This article has been
cited by other articles in ScienceCentral.
Abstract
Background
Current therapies are quite unsuccessful in the management of neuropathic pain. Therefore, considering the inhibitory characteristics of GABA mediators, the present systematic review and meta-analysis aimed to determine the efficacy of GABAergic neural precursor cells on neuropathic pain management.
Methods
Search was conducted on Medline, Embase, Scopus, and Web of Science databases. A search strategy was designed based on the keywords related to GABAergic cells combined with neuropathic pain. The outcomes were allodynia and hyperalgesia. The results were reported as a pooled standardized mean difference (SMD) with a 95% confidence interval (95% CI).
Results
Data of 13 studies were analyzed in the present meta-analysis. The results showed that administration of GABAergic cells improved allodynia (SMD = 1.79; 95% CI 0.87, 271; P < 0.001) and hyperalgesia (SMD = 1.29; 95% CI 0.26, 2.32; P = 0.019). Moreover, the analyses demonstrated that the efficacy of GABAergic cells in the management of allodynia and hyperalgesia is only observed in rats. Also, only genetically modified cells are effective in improving both of allodynia, and hyperalgesia.
Conclusions
A moderate level of pre-clinical evidence showed that transplantation of genetically-modified GABAergic cells is effective in the management of neuropathic pain. However, it seems that the transplantation efficacy of these cells is only statistically significant in improving pain symptoms in rats. Hence, caution should be exercised regarding the generalizability and the translation of the findings from rats and mice studies to large animal studies and clinical trials.
Go to :

Keywords: Bibliometrics, Cell Engineering, Chronic Pain, GABAergic Neurons, Gamma-Aminobutyric Acid, Hyperalgesia, Neural Stem Cells, Neuralgia, Pain Management, Spinal Cord Injuries
INTRODUCTION
Neuropathic pain is caused by a primary lesion or dysfunction in the nervous system and is subsequent to an injury to the peripheral or central nervous system (CNS). Hyperalgesia and allodynia are two main symptoms of neuropathic pain [
1]. Spinal cord injury (SCI) is an important mechanism causing neuropathic pain, which is very challenging to manage. Following SCI, a majority of the patients suffer from chronic pain with a severity ranging from moderate to severe [
2-
5]. The current findings indicate that the experienced pain in regions below the injury has a central origin and is associated with functional impairments in the white and gray matter of the spinal cord. This pain is characterized by inappropriate responses to painful or non-painful stimulations of the skin [
5].
Medication therapy is the main basis of neuropathic pain management in the clinic [
6]. But these medications can only alleviate the pain, temporarily [
7]. Additionally, the undesirable complications, accompanied by the long-term use of medications, pose major obstacles to the use of these treatments [
8]. Since neuropathic pain is caused by an injury to the central or peripheral nervous system, the pain will continue to affect patients, unless the injured area is somehow repaired or neural pathways are strengthened. Gene therapy is one appropriate strategy which has shown to be beneficial in delivering active biological molecules [
9]. Hence, extensive research is being conducted on the management of neuropathic pain through transplantation of genetically-modified cells.
GABA and glycine are two key CNS mediators, with inhibitory effects on pain transmission pathways in the central cord. Injection of GABA antagonists stimulates severe pain, similar to allodynia and hyperalgesia [
10]. On the other hand, administration of GABA neurotransmitter helps with pain management in a dose-dependent manner. However, systemic administration of GABA agonists has shown unsatisfactory results [
11]. One of the solutions to increase the efficacy of these neurotransmitters is through intrathecal administration, and transplantation of GABAergic neural precursor cells may be beneficial in this regard [
12,
13]. Recent findings emphasize on the favorable effects of using these cells in alleviating chronic pain. Moreover, injection of these cells has improved and reduced neuropathic pain in animal models [
14-
16].
Although pre-clinical studies have demonstrated that administration of GABAergic neural precursor cells has significant effects on the management of neuropathic pain, there is no consensus over the matter. Conducting a systematic review and meta-analysis is one way to reach a complete and comprehensive conclusion. Accordingly, the present study was conducted to determine the efficacy of GABAergic neural precursor cells on animal models of neuropathic pain.
Go to :

MATERIALS AND METHODS
The current study was designed based on instructions for performing meta-analysis on clinical trials in animal studies. Our meta-analysis aimed to review animal studies. Therefore, PICO in the current study is as follows: P: Animal models (rats and mice) with neuropathic pain, I: Intrathecal or intraspinal transplantation of GABAergic cells, C: Comparison with the control group (without treatment or treatment with drug vehicle) and O: pain alleviation in the studied animals based on Allodynia and Hyperalgesia assessment tests.
On the basis of achieving the above-mentioned aims, an extensive search was performed in Medline (using PubMed), ISI Web of Science, Embase, and Scopus electronic databases, using related keywords and their proper combinations, searching for the articles published through the end of January 20, 2021. The search strategy was designed based on the keywords presented in Appendix 1.
Keywords were selected according to the words related to neuropathic pain and GABAergic neural precursor cells’ transplantation, and were chosen on three bases. First, MeSH and Emtree were searched for the keywords. Then, titles and abstracts of the relevant articles were screened to find additional keywords. Finally, with the help of experts in the field of SCI research, the keywords list was completed. Combining the selected keywords to design search terms, the search was performed in the electronic databases. Moreover, a hand search was performed using relevant articles’ references and related journals, to find additional and possibly missing articles. Finally, for finding gray literature, three strategies were adopted including searching through ProQuest’s Dissertations and Theses section, contacting authors of the relevant articles to access their unpublished or preprint data and searching in the Google and Google Scholar search engines.
Controlled studies assessing the effects of GABAergic neural precursor cells’ transplantation in managing neuropathic pain were included. The term “controlled studies” refers to the studies which performed their experiments on a no-treatment control group (placebo group or vehicle group), in addition to performing experiments on the group treated with the transplanted cells. Including articles was not limited to their published date or their language, as part of the existing literature was in Chinese or Japanese. The target was animal studies performed on mice and rats, irrespective of the animals’ age, sex, and strain, in which neuropathic pain was created using central or peripheral mechanisms. The assessed intervention was intraspinal or intrathecal transplantation of GABAergic neural precursor cells. The animals’ pain was considered to be the assessed outcome.
Studies not having a control group, studies in which pain was not evaluated, and studies in which the number of transplanted GABAergic neural precursor cells was not reported were excluded. It is worth mentioning that review articles were excluded as well. Since, the onset of pain in the animal models occurs at least four weeks after the injury, studies which performed less than four weeks of follow up were also excluded.
The articles obtained from the hand search and systematic search were gathered using the EndNote program (version X7; Thomson Reuters, Toronto, ON, Canada), and duplicates were eliminated. Two independent reviewers performed the initial screening, reviewing titles and abstracts of the gathered articles. Next, full texts of the relevant articles were studied, and, based on the inclusion and exclusion criteria, included articles were selected. Every disagreement was resolved, using a third reviewer’s opinion.
The extracted data from the articles included information related to the study design, sample, and control groups’ characteristics (age, sex, mechanism of neuropathic pain inducement), sample size, type of the donated cell, species which transplanted cells were obtained from, number of the transplanted cells, interval time between inducement of the neuropathic pain and transplantation of the cells, follow-up period, and the severity of the animals’ pain. Since in most cases, the assessed outcome was reported in multiple stages, the last report in the article was taken as input to the present meta-analysis. In cases of the results being reported in graphs, the graph data extraction method by Sistrom and Megro [
17] was used. Since, some included studies had more than one experiment, we decided to record and analyze data by separate experiments. Therefore, the number of experiments exceeded the number of studies.
