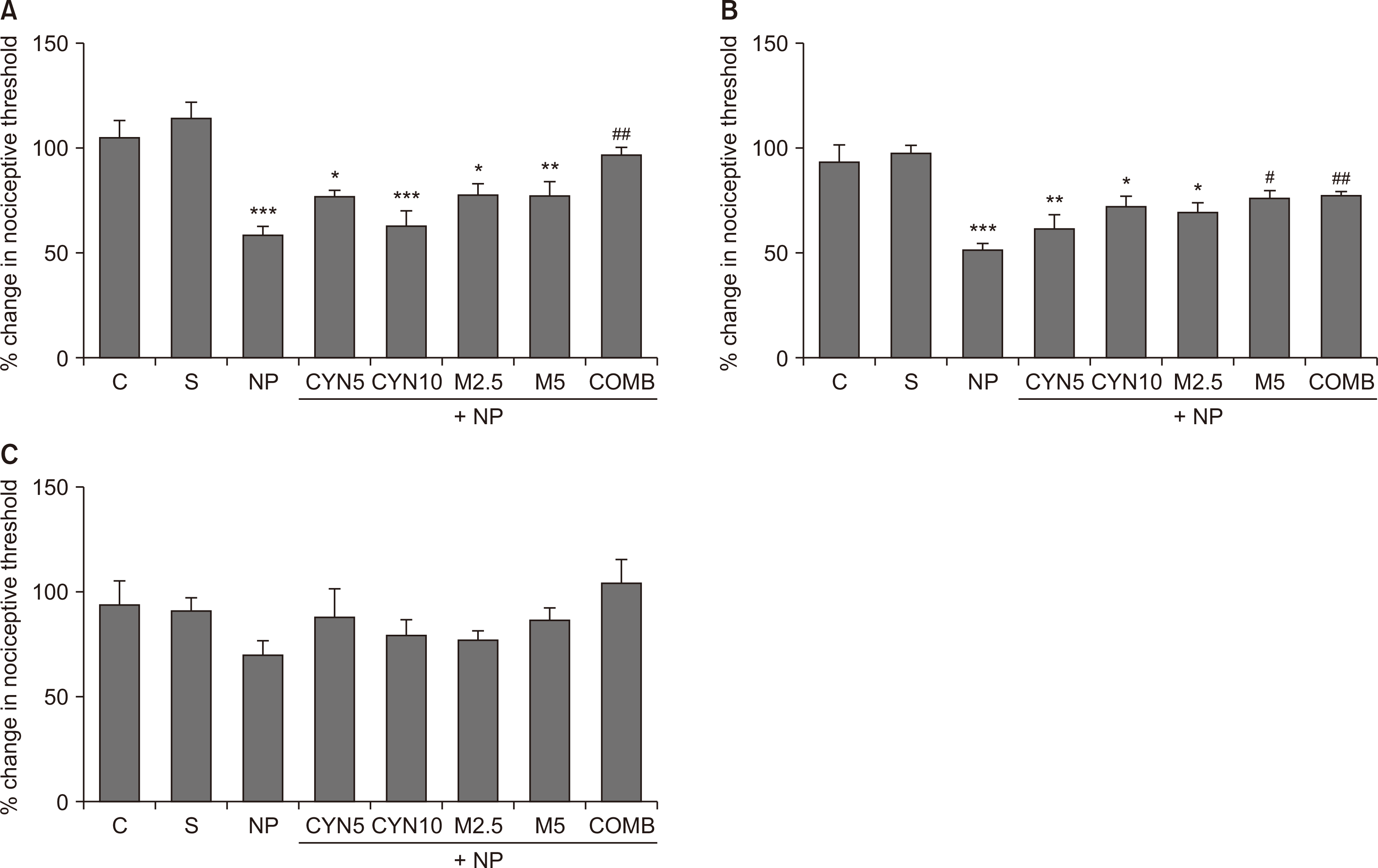 | Fig. 3Nociceptive thresholds of lesioned paws were expressed on the 14th postoperative day as the percentage change in the threshold, calculated using the following formula: % change in nociceptive threshold = (Postoperative paw withdrawal latency/Basal paw withdrawal latency) × 100. Mechanical hyperalgesia, tactile allodynia, and thermal hyperalgesia are shown by using (A) analgesia meter, (B) dynamic plantar esthesiometer, and (C) plantar test. Statistical analyzes of data obtained from behavioral pain tests were performed using one-way analysis of variance followed by posthoc Bonferonni test (n = 7-14). Data are presented as mean ± standard error of mean. (A) Analgesia meter. ***P < 0.001, *P = 0.021, ***P < 0.001, *P = 0.015, **P = 0.005; NP, CYN5, CYN10, M2.5, and M5, respectively, vs. S group. ##P = 0.002, COMB vs. NP group. (B) Dynamic plantar esthesiometer. ***P < 0.001, **P = 0.001, *P = 0.019, *P = 0.016; NP, CYN5, CYN10, and M2.5, respectively, vs. S group. #P = 0.014, ##P = 0.006; M5 and COMB, respectively, vs. NP group. C: control, S: Sham, NP: saline-injected