Abstract
Background
Prior studies have reported that 40%-90% of the patients with celiac plexus-mediated visceral pain benefit from the neurolytic celiac plexus block (NCPB), but the predictive factors of response to NCPB have not been evaluated extensively. This study aimed to identify the factors associated with the immediate analgesic effectiveness of NCPB in patients with intractable upper abdominal cancer-related pain.
Methods
A retrospective review was performed of 513 patients who underwent NCPB for upper abdominal cancer-related pain. Response to the procedure was defined as (1) a decrease of ≥ 50% or ≥ 4 points on the numerical rating scale (NRS) in pain intensity from the baseline without an increase in opioid requirement, or (2) a decrease of ≥ 30% or ≥ 2 points on the NRS from the baseline with simultaneously reduced opioid consumption after NCPB. Logistic regression analysis was performed to determine the factors associated with successful responses to NCPB.
Results
Among the 513 patients included in the analysis, 255 (49.8%) and 258 (50.2%) patients were in the non-responder and responder group after NCPB, respectively. Multivariable logistic regression analysis showed that diabetes (odds ratio [OR] = 0.644, P = 0.035), history of upper abdominal surgery (OR = 0.691, P = 0.040), and celiac metastasis (OR = 1.496, P = 0.039) were the independent factors associated with response to NCPB.
Go to : 
The prevalence of cancer pain is as high as 51% among patients diagnosed with cancer; the estimates may vary with patient groups stratified by the type of cancer, phases of illness, and/or treatment [1]. For example, 80% of the patients with pancreatic cancer and 90% of the patients in advanced stages of the disease experience pain [2]. As the World Health Organization analgesic ladder is generally accepted for cancer pain management, systemic analgesics are preferentially used to manage cancer pain initially. However, such medical management is sometimes not enough to reduce pain effectively, and is accompanied by intolerable side effects such as drowsiness, somnolence, confusion, delirium, dry mouth, anorexia, and constipation. In this context, neurolytic celiac plexus block (NCPB) can be considered as an alternative pain management strategy.
Since the first experience reported by Kappis in 1914, NCPB has become widely known as an effective and minimally invasive method for the pain management of patients with cancer-related visceral pain. This technique involves the use of a long-acting local anesthetic, with or without a steroid or neurolytic agent, that interrupts the neural transmission of pain signals from the celiac plexus, which innervates the upper abdominal visceral organs from the distal esophagus to the transverse colon [2-4]. Although several studies have shown that NCPB significantly relieves certain types of visceral pain, pain relief is not guaranteed in all cases. Prior studies have reported that 40%-90% of the patients benefit from this intervention, and the efficacy of pain relief after NCPB may vary among patients [4-10]. Thus, it is important to identify the factors associated with the effectiveness of NCPB, as this will enable physicians to determine whether the procedure will benefit their patients. Several studies have investigated the predictive factors for several years. However, these studies are insufficient for establishing the factors because other potential factors exist but have not been illuminated, and the roles of some specific factors are still debated [2,7,10-16].
This retrospective study aimed to identify the factors associated with the immediate analgesic effectiveness of NCPB, including patient and cancer characteristics, and the pain profiles of patients with intractable upper abdominal cancer-related pain.
Go to : 
This study was approved by the Institutional Review Board of Asan Medical Center (Registry number: 2018-1157), and the requirement of informed consent was waived, as the recorded data was reviewed only retrospectively.
The authors reviewed the electronic medical records of the patients treated at their institution. The records of all the patients who received NCPB at their pain clinic (Asan Medical Center, Seoul, Korea) between 2007 and 2018 were reviewed. The inclusion criteria for the analysis were as follows: (1) age ≥ 20 years and (2) intractable abdominal or back pain related to upper abdominal cancer. Intractable cancer pain, in the present study, was defined as a state of cancer pain that cannot be controlled by sufficient high-dose administration of opioids, or a state in which adequate administration of opioid cannot be performed for pain control due to intolerable adverse effects. The exclusion criteria were as follows: (1) abdominal or back pain not related to cancer in the upper abdomen (e.g., metastases of bone or other organs, especially pelvic organs), (2) insufficient medical records, (3) loss to follow-up after the procedure, (4) receiving other procedures such as epidural patient-controlled analgesia by changing the treatment plan, (5) non-execution for other reasons, such as patient refusal or death, and (6) non-completion of the procedure due to poor cooperation.