The quality assessment of the included studies was performed using the proposed guidelines of SYRCLE’s risk of bias assessment tools [
18]. This tool evaluates the risk of bias among the studies in terms of sequence generation, baseline characteristics, allocation concealment, random housing, caregiver blinding, random outcome assessment, blinding of the outcome assessor, incomplete outcome assessment, selective outcome assessment, and other risk of bias. In cases of disagreement, a third reviewer’s opinion was adopted. Level of evidence was assessed using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) framework [
19].
All studies were summarized and categorized based on the pain’s severity. Data are presented as means ± standard deviation. Analyses were performed using the “meta” command in the STATA 17.0 statistical program (Stata Corp., College Station, TX), and the results are reported as standardized mean difference (SMD) and 95% confidence interval (CI) by calculating Hedges’ g. SMD was used to standardize the data of the different tests to a uniform scale, because of the variations between the scales of pain assessment tests performed in the studies. Since, the sample size was small and varied among included studies, we used the method proposed by Hartung, Knapp, Sidik, and Jonkman for calculating 95% CI to avid type 1 error [
20].
Neuropathic pain has two main symptoms: allodynia and hyperalgesia. Since the underlying mechanism causing the allodynia and hyperalgesia differs and various pathways are involved in their development, analyses were performed with respect to allodynia and hyperalgesia and the results are presented separately. It is worth mentioning that two types of allodynia and three types of hyperalgesia exist in the eligible articles, including mechanical allodynia, cold allodynia, mechanical hyperalgesia, heat hyperalgesia, and chemical hyperalgesia. Since the performed meta regression showed that the efficacy of the GABAergic cells’ transplantation on the management of hyperalgesia and allodynia is independent of the nature of their cause, the results of the different origins of allodynia were pooled in one analysis. The same approach was adopted regarding hyperalgesia’s results, as well.
Heterogeneity between the studies was evaluated using I
2 statistics, and I
2 values higher than 50% were considered significant (indicating heterogeneity). In cases of heterogeneity, subgroup analysis and meta-regression were performed to find the origin of the heterogeneity. In assessment of possible source of heterogeneity, a
P value less than 0.1 was considered significant (presence of heterogeneity). A random effect model was performed, and a 95% CI was calculated according to the method proposed by Hartung, Knapp, Sidik, and Jonkman [
20]. Outliers were identified using Galbraith plot [
21]. Sensitivity analysis was performed by using the leave-one-out approach to find any possible individual studies’ effect on pooled effect size. Meta-analyses were performed only when data were presented in at least two experiments. Eventually, publication bias was assessed, using the trim-and-fill method, and presenting a funnel plot.
Go to :

RESULTS
1. Study characteristics
The systematic search in the electronic databases resulted in 2,644 articles, 1,760 of which were non-duplicate records. One thousand seven hundred twenty one studies were excluded by screening the titles and abstracts and the full texts of the remaining 39 were studied (Appendix 2). Finally, the data of 13 studies were included in the present meta-analysis [
15,
16,
22-
32] (
Fig. 1). These studies consisted of 17 separate experiments and involved 317 animals, 176 of which were controls and 141 animals were treated with GABAergic cells. The neuropathy pain model was peripheral in one and central in two studies, and one study used both models. The time interval between injury to transplantation of the cells ranged from three to 21 days. The site of injection was intraspinal in nine, intrathecal in three, and intracranial in one study. The origin of GABAergic cells was from the medial ganglionic eminence (intrinsic GABAergic cells) in one study, genetically modified GABAergic cells in six studies, and stem cell-derived GABAergic cells in two. The number of transplanted cells varied from 5 × 10
4 to 1 × 10
6 cells. The follow-up time was between 4 and 8 weeks.
Table 1 summarizes the characteristics of the included studies.
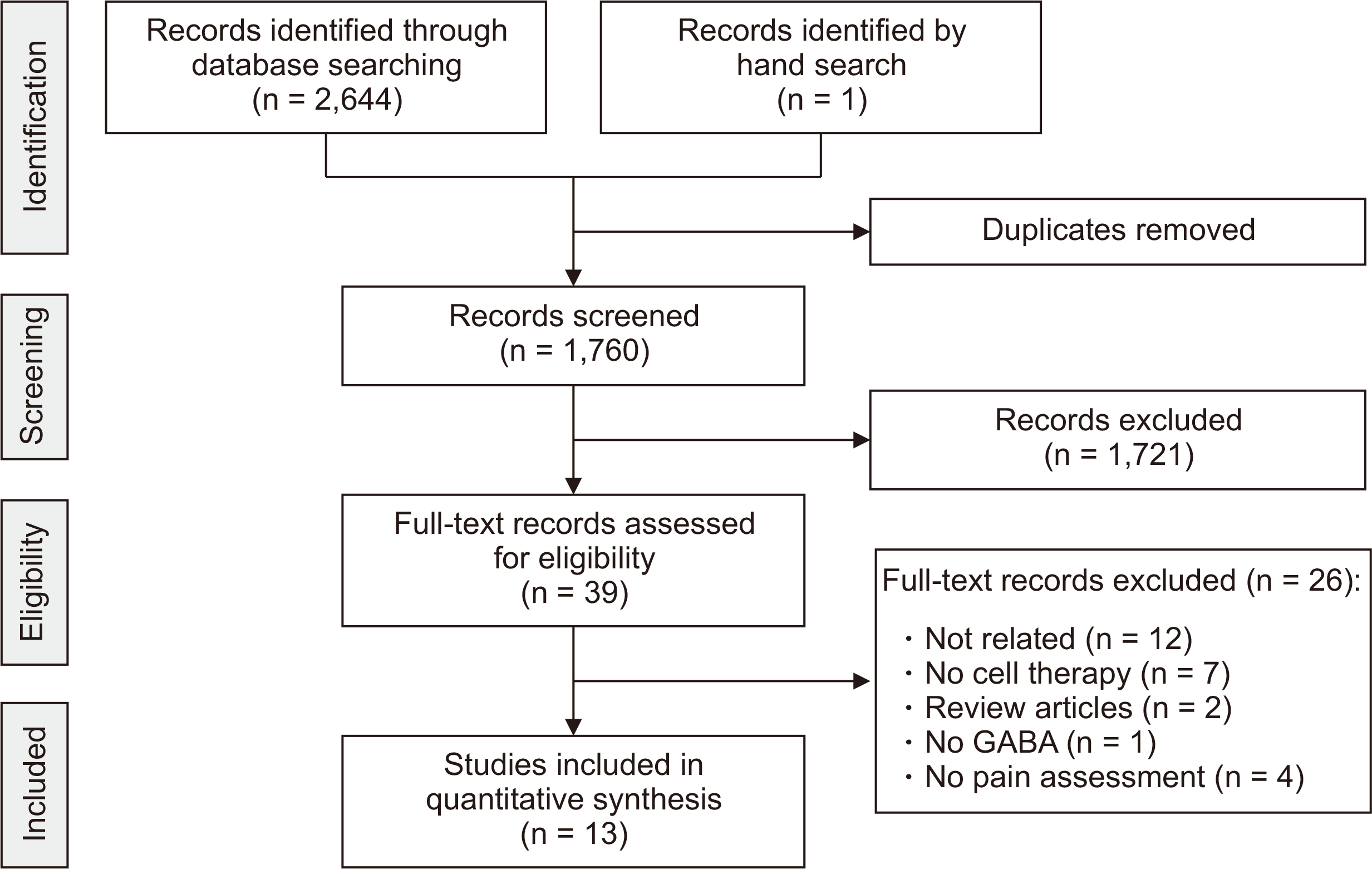 | Fig. 1PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow diagram of present study. 