neuropathic pain, CYN5 and CYN10: NP + 5 and 10 mg/kg/day cyanocobalamin, respectively, M2.5 and M5: NP + 2.5 and 5 mg/kg/day morphine, respectively, COMB: NP + 10 mg/kg/day cyanocobalamin + 5 mg/kg/day morphine. |
2. Assessment of TSP4 protein expression in the lumbar spinal cord
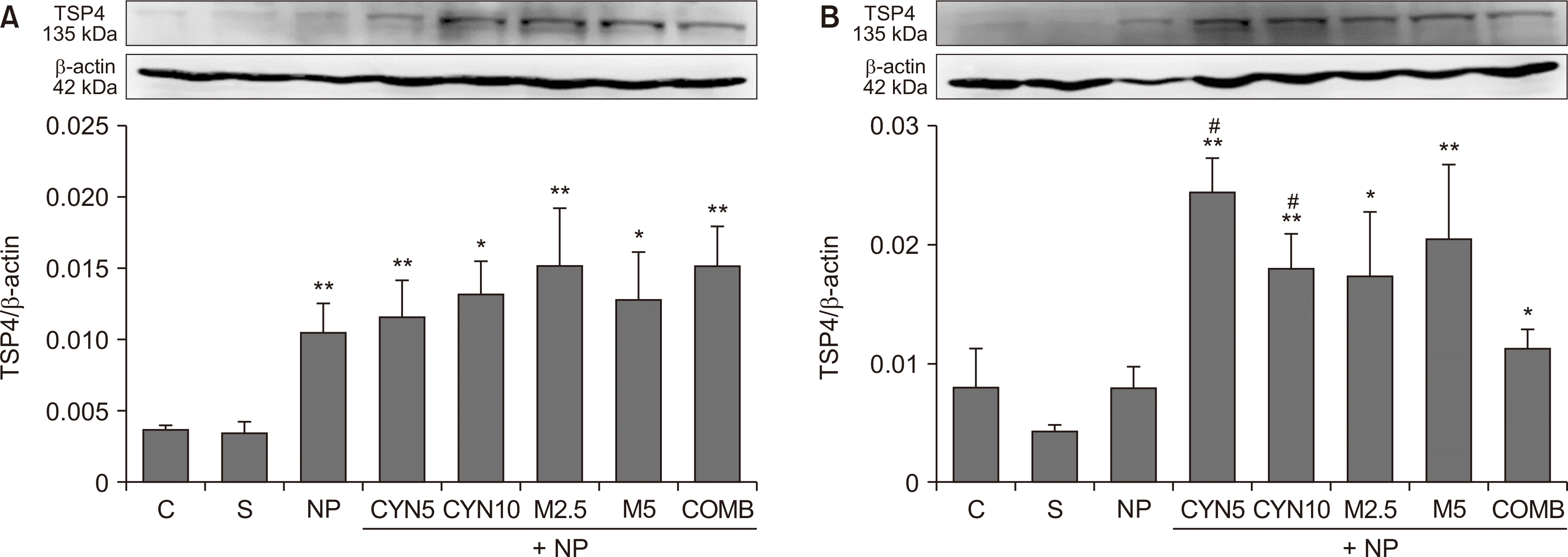 | Fig. 4Thrombospondin-4 (TSP4) protein expressions in L4-L6 spinal cord segments taken from rats on the (A) 4th and (B) 14th postoperative days. Bar graphs indicate results of densitometric analysis of the TSP4 protein (135 kDa) bands as normalized to the quantity of β-actin (42 kDa) protein. Statistical analyses of densitometric data of protein bands obtained from Western blotting were performed using the Kruskal–Wallis test followed by the Mann–Whitney U-test (n = 4-6). Data are presented as mean ± standard error of mean. (A) **P = 0.004, **P = 0.009, *P = 0.019, **P = 0.006, *P = 0.019, **P = 0.002; NP, CYN5, CYN10, M2.5, M5, and COMB, respectively, vs. S group. (B) **P = 0.007, **P = 0.007, *P = 0.031, **P = 0.007, *P = 0.039; CYN5, CYN10, M2.5, M5, and COMB, respectively, vs. S group. #P = 