Demographic data such as age, sex, and body mass index (BMI) were collected. Clinical data such as underlying diabetes and hypertension, a history of previous upper abdominal operation, cancer origin (biliary, pancreas, liver, stomach, others), metastasis, tumor invasion and metastasis in upper abdomen, celiac axis metastasis, pain location (abdominal, back, or both), pain duration, pre/post-procedural pain intensity (after 24–48 hours) measured using the numerical rating scale (NRS), and pre/post-procedural (after 24–48 hours) oral morphine equivalent dose (MED) were obtained. Data were extracted by clinical assistants who were not involved with the study or data analysis. Clinical physicians clarified the queries and assisted in data extraction when necessary.
All the procedures in this study were performed by four pain physicians. Prior to the procedure, all the patients were hydrated with intravenous crystalloid to prevent hypotension secondary to the sympathetic block. In the operation room, the patient was placed in prone position with vital signs monitoring throughout the procedure. Under fluoroscopic guidance, the posterior approach was standardized for all cases. Depending on the patient’s anatomy, either a paravertebral or transdiscal approach was selected. The transdiscal approach was selected for patients with suspected hydronephrotic kidneys, a prominent transverse process of the L1 vertebra or an osteophyte interfering with the advancement of the needle and proper positioning. The paravertebral approach was used for patients with a degenerated disc with calcification. In addition, the approach was chosen according to the physician’s preference.
For the paravertebral approach, the T12 and L1 vertebrae, and the 12th ribs were first identified under an anteroposterior fluoroscopic view. The C-arm was rotated 30°-45° until the transverse process of L1 was within the silhouette of the vertebral body. The needle entry site was the anterior margin of the L1 vertebral body, which was within 7-8 cm of the midline. Once the skin entry point was infiltrated with 2 to 5 mL of lidocaine 2%, a curved 22-gauge 152-mm Chiba needle (Green Medical Supply, Seoul, Korea) was inserted and advanced toward the upper third and anterior third of the first lumbar vertebral body in tunnel view. Once the bony contact was made, under the lateral fluoroscopic view, the needle advanced further (sliding on the lateral vertebral body) until the tip of the needle was 0.5 cm anterior to the vertebral body. Similar steps were carried out on the contralateral side.
For the transdiscal approach, a virtual transdiscal needle pathway was drawn and the oblique angle from the axis was measured in advance, using the most recent abdominal computed tomographic (CT) image before the procedure [17,18]. After positioning the needle obliquely to an angle measured from the anteroposterior view under fluoroscopic guidance, local skin infiltration was performed with 2% lidocaine. A 22-gauge 152-mm Chiba needle was inserted and advanced through the T12-L1 intervertebral disc along the CT-simulated pathway using the tunnel vision technique. Then, the needle advanced further through the intervertebral disc until the tip of the needle was 0.5 cm anterior to the vertebral body under the lateral fluoroscopic view and at the midline of the disc in the anteroposterior view [19].