|
Table 1
Characteristics of included studies
|
Study |
Sex/Species/Strain/Weight |
SCI/Treat (n) |
Model of injury |
Interval time injury to treat (day) |
Transplantation location |
GABAergic cell type |
Cell type |
Immunosuppressive/Antibiotic/Blindinga
|
Transplantation
methods
cell/Type graft |
FU (day) |
|
Bráz et al., 2012 [22] |
Male/Mice/C57BL6/J/6-8 week |
13/10 |
SNI |
7 |
Dorsal horn of lumbar spinal cord |
GABAergic MGE interneurons |
Intrinsic GABAergic cell |
No/No/Yes |
Intraspinal: 5 × 104 cell/Allograft |
28 |
|
Bráz et al., 2015 [23] |
Male/Mice/C57BL6/J/8 weeks |
5/5 |
Paclitaxel induce pain |
7 |
Dorsal horn of lumbar spinal cord |
GABAergic MGE interneurons |
Intrinsic GABAergic cell |
No/No/Yes |
Intraspinal: 5 × 104 cell/Allograft |
28 |
|
Eaton et al., 1999 [25] |
Female/Rat/Wistar/180 g |
14/14 |
CCI |
7 |
L1 |
33G10.17 |
Genetically modified cell |
No/No/No |
Intraspinal: 1 × 106 cell/Allograft |
56 |
|
Eaton et al., 2012 [24] |
Female/Rat/NR/Adult |
15/15 |
Streptozotocin induce neuropathic pain, CCI, excitotoxic spinal cord injury |
14 |
Subarachnoid space of dorsal horn |
Viable hNT2.17 cells |
Genetically modified cell |
Yes/Yes/Yes |
Intrathecal: 1 × 106 cell/Xenograft |
56 |
|
Etlin et al., 2016 [26] |
Male/Mice/C57BL6/J/6-8 weeks |
10/10 |
SNI |
3 |
Dorsal horn on one side of the spinal |
GABAergic interneurons from the mouse medial ganglionic eminence |
Intrinsic GABAergic cell |
No/No/Yes |
Intraspinal: 5 × 104 cell/Allograft |
21 |
|
Hwang et al., 2016 [16] |
NR/Rat/SD/Adult |
7/7 |
SCI contusion injury |
21 |
Intrathecal injection |
mESC-NPCs |
Intrinsic GABAergic cell |
No/No/Yes |
Intrathecal: 1 × 106 cell/Allograft |
56 |
|
Jergova et al., 2012 [27] |
Male/Rat/SD/140-160 g |
10/10 |
CCI |
7 |
L3-L4 |
GABAergic NPCs |
Genetically modified cell |
No/No/Yes |
Intraspinal: 3 × 105 cell/Allograft |
35 |
|
Jergova et al., 2016 [15] |
Male/Rat/SD/140-160 g |
6/6 |
CCI |
7 |
L3-L4 |
GABAergic NPCs |
Genetically modified cell |
No/No/Yes |
Intraspinal: 5 × 105 cell/Allograft |
35 |
|
Juarez-Salinas et al., 2019 [28] |
Male/Mice/C57BL6/J/6-8 weeks |
7/9 |
Paclitaxel induce pain |
7 |
Anterior cingulate cortex |
GABAergic interneurons from the mouse medial ganglionic eminence |
Intrinsic GABAergic cell |
No/No/Yes |
Intracranial; 5 × 104 cell; Allograft |
30 |
|
Kim et al., 2010 [29] |
Male/Rat/SD/170-200 g |
76/40 |
Hemisection injury |
14 |
T13-L1 |
ESC-derived GABAergic cell |
Stem cell derived |
No/No/Yes |
Intraspinal: 5 × 105 cell/Xenograft |
70 |
|
Manion et al., 2020 [32] |
Male/Mice/NOD prkdSCID/10 weeks |
21/29 |
SNI |
7 |
L1 |
ATCC-BXS0116 hiPSC |
Genetically modified cell |
No/Yes/Yes |
Intraspinal: 2 × 105 cell/Xenograft |
63 |
|
Mukhida et al., 2007 [30] |
Female/Rat/Wistar/175-200 g |
5/7 |
L5-L6 nerve ligation |
10 |
L1 |
NPC-derived GABAergic cells |
Stem cell derived |
NR/NR/Yes |
Intraspinal: 2 × 105 cell/Xenograft |
42 |
|
Vaysse et al., 2011 [31] |
Male/Rat/SD/225-250 g |
8/8 |
CCI |
14 |
Intrathecal injection |
hNT2.17 |
Genetically modified cell |
Yes/No/No |
Intrathecal: 1 × 106 cell/Xenograft |
42 |

2. Risk of bias assessment
Quality assessment of the studies revealed that out of all the 10 items reviewed, all articles had a high risk of bias in random outcome assessment. Additionally, 12 studies had a high risk of bias in random housing. Allocation concealment in 11 studies, sequence generation in nine studies, and caregivers’ and/or investigators’ blinding status in nine studies a had high risk of bias. Finally, it was found that the risk of bias in selective reporting was unclear in all of the studies (
Table 2).
Table 2
Quality control of included studies
|
Study |
Item 1 |
Item 2 |
Item 3 |
Item 4 |
Item 5 |
Item 6 |
Item 7 |
Item 8 |
Item 9 |
Item 10 |
|
Bráz et al., 2012 [22] |
✘ |
✔ |
✘ |
✘ |
✔ |
✘ |
✔ |
✔ |
? |
✔ |
|
Bráz et al., 2015 [23] |
✘ |
✔ |
✘ |
✘ |
✘ |
✘ |
✔ |
✔ |
? |
✔ |
|
Eaton et al., 1999 [25] |
✘ |
✔ |
✘ |
✘ |
✘ |
✘ |
✘ |
? |
? |
✔ |
|
Eaton et al., 2012 [24] |
✘ |
✘ |
✘ |
✘ |
✘ |
✘ |
✔ |
✔ |
? |
✔ |
|
Etlin et al., 2016 [26] |
✘ |
✔ |
✘ |
✘ |
✘ |
✘ |
✔ |
? |
? |
✔ |
|
Hwang et al., 2016 [16] |
✔ |
✘ |
✘ |
✘ |
✘ |
✘ |
✔ |
✔ |
? |
✔ |
|
Jergova et al., 2012 [27] |
✘ |
✔ |
✘ |
✘ |
✘ |
✘ |
✔ |
? |
? |
✔ |
|
Jergova et al., 2016 [15] |
✘ |
✔ |
✘ |
✘ |
✘ |
✘ |
✔ |
✔ |
? |
✔ |
|
Juarez-Salinas et al., 2019 [28] |
✘ |
✔ |
✘ |
✘ |
✘ |
✘ |
✔ |
? |
? |
✔ |
|
Kim et al., 2010 [29] |
✘ |
✔ |
✔ |
✘ |
✔ |
✘ |
✔ |
✔ |
? |
✔ |
|
Manion et al., 2020 [32] |
✔ |
✔ |
✔ |
✔ |
✔ |
✘ |
✔ |
✔ |
? |
✔ |
|
Mukhida et al., 2007 [30] |
✔ |
✔ |
✘ |
✘ |
✔ |
✘ |
✔ |
? |
? |
✔ |
|
Vaysse et al., 2011 [31] |
✔ |
✔ |
✘ |
✘ |
✘ |
✘ |
✘ |
✔ |
? |
✔ |

3. Publication bias
The present study was conducted in two sections. In the first part, the efficacy of the transplantation of GABAergic cells on allodynia alleviation, and in the second part, the efficacy of these cells on hyperalgesia was investigated. The trim-and-fill method did not show any evidence of publication bias since there was no possibly missing study (
Fig. 2).