0.015, #P = 0.031; CYN5 and CYN10, respectively, vs. NP group. C: control, S: Sham, NP: saline-injected neuropathic pain, CYN5 and CYN10: NP + 5 and 10 mg/kg/day cyanocobalamin, respectively, M2.5 and M5: NP + 2.5 and 5 mg/kg/day morphine, respectively, COMB: NP + 10 mg/kg/day cyanocobalamin + 5 mg/kg/day morphine. |
3. Assessment of TSP4 and GFAP immunoreactivities in the lumbar spinal cord
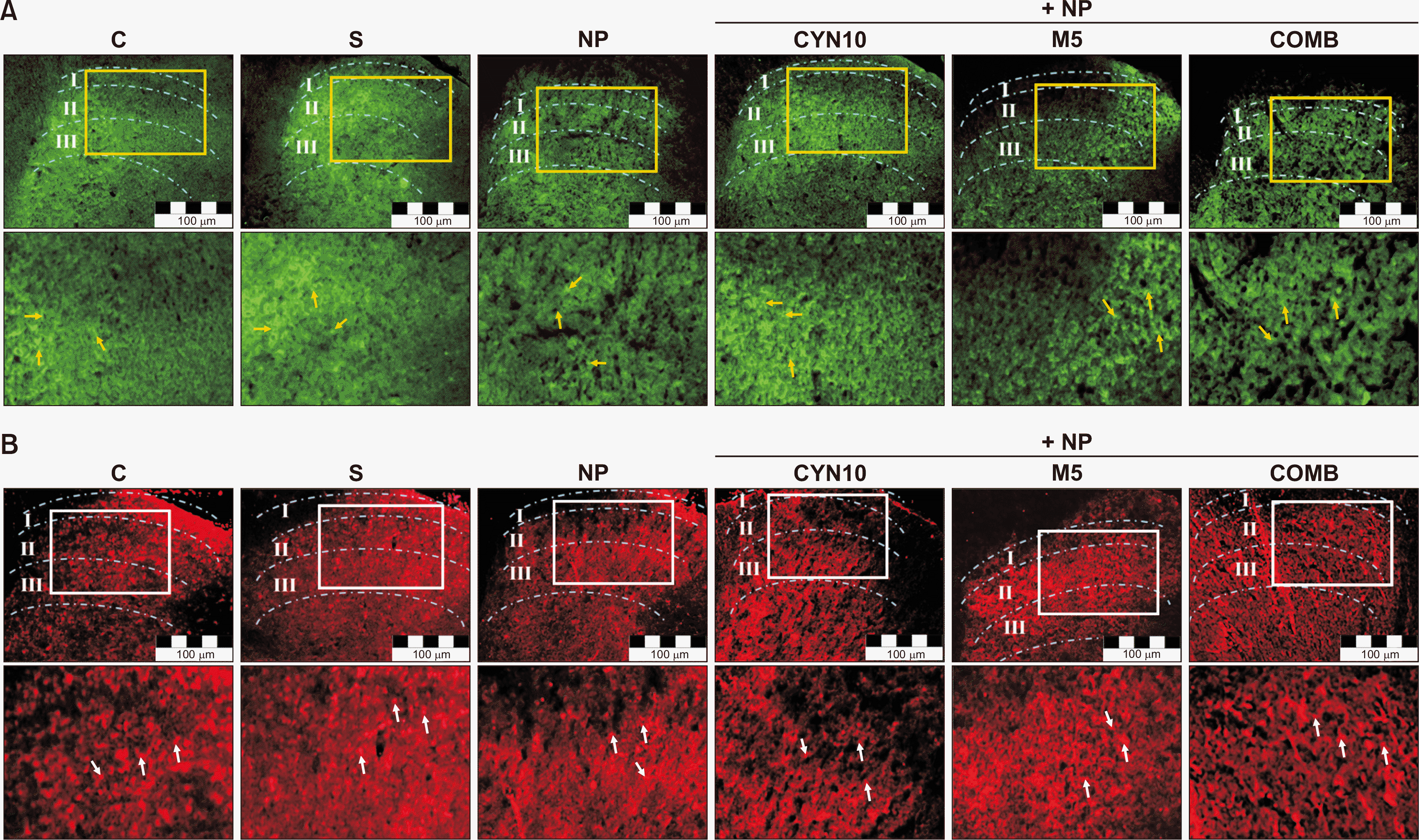 | Fig. 5Representative images of (A) thrombospondin-4 (TSP4) and (B) glial fibrillary acidic protein (GFAP) immunoreactivities in the ipsilateral dorsal horns of L4-L6 spinal cord transverse sections of experimental groups (Scale bar: 100 μm). The analyzed cross-sectional areas contain lamina I-II-III in the dorsal horn and have been marked over the images. The areas marked in the above images are magnified just below, and TSP4 proteins are indicated by yellow arrows and GFAP immunoreactive cells by white arrows. C: control, S: Sham, NP: saline-injected neuropathic pain, CYN10: NP + 10 mg/kg/day cyanocobalamin, M5: NP + 5 mg/kg/day morphine, COMB: NP + 10 mg/kg/day cyanocobalamin + 5 mg/kg/day morphine. |