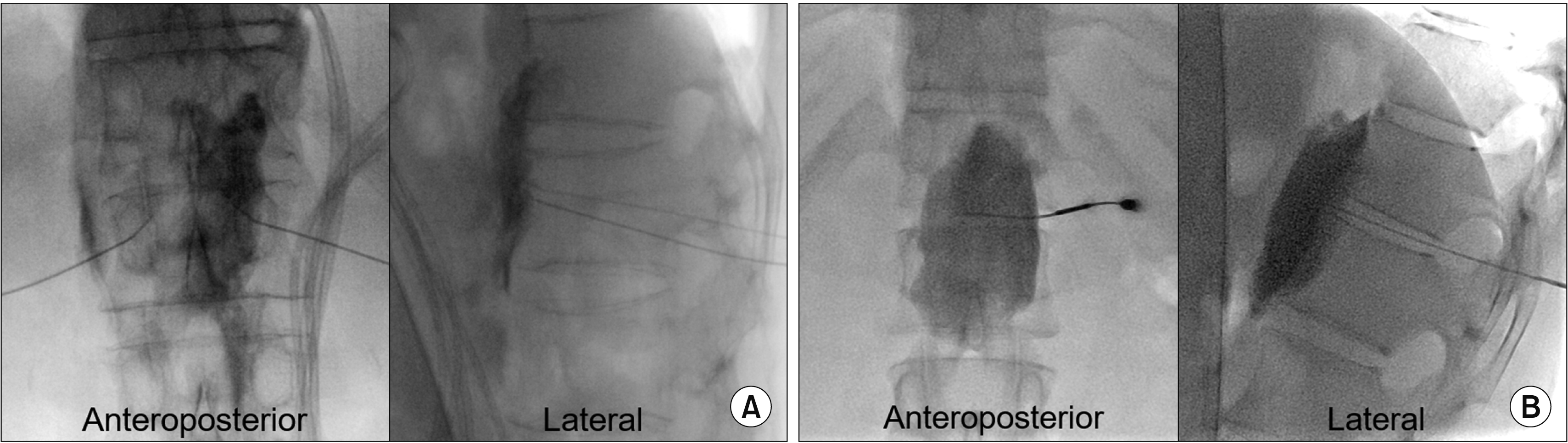
An aspiration test was carried out to exclude blood, cerebrospinal fluid, and chyle prior to contrast dye injection. Subsequently, 1% lidocaine or 0.25% bupivacaine mixed with contrast dye was injected for a temporary block and simultaneous identification of contrast dispersion. The contrast dye spread should be anterolateral to the vertebral body in the lateral view and within the contour of the spine in the anteroposterior view (Fig. 1). Next, 7% phenol or 99% alcohol neurolytic agent was injected slowly (over 2 minutes). The volume of the injectate varied, but both the local anesthetic and neurolytic agent were typically within the range of 8-12 mL based on the spread of contrast dye during injection. The patient was transferred to the recovery unit for an hour to check for signs of adverse effects such as hypotension and neurologic deficits. The patients were required to be in the prone or supine position for 2 hours, as appropriate.
The outcome was evaluated at baseline and 24–48 hours after the NCPB to eliminate the immediate post-procedural pain associated with neurolysis [7,20]. The pain intensity was rated for each patient using the NRS from 0 to 10 (0 denotes no pain and 10 denotes the worst pain imaginable). Daily opioid consumption was converted to oral MED for outcome assessment [21]. Depending on the outcomes, the patients were divided into non-responder and responder groups. Response to the procedure was defined as (1) a decrease of ≥ 50% or ≥ 4 points on the NRS in pain intensity from the baseline without an increase in opioid requirement or (2) a decrease of ≥ 30% or ≥ 2 points on the NRS from the baseline with simultaneously reduced opioid consumption after the NCPB [22]. Data on adverse effects (for example, procedure-related pain, diarrhea, and hypotension) were collected as secondary outcomes.
Continuous variables were expressed as means ± standard deviations or medians with interquartile ranges as appropriate, and categorical variables were expressed as numbers (percentages). Continuous variables pertaining to the non-responders and successful responders were compared using the Student’s t-test or Mann–Whitney U-test. Categorical variables were compared using the chi-squared test or Fisher’s exact test, as appropriate. Univariable and multivariable logistic regression analyses were performed to determine the factors associated with a successful response to the NCPB. The variables associated with successful responses that showed P values of < 0.05 on univariable logistic regression analyses were included in the multivariable logistic regression analyses. The odds ratios (ORs) for successful response in the presence of independent predictors of NCPB were calculated by logistic regression analyses. All statistical analyses were performed using the SPSS version 21.0 (IBM Co., Armonk, NY).