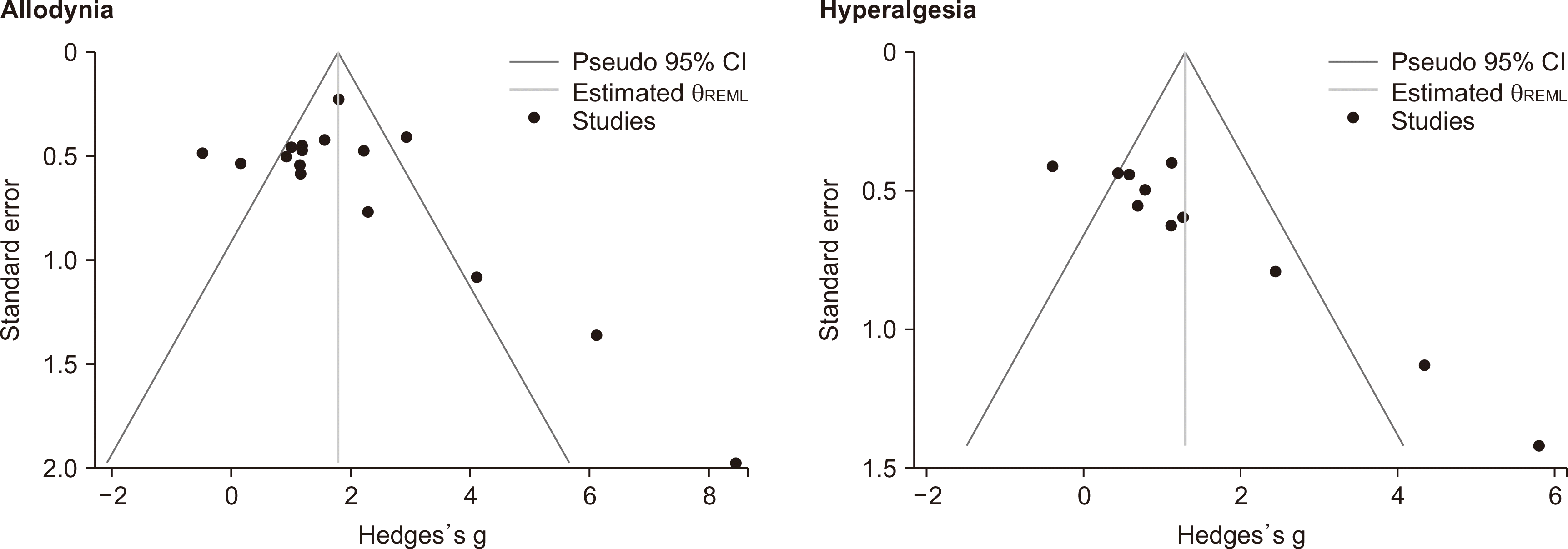 | Fig. 2Publication bias assessment across included studies. CI: confidence interval. 
|
4. The efficacy of administration of GABAergic cells on allodynia
The Galbraith plot showed that the allodynia assessment in chronic constriction injury (CCI) of the sciatic nerve in Eaton et al. [
24] is an outlier (SMD = 19.80; 95% CI: 11.05 to 28.55), therefore we excluded this analysis from our meta-analysis. The present meta-analysis showed that administration of GABAergic neural precursor cells improved allodynia as one of the symptoms of neuropathic pain (SMD = 1.79; 95% CI: 0.87 to 2.71;
P < 0.001) (
Fig. 3). However, high heterogeneity was observed between the studies (I
2 = 87.08%;
P < 0.001).
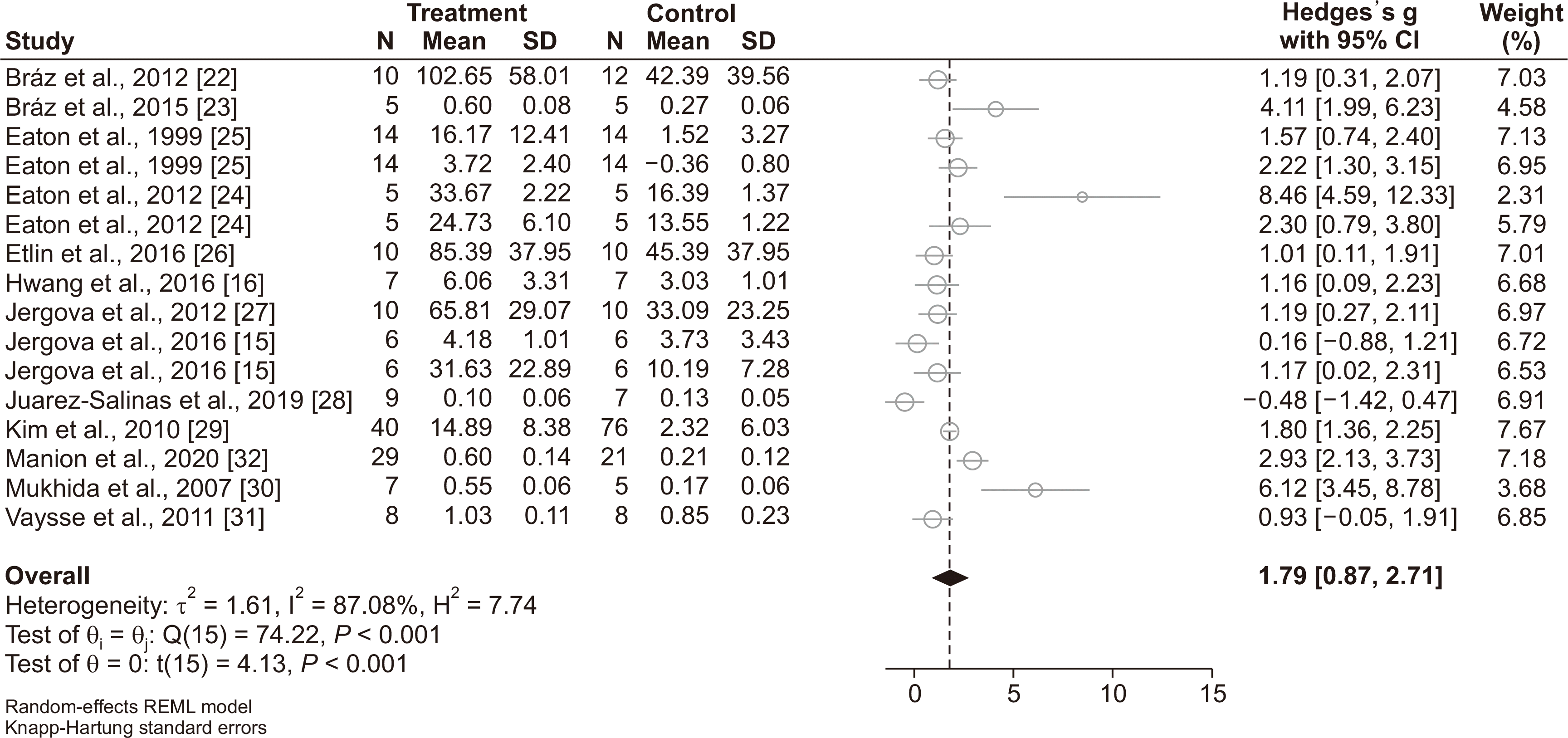 | Fig. 3Forest plot of GABAergic cell transplantation on improvement of allodynia. Since, some included studies had more than one experiment, the number of experiments exceeded the number of studies. CI: confidence interval, SD: standard deviation. 
|
Meta-regression demonstrated that the main sources of this heterogeneity among the studies that had evaluated the efficacy of GABAergic cells’ transplantation on allodynia, were due to the administration of antibiotics (coefficient = –1.96;
P = 0.033) and the type of graft (coefficient = 1.56;
P = 0.075) (
Table 3).