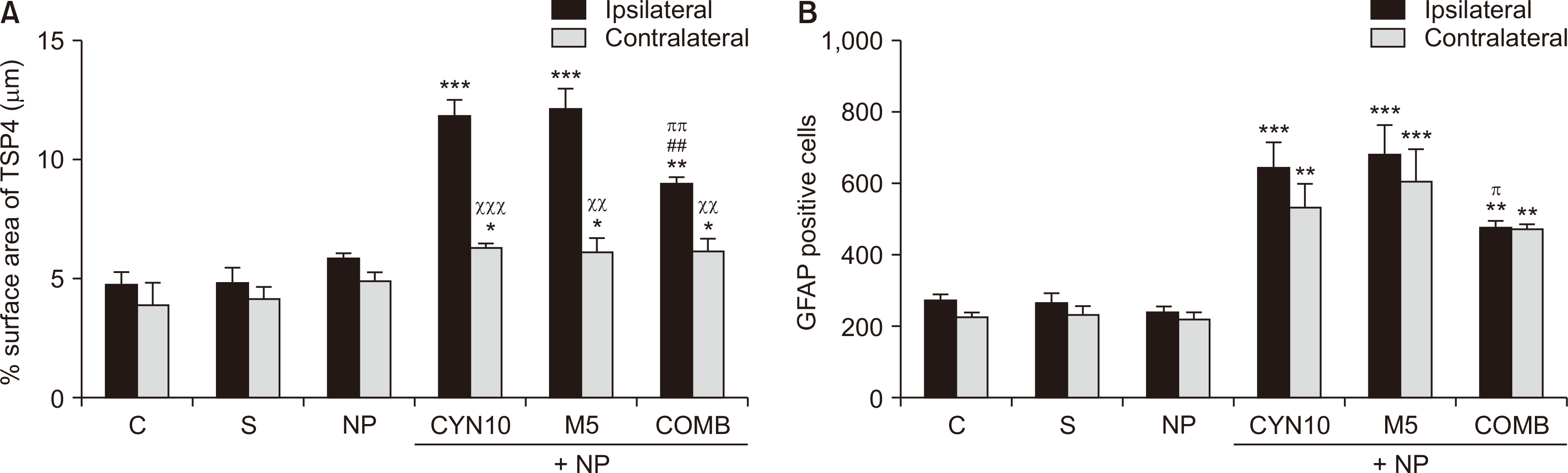 | Fig. 6(A) Percentage of thrombospondin-4 (TSP4) surface area (μm) and (B) glial fibrillary acidic protein (GFAP) immunoreactive cells (number) in the lumbar dorsal horn on the 14th postoperative day. The analyzed cross-sectional areas contain lamina I-II-III in the dorsal horn. Statistical analyzes of data obtained from immunostained sections were performed using one-way analysis of variance followed by a posthoc LSD test (n = 3-4). Each bar represents the mean ± standart error of the mean. (A) ***P < 0.001, ***P < 0.001, **P = 0.001; ipsilateral sides of CYN10, M5, and COMB, respectively, vs. ipsilateral side of NP group. ##P = 0.001, ipsilateral side of COMB vs. ipsilateral side of CYN10 group. ππP = 0.002, ipsilateral side of COMB vs. ipsilateral side of M5 group. *P = 0.017, *P = 0.021, *P = 0.022; contralateral sides of CYN10, M5, and COMB, respectively, vs. contralateral side of NP group. xxxP < 0.001, xxP = 0.006, xxP = 0.004; contralateral sides of CYN10, M5, and COMB, respectively, vs. own ipsilateral sides. (B) ***P < 0.001, ***P < 0.001, **P = 0.005; ipsilateral sides of CYN10, M5, and COMB, respectively, vs. ipsilateral side of NP group. πP = 0.029, ipsilateral side of COMB vs. ipsilateral side of M5 group. **P = 0.001 , ***P < 0.001, **P = 0.005; contralateral sides of CYN10, M5, and COMB, respectively, vs. contralateral side of NP group. C: control, S: Sham, NP: Saline-injected neuropathic pain, CYN10: NP + 10 mg/kg/day cyanocobalamin, M5: NP + 5 mg/kg/day morphine, COMB: NP + 10 mg/kg/day cyanocobalamin + 5 mg/kg/day morphine. |
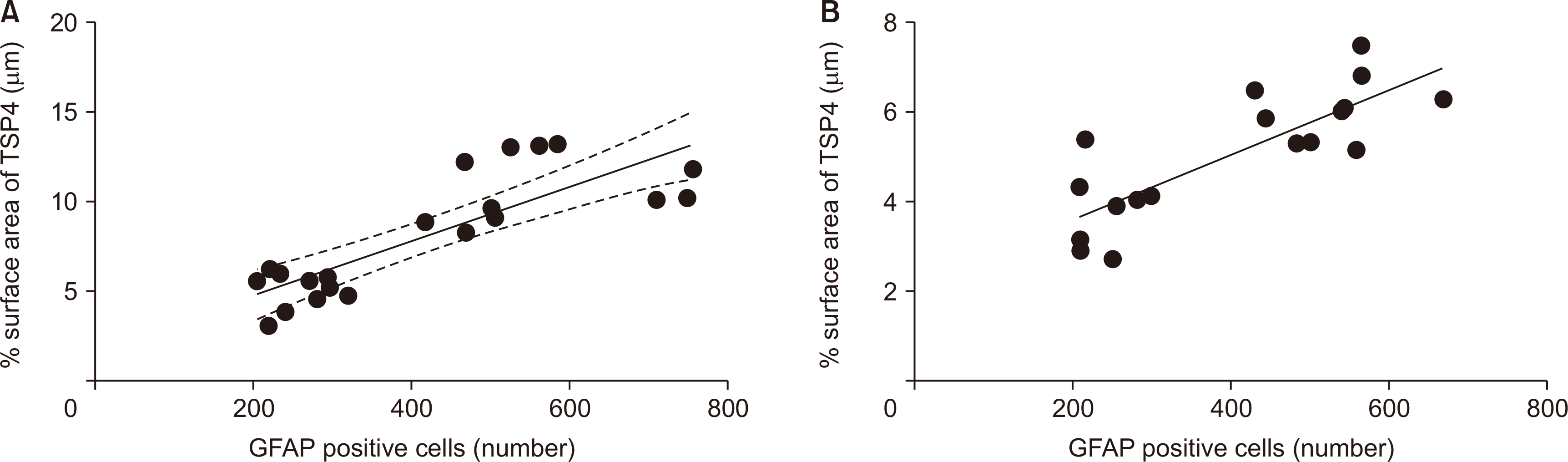 | Fig. 7Pearson’s correlation shows a linear relationship between glial fibrillary acidic protein (GFAP) immunoreactive cells and thrombospondin-4 (TSP4) protein surface area in both (A) ipsilateral (P < 0.001, r = 0.826, n = 21) and (B) contralateral (P < 0.001, r = 0.811, n = 18) spinal cord sections. The data of each group show a normal distribution pattern. GFAP positive cell number is accepted as the dependent variable (X-axis), and the percentage of TSP4 surface area is accepted as the independent variable (Y-axis). |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download