Go to : 
Of the 811 patients whose records were reviewed, 513 with severe abdominal or back pain related to upper abdominal cancer were selected, and their data were analyzed; 298 patients were excluded as per the exclusion criteria. Further, 255 patients (49.8%) were identified as the non-responder group and 258 patients (50.2%) were identified as the responder group after NCPB (Fig. 2).
The demographic characteristics of the non-responders and responders are shown in Table 1. On average, diabetes mellitus and history of previous upper abdominal operation were more common in non-responders than in responders (P = 0.043 and P = 0.019, respectively). Diagnostic imaging showed celiac metastasis more frequently in the responder group than in the non-responder group (P = 0.036). The differences in other data such as age, sex, BMI, hypertension, origin of the tumor, presence of metastasis, tumor invasion and metastasis in upper abdomen, pain location, pain duration, basal pain intensity, and opioid consumption in oral MED were not statistically significant between the groups.
Demographics of the study population
After NCPB, the pain intensity and opioid intake were found to be significantly lower in the responder group than in the non-responder group (Table 2). Moreover, the number of patients with decreased opioid intake after NCPB was significantly greater in the responder group (51.9%) than in the non-responder group (34.9%) (P < 0.001).
Comparison of pain intensity and opioid consumption between non-responder and responder groups after neurolytic celiac plexus block
The results of the logistic regression analysis carried out to determine the factors associated with successful response to NCPB are shown in Table 3. Univariable analysis demonstrated that diabetes, a history of upper abdominal surgery, and celiac metastasis were significantly associated with successful response to NCPB. In addition, multivariable logistic regression analysis showed that diabetes (OR = 0.644, 95% confidence interval [CI] = 0.427-0.970, P = 0.035), history of upper abdominal surgery (OR = 0.691, 95% CI = 0.486-0.983, P = 0.040), and celiac metastasis (OR = 1.496, 95% CI = 1.021-2.190, P = 0.039) (Table 3) were independent factors significantly associated with successful response to NCPB.
Logistic regression analysis of factors associated with successful response after neurolytic celiac plexus block
Serious adverse effects were not observed in any patient, and all adverse effects that were observed, during and after NCPB, were mild and transient. Several patients reported temporary pain during needle insertion or injection of the neurolytic agent during the NCPB, which was tolerable and did not require additional medications or discontinuation of the procedure. Transient hypotension after the procedure requiring hydration and medication was observed in some patients, who recovered immediately after conservative treatment. No other complications such as neurological deficit or infection were reported.
Go to : 
In the present study, it was observed that the presence of celiac plexus metastases, absence of diabetes, and absence of any history of prior upper abdominal surgery may be independently associated with better response to NCPB in patients with upper abdominal cancer-related abdominal or back pain.
Several studies have demonstrated the benefit of NCPB by comparing it with standard systemic medical therapy; NCPB can provide analgesia more effectively and lead to a reduction in the use of opioids and their related side effects [8,11,17,23-26]. Wong et al. [8] demonstrated that NCPB could provide more effective analgesia than a sham block and systemic therapy. Polati et al. [25] also found that NCPB facilitated short-term analgesia and low opioid requirement, and had very few side effects. The response to NCPB, however, is not consistent across all cases reported in the literature on the effectiveness of NCPB for the treatment of upper abdominal cancer-related pain [5,9,27]. According to previous studies, NCPB is expected to work for a large proportion (40%-90%) of patients with cancer-related visceral pain [5,9,12,13]. In the present study, it was also found that the proportion of non-responders was 49.8%. Therefore, exploring the factors that influence the effectiveness of NCPB is important, as the findings will guide the selection of patients who are likely to benefit from NCPB.
Previous studies suggested that a small dosage of pre-block opioid [2,5,12,28], short duration of pain before the block [2,3,5,14,28], low baseline pain score [3], tumor location [7], small tumor size, and low TNM staging [3] are factors associated with successful response after NCPB. In the present study, celiac metastasis, absence of diabetes, and no history of abdominal surgery were found to be independently associated with a successful response to NCPB in patients with upper abdominal cancer pain, although the current study did not find any association between previously mentioned factors and the success of NCPB. This discrepancy may be attributed to the different stages of disease of the enrolled patients [17] or the different times of outcome assessments. Most patients enrolled in the present study experienced severe abdominal pain and their disease was in the advanced stages. In addition, the outcome assessments were conducted 24–48 hours after the procedure in this study. Although the individual approaches and agents used were not uniform in this study, there was no difference between the various techniques and agents in previous studies [5,9,10,23,29-31].