Table 3
Subgroup analysis for assessment of application of GABAergic cell on allodynia improvement in 16 experiments
|
Subgroup |
No. of experimenta
|
Effect size |
Heterogeneity
(P value) |
Meta-regression |
|
SMD (95% CI) |
P value |
Coef. (95% CI) |
P value |
|
Species |
|
|
|
|
|
|
|
Mice |
5 |
1.63 (–0.50, 3.76) |
0.101 |
90.7% (< 0.001) |
Ref. |
|
|
Rat |
11 |
1.83 (0.66, 3.00) |
0.006 |
83.3% (< 0.001) |
0.32 (–1.78, 2.42) |
0.749 |
|
Pain inducing model |
|
|
|
|
|
|
|
Peripheral |
13 |
1.92 (0.66, 3.18) |
0.006 |
90.4% (< 0.001) |
Ref. |
|
|
Central |
3 |
1.75 (0.94, 2.56) |
0.012 |
0.0% (0.419) |
–0.11 (–2.58, 2.36) |
0.923 |
|
Route of transplantation |
|
|
|
|
|
|
|
Intraspinal |
11 |
1.83 (0.93, 2.73) |
0.001 |
82.2% (< 0.001) |
Ref. |
|
|
Intrathecal |
4 |
2.76 (–2.36, 7.89) |
0.184 |
93.9% (< 0.001) |
0.25 (–2.00, 2.50) |
0.816 |
|
Intracranial |
1 |
NA |
NA |
NA |
NA |
NA |
|
Cell source |
|
|
|
|
|
|
|
Intrinsic GABAergicb
|
5 |
1.19 (–0.67, 3.05) |
0.149 |
83.9% (0.001) |
Ref. |
|
|
Genetically modified |
9 |
2.12 (0.60, 2.94) |
0.008 |
77.1% (< 0.001) |
0.64 (–1.47, 2.75) |
0.522 |
|
Stem cell derived |
2 |
3.75 (–23.53, 31.03) |
0.331 |
89.8 (0.002) |
2.03 (–1.26, 5.32) |
0.206 |
|
Immunosuppressive |
|
|
|
|
|
|
|
Yes |
3 |
3.53 (–6.01, 13.09) |
0.252 |
94.4% (< 0.001) |
Ref. |
|
|
No |
12 |
1.42 (0.73, 2.10) |
< 0.001 |
79.8% (< 0.001) |
–1.02 (–3.29, 1.25) |
0.349 |
|
NR |
1 |
NA |
NA |
NA |
NA |
NA |
|
Antibiotic |
|
|
|
|
|
|
|
Yes |
3 |
4.11 (–3.67, 11.80) |
0.151 |
91.7% (0.014) |
Ref. |
|
|
No |
12 |
1.22 (0.64, 1.83) |
< 0.001 |
68.6% (< 0.001) |
–1.96 (–3.73, –0.19) |
0.033 |
|
NR |
1 |
NA |
NA |
NA |
NA |
NA |
|
Blinding of outcome assessor |
|
|
|
|
|
|
|
Yes |
13 |
1.96 (0.71, 3.22) |
0.005 |
91.4% (< 0.001) |
Ref. |
|
|
No |
3 |
1.59 (0.02, 3.15) |
0.049 |
42.7% (0.170) |
–0.32 (–2.75, 2.12) |
0.785 |
|
Type of graft |
|
|
|
|
|
|
|
Allograft |
10 |
1.18 (0.44, 1.92) |
0.005 |
69.1% (< 0.001) |
Ref. |
|
|
Xenograft |
6 |
3.28 (0.53, 6.04) |
0.028 |
94.7% (< 0.001) |
1.56 (–0.18, 3.31) |
0.075 |
|
Injury to transplant |
|
|
|
|
|
|
|
0 to 7 days |
10 |
1.41 (0.54, 2.27) |
0.005 |
81.2% (< 0.001) |
Ref. |
|
|
8 days and over |
6 |
3.02 (0.05, 5.99) |
0.048 |
94.8% (< 0.001) |
1.13 (–0.84, 3.10) |
0.238 |
|
No. of cell (cell/kg) |
|
|
|
|
|
|
|
≤ 5 × 105
|
10 |
1.68 (0.43, 2.92) |
0.014 |
90.6% (< 0.001) |
Ref. |
|
|
5 × 105 to 1 × 106
|
6 |
1.79 (0.35, 3.23) |
0.024 |
50.7% (0.005) |
0.44 (–1.60, 2.47) |
0.653 |
|
Follow up duration |
|
|
|
|
|
|
|
4 to 7 weeks |
9 |
1.41 (0.03, 2.80) |
0.046 |
87.3% (< 0.001) |
Ref. |
|
|
8 weeks and over |
7 |
2.12 (1.05, 3.19) |
0.003 |
60.3% (0.002) |
0.97 (–0.82, 2.76) |
0.265 |

Subgroup analysis showed that the alleviating effect of GABAergic cell transplantation on allodynia is observed in rat (SMD = 1.83; 95% CI: 0.66 to 3.00;
P = 0.006) not mouse studies (SMD = 1.63; 95% CI: –0.50 to 3.76;
P = 0.101). In addition, it seems that intraspinal transplantation (SMD = 1.83; 95% CI: 0.93 to 2.73;
P = 0.001) is effective in alleviating allodynia, while intrathecal transplantation did not have a significant effect (SMD = 2.76; 95% CI: –2.36 to 7.89;
P = 0.184). Cell source was another possible moderator for the efficacy of GABAergic cell transplantation. The results showed that genetically modified GABAergic cells (SMD = 2.12; 95% CI: 0.60 to 2.94;
P = 0.008) can improve allodynia, while intrinsic GABAergic cells (SMD = 1.19; 95% CI: –0.67 to 3.05;
P = 0.149) and stem cell derived GABAergic cells (SMD = 3.75; 95% CI: –23.53 to 31.03;
P = 0.331) did not have a significant effect on allodynia. Immunosuppressive (SMD = 3.53; 95% CI: –6.01 to 13.09;
P = 0.252) and antibiotic (SMD = 4.11; 95% CI: –3.67 to 11.80;
P = 0.151) administration abolished any significant effect of GABAergic cells transplantation on allodynia compared to the non-transplanted group (animals receiving immunosuppressive/antibiotic without cell transplantation) (
Table 3).
5. The efficacy of administration of GABAergic cells on hyperalgesia
The Galbraith plot showed that the heat hyperalgesia assessment in Eaton et al. [
10] is an outlier (SMD = 7.10; 95% CI: 5.11 to 9.10), therefore we excluded this analysis from our meta-analysis. The meta-analysis showed that transplantation of GABAergic cells improves hyperalgesia in the subjects, as well (SMD = 1.29; 95% CI: 0.26 to 2.32;
P = 0.019) (
Fig. 4). Nonetheless, high heterogeneity was found among the studies (I
2 = 81.5%;
P < 0.001).
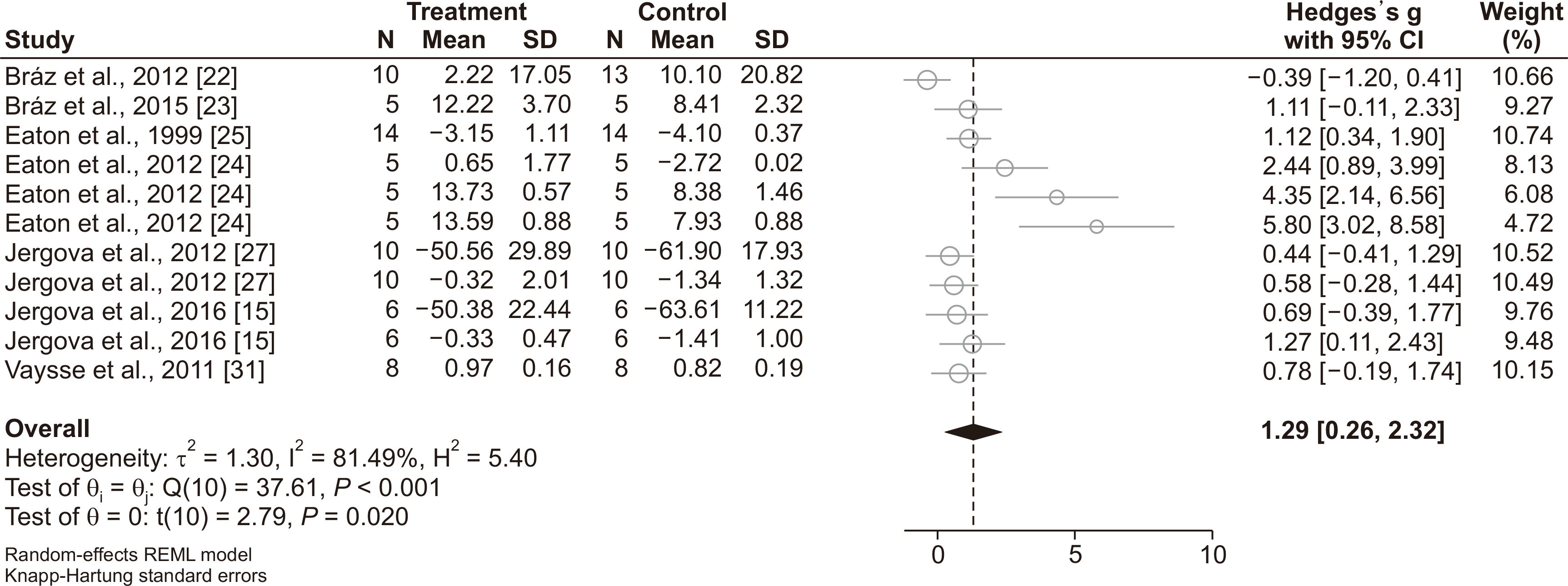 | Fig. 4Forest plot of GABAergic cell transplantation on improvement of hyperalgesia. Since, some included studies had more than one experiment, the number of experiments exceeded the number of studies. CI: confidence interval, SD: standard deviation. 
|
Meta-regression demonstrated that the possible sources of heterogeneity among the studies that had evaluated the efficacy of GABAergic cells’ transplantation on hyperalgesia were the route of administration (coefficient = 1.94;
P = 0.035), immunosuppressive (coefficient = –1.94;
P = 0.035) and antibiotic (coefficient = –2.97;
P = 0.002) administration, the type of graft (coefficient = 1.94;
P = 0.035), injury to transplantation interval (coefficient = 1.94;
P = 0.035), number of transplanted cells (coefficient = 1.62;
P = 0.065) and follow up duration (coefficient = 2.00;
P = 0.022) (
Table 4). It seems that a possible confounding effect was present among above moderators, since seven studies did not administer immunosuppressives, their route of GABAergic transplantation was intraspinal, their type of graft was xenograft and the injury to treatment interval was 0 to 7 days. Therefore, we performed a multiple meta-regression to find independent sources of heterogeneity for the efficacy of GABAergic cells on hyperalgesia. The analysis showed that antibiotic administration is the main source of heterogeneity among included studies (coefficient = –3.51;
P = 0.076).
Table 4
Subgroup analysis for assessment of application of GABAergic cell on hyperalgesia improvement in 11 experiments
|
Subgroup |
No. of experimenta
|
Effect size |
Heterogeneity (P value) |
Meta-regression |
|
SMD (95% CI) |
P value |
Coef. (95% CI) |
P value |
|
Species |
|
|
|
|
|
|
|
Mice |
2 |
0.29 (–9.24 to 9.81) |
0.768 |
75.5% (0.043) |
Ref. |
|
|
Rat |
9 |
1.56 (0.32 to 2.78) |
0.019 |
81.9% (< 0.001) |
1.23 (–1.36, 3.82) |
0.312 |
|
Origin of pain |
|
|
|
|
|
|
|
Peripheral |
10 |
0.97 (0.13 to 1.81) |
0.028 |
65.4% (< 0.001) |
NA |
NA |
|
Central |
1 |
NA |
NA |
NA |
NA |
NA |
|
Route of transplantation |
|
|
|
|
|
|
|
Intraspinal |
7 |
0.63 (0.08 to 1.17) |
0.030 |
40.2% (0.137) |
Ref. |
|
|
Intrathecal |
4 |
3.08 (–0.38 to 6.55) |
0.066 |
83.8% (< 0.001) |
1.94 (0.17, 3.72) |
0.035 |
|
Intracranial |
0 |
NA |
NA |
NA |
NA |
NA |
|
Cell source |
|
|
|
|
|
|
|
Intrinsic GABAergicb
|
2 |
0.29 (–9.24 to 9.81) |
0.768 |
75.5% (0.043) |
Ref. |
|
|
Genetically modified |
9 |
1.56 (0.32 to 2.78) |
0.019 |
81.9% (< 0.001) |
1.23 (–1.36, 3.82) |
0.312 |
|
Stem cell derived |
0 |
NA |
NA |
NA |
NA |
NA |
|
Immunosuppressive |
|
|
|
|
|
|
|
Yes |
4 |
3.33 (–0.38 to 6.55) |
0.066 |
83.8% (< 0.001) |
Ref. |
|
|
No |
7 |
0.63 (0.08 to 1.17) |
0.030 |
40.2% (0.137) |
–1.94 (–3.72, –0.17) |
0.035 |
|
Antibiotic |
|
|
|
|
|
|
|
Yes |
3 |
3.94 (–0.25 to 8.12) |
0.056 |
59.5% (0.082) |
Ref. |
|
|
No |
8 |
0.64 (0.18 to 1.10) |
0.013 |
33.0% (0.198) |
–2.97 (–4.57, –1.37) |
0.002 |
|
Blinding of outcome assessor |
|
|
|
|
|
|
|
Yes |
9 |
1.50 (0.10 to 2.89) |
0.039 |
86.8% (< 0.001) |
Ref. |
|
|
No |
2 |
0.99 (–1.14 to 3.11) |
0.107 |
0.0% (0.588) |
–0.49 (–3.28, 2.31) |
0.702 |
|
Type of graft |
|
|
|
|
|
|
|
Allograft |
7 |
0.63 (0.08 to 1.17) |
0.030 |
40.2% (0.138) |
Ref. |
|
|
Xenograft |
4 |
3.08 (–0.38 to 6.55) |
0.066 |
83.8% (< 0.001) |
1.94 (0.17, 3.72) |
0.035 |
|
Injury to transplant |
|
|
|
|
|
|
|
0 to 7 days |
7 |
0.63 (0.08 to 1.17) |
0.030 |
40.2% (0.138) |
Ref. |
|
|
8 days and over |
4 |
3.08 (–0.38 to 6.55) |
0.066 |
83.8% (< 0.001) |
1.94 (0.17, 3.72) |
0.035 |
|
No. of cell (cell/kg) |
|
|
|
|
|
|
|
≤ 5 × 105
|
6 |
0.52 (–0.11 to 1.14) |
0.087 |
35.7% (0.183) |
Ref. |
|
|
5 × 105 to 1 × 106
|
5 |
2.54 (0.04 to 5.14) |
0.048 |
87.6% (< 0.001) |
1.62 (–0.12, 3.37) |
0.065 |
|
Follow up duration |
|
|
|
|
|
|
|
4 to 7 weeks |
7 |
0.55 (0.03 to 1.06) |
0.041 |
28.2% (0.246) |
Ref. |
|
|
8 weeks and over |
4 |
3.14 (–0.10 to 6.38) |
0.054 |
82.9% (< 0.001) |
2.00 (0.36, 3.63) |
0.022 |

Subgroup analysis showed that the alleviating effect of GABAergic cell transplantation on hyperalgesia was observed only in rats (SMD = 1.56; 95% CI: 0.32 to 2.78;
P = 0.019) and not in mice (SMD = 0.29; 95% CI: –9.24 to 9.81;
P = 0.768). Intraspinal transplantation of GABAergic cells improved hyperalgesia (SMD = 0.63; 95% CI: 0.08 to 1.17;
P = 0.030), but its intrathecal transplantation did not show a significant effect (SMD = 3.08; 95% CI: –0.38 to 6.55;
P = 0.066). Cell source was another possible moderator for the efficacy of GABAergic cell transplantation. The results showed that genetically modified GABAergic cells (SMD = 1.56; 95% CI: 0.32 to 2.78;
P = 0.019) can improve hyperalgesia, while intrinsic GABAergic cells (SMD = 0.29; 95% CI: –9.24 to 9.81;
P = 0.768) did not have significant effect on hyperalgesia. Immunosuppressives (SMD = 3.33; 95% CI: –0.38 to 6.55;
P = 0.066) and antibiotics (SMD = 3.94; 95% CI: –0.25 to 8.12;
P = 0.056) abolished any significant effect of GABAergic cells transplantation on hyperalgesia compared to the non-transplanted groups (animals receiving an immunosuppressive/antibiotic without cell transplantation). In addition, allograft transplantation of GABAergic cells can improve hyperalgesia and their efficacy was observed when the GABAergic cells were transplanted within the first seven days after induction of the neuropathic pain model (SMD = 0.63; 95% CI: 0.08 to 1.17). The number of cells was another moderator of the GABAergic cells. These cells can alleviate hyperalgesia when transplanted in doses above 5 × 10
5. It seems that the blinding status of the outcome assessor can affect the reported efficacy of GABAergic cells. The observed efficacy of GABAergic cell transplantation on hyperalgesia is significant when outcome assessor blinding was performed, while the result was not significant in unblinded outcome assessment (
Table 4).
6. Sensitivity analysis
The leave-one-out approach was used to assess the individual studies’ effect on the findings. As
Fig. 5 and
Fig. 6 show, omitting any of the included articles does not statistically change the effect size of GABAergic cells transplantation.
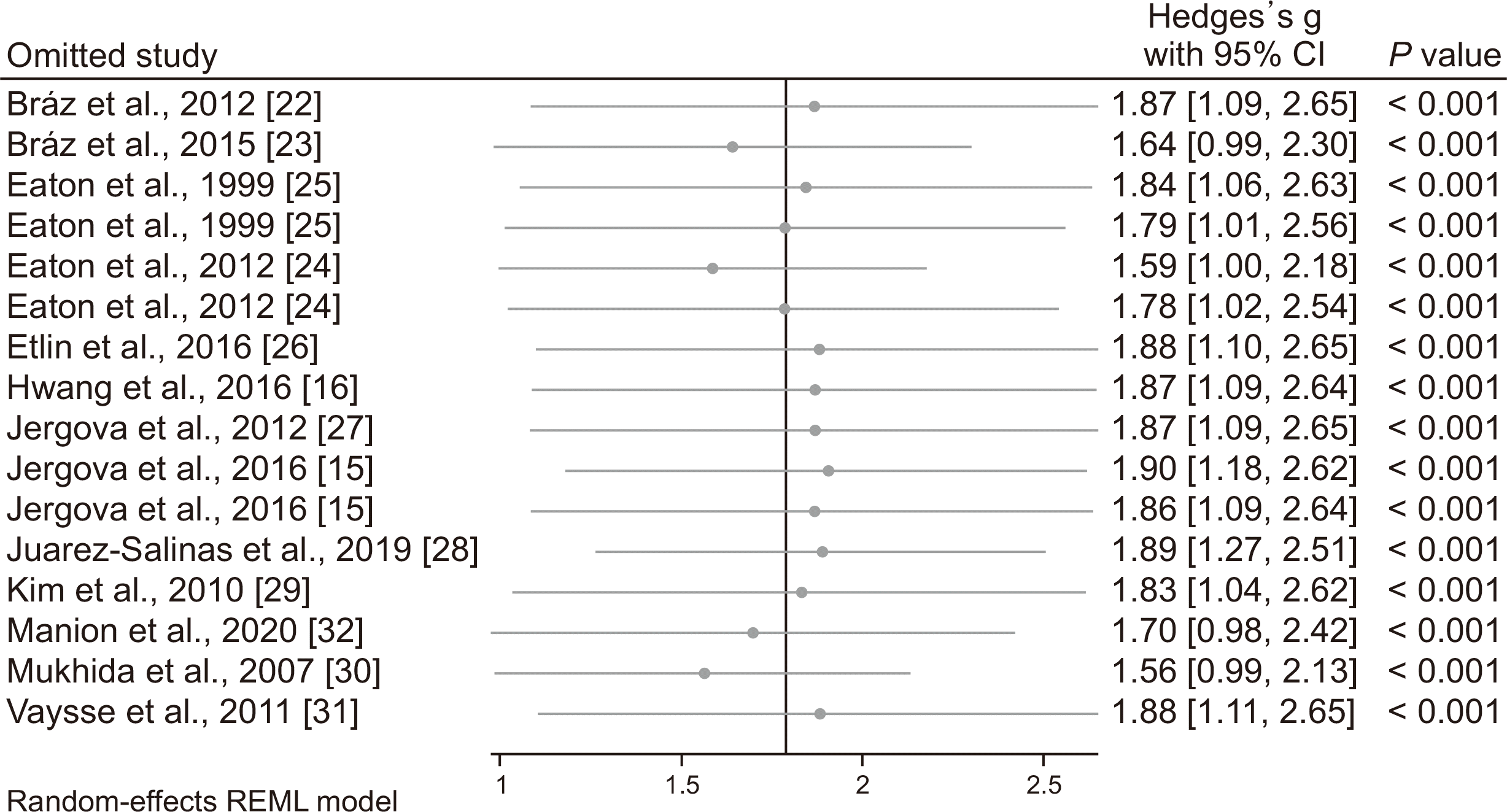 | Fig. 5Sensitivity analysis to assess individual studies’ effect on efficacy of GABA cell transplantation on improvement of allodynia. Since, some included studies had more than one experiment, the number of experiments exceeded the number of studies. CI: confidence interval. 
|
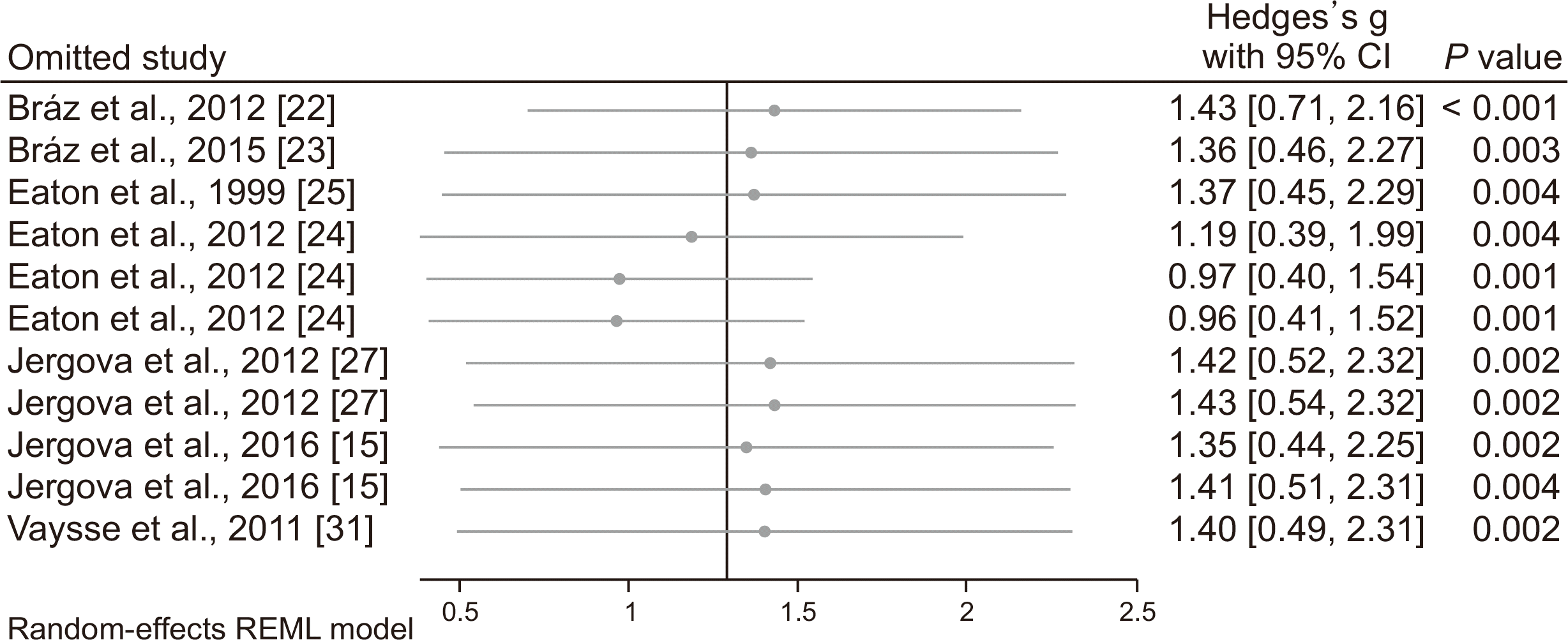 | Fig. 6Sensitivity analysis to assess individual studies’ effect on efficacy of GABA cell transplantation on improvement of hyperalgesia. Since, some included studies had more than one experiment, the number of experiments exceeded the number of studies. CI: confidence interval. 
|
7. Certainty of evidence
The assessment of the evidence level according to the GRADE framework showed that the overall certainty of the evidence evaluating the efficacy of GABAergic cell transplantation on alleviating allodynia and hyperalgesia was moderate (
Table 5).
Table 5
Pain
symptom |
No. of analysesa
|
Risk of bias |
Imprecision |
Inconsistency (I2 range) |
Indirectness |
Publication bias |
Judgment |
Level of evidence |
|
Allodynia |
16 |
High |
Not serious imprecision |
Not seriousb
|
Not serious indirectness |
Not present |
Level of evidence was rated down one grade since there is possible risk of bias |
Moderate |
|
Hyperalgesia |
11 |
High |
Not serious imprecision |
Not seriousb
|
Not serious indirectness |
Not present |
Level of evidence was rated down one grade since there is possible risk of bias |
Moderate |

Go to :

DISCUSSION
A moderate level of pre-clinical evidence showed that the administration of GABAergic cells improves allodynia and hyperalgesia in neuropathic pain animal models. However, the considerable heterogeneity among the studies led the researchers to perform subgroup analysis to find the main sources of this heterogeneity. These analyses revealed that the transplanted GABAergic cells improved neuropathic pain’s symptoms only in rat studies, whereas such a positive effect was not observed in mice. It was also demonstrated that only genetically modified GABAergic cells alleviate both of neuropathic pain’s symptoms. Stem cell-derived cells had no significant effect on allodynia. Also, it was concluded that GABAergic interneurons from the mouse medial ganglionic eminence are not effective on the management of hyperalgesia. Consequently, it seems that the best sources of GABAergic cells in the management of neuropathic pain are genetically modified cells.
Nonetheless, concerns still exist regarding genetically modified cells. The possibility of genetic defects following genetic modification is still debated [
33]. Adding a synthetic DNA sequence could cause dangerous mutations which are difficult to manage and may lead to serious consequences. Still, advances in tissue and gene engineering show promising results for adopting these techniques in the future.
This study has taken advantage of multiple subgroup analyses, performed to identify and possibly eliminate the significant difference in the articles, from a methodological point of view, to draw a conclusion with regards to these differences. The present study revealed that the efficacy of GABAergic cells in the management of hyperalgesia is only seen in rat subjects. This causes serious limitations to the generalization of the findings to other species. If such differences are observed between the two morphologically and genetically close species as rats and mice, then caution should be exercised in generalizing and translating these findings to future large animal studies or clinical trials.
The route of administration was also evaluated in in the present study. Overall, the method of injection was intraspinal in nine studies [
15,
22-
24,
26,
27,
29,
30,
32] and intrathecal in three studies, while one study had transplanted the cells in the anterior cingulate cortex. Overall, the analyses revealed that the intraspinal administration method might be the desired route for the transplantation of GABAergic cells. However, the number of included studies administrating the cells intrathecally was low. Since intrathecal injection causes the least damage to the neural tissue, future studies may adopt this method and compare its efficacy with that of intraspinal or intracranial injection.
Various mechanisms are involved in the occurrence of neuropathic pain, the most important of which are neuropathy following SCI, diabetes, and chemotherapy [
34,
35]. Studies regarding all of these three mechanisms were included in the present study, and it was revealed that GABAergic cells improve the symptoms of neuropathic pain caused by all of the aforementioned mechanisms. In this setting, Eaton et al. [
24] compared the effect of the transplantation of GABAergic cells in the following three neuropathic pain models in animals: streptozotocin-induced neuropathic pain (diabetic induced neuropathic pain), CCI (peripheral neuropathic pain) and excitotoxic SCI (central neuropathic pain). The findings of this study showed that the administration of GABAergic cells alleviated neuropathic pain in all of the three models [
24].
Regarding the effects of antibiotic and immunosuppressive administration on the efficacy of GABAergic precursor cells’ transplantation, one could speculate that because of the possible beneficial effects of these two agents on alleviating the neuropathic pain, the difference of pain alleviation, in terms of allodynia and hyperalgesia, between the animals in the cell-treated group and the non-treated group was not significant. However, data regarding the matter is somewhat scarce, as few studies administered antibiotic and immunosuppressive treatments, which could be counted as a limitation of the present study. Hence, researchers are encouraged to gather more evidence about the effects of antibiotic and immunosuppressive administration on the efficacy of the GABAergic precursor cells’ transplantation in the animal models.
As the paramount limitation of the present study, the number of studied animals, as well as the number of studies was significantly low, as only 317 animals were studied overall. Furthermore, much of the data was gathered from the same labs, which may affect the results regarding the efficacy of the GABAergic precursor cells’ transplantation. As a result, to better reach a conclusion, a greater number of studies and experiments are needed.
Another limitation of the present systematic review was the low number of studies on the efficacy of GABAergic cells’ transplantation in the management of central hyperalgesia and on the comparison of stem cell-derived GABAergic cells with other cell origins in analyses related to hyperalgesia. Hence, meta-analysis could not be performed in these two areas. Moreover, the quality assessment of the articles demonstrated that most of the included studies had high risk of bias in sequence generation, random housing, as well as caregiver and/or investigator blinding and random outcome assessment. However, these items are overlooked in most animal studies, and a review on all of these studies reveals that not reporting allocation concealment, random housing, caregivers and/or investigators blinding and random outcome assessment is common. Thus, it is recommended that researchers be informed in this regard. Additionally, the risk of bias was unclear in selective outcome reporting in all the included studies. This could be attributable to the lack of an animal registry database. If such databases, similar to clinical trial registries, are created, selective outcome reporting could be largely monitored and reduced.
In conclusion, the present meta-analysis showed that administration of GABAergic cells alleviates allodynia and hyperalgesia in neuropathic pain models. However, it seems that the transplantation efficacy of these cells is only statistically significant in improving allodynia and hyperalgesia in rats, while such a positive effect was not observed in mouse studies. Therefore, caution should be exercised regarding the generalizability of findings from rat and mice studies to large animals and subsequent clinical trials. It was also concluded that genetically modified cells are the better source of GABAergic cells for management of neuropathic pain, compared to intrinsic GABAergic cells.
Go to :






 PDF
PDF Citation
Citation Print
Print








 XML Download
XML Download