In the present study, the absence of diabetes was related to a better response to NCPB. It has been reported that poorly controlled diabetes is related to poor analgesic control and high analgesic consumption in the postoperative period [32]. It was also reported that the absence of diabetes was an independently predictive factor of successful response after epidural procedures in patients with chronic lumbar spinal stenosis [33]. These results may be associated with the chronic neuropathic component of diabetes because of the long-standing microvascular effect of diabetes on the nervous system, but this was not examined in this study.
It was also found that patients with a history of upper abdominal surgery may be less likely to respond to NCPB than those without such a history. Although a direct relationship between previous abdominal surgery and the effects of NCPB was not found in other studies, generally, tissue trauma in abdominal surgery triggers an inflammatory cascade, which leads to scar tissue formation. Changes in the anatomic relationships around the celiac plexus may disturb the spread of the injectate during NCPB. Previously, De Cicco et al. [13] analyzed the efficacy of the celiac plexus block in those who had anatomical abnormalities of the celiac area on prior CT and found an inverse relationship between the extent of spread and the number of abnormal anatomical areas. In addition, the mechanism underlying the pain in those patients may be very complex; it may include the somatosensory component that is thought to be unresponsive to NCPB [34,35]. Nonetheless, further investigation is needed to verify this result.
Our results also revealed that celiac metastasis was associated with the better response to NCPB in patients with intractable cancer-related abdominal and back pain. There was a discrepancy between the present findings and those of previous reports. Direct celiac invasion may adversely affect the effectiveness of endoscopic ultrasound (EUS)-guided NCPB [36,37]. EUS-NCPB refers to the injection of a neurolytic agent at the celiac plexus or ganglia under EUS guidance in the antecrural approach. It is presumed that cancer invasion of the celiac plexus may restrict the spread of neurolytic agents to the target site in the antecrural approach. Most procedures in the pain clinic used the retrocrural approach. It is postulated that a retrocrural block prevents the injectate from spreading to the retroperitoneum beyond the diaphragm crura, which helps the injectate to be distributed relatively well within the target neural structure. In addition, it may work effectively by blocking the pain caused by the celiac plexus invasion. Levy et al. [38] found tumor cells in the celiac ganglia of patients with pancreatic cancer using EUS-guided fine needle aspiration, and they attributed nociception in pancreatic cancer-related pain to the perineural invasion by the tumor as the most important mechanism underlying the experience of pain. However, this result is inconsistent with that of a prior study conducted by Koyyalagunta et al. [10] who analyzed only patients with splanchnic nerve neurolysis and found that celiac axis tumor infiltration did not change the efficacy of the procedure. Further research on this aspect is needed.
The present study has several limitations. As the data were collected retrospectively, accurate procedural information was often difficult to obtain for analysis. This study was carried out with only about two-thirds of the total cases available for analysis and not formally powered to detect predefined clinically important differences. Second, interpersonal differences in terms of approaches, sides, volume, and type of neurolytic agents used between the physicians might have had some impact on the final outcome of this study. However, NCPB is almost standardized at the authors’ institution, and the inter-physician differences are considered to be minimal. Third, the follow-up duration for evaluating effectiveness varied. In the present study, the outcome variables were measured for a short period. Long-term follow-up could not be conducted because of deaths or incomplete medical records. Only the immediate effect of NCPB was recorded, which may not accurately reflect the long-term efficacy of NCPB. If the time frame for evaluating the outcomes is different, different results may be obtained. Finally, the definition of successful response used in the present study can be criticized. Different results could be obtained with other definitions of successful response.
In conclusion, the evidence of celiac plexus metastasis confirmed by imaging analysis, absence of diabetes mellitus, and absence of previous upper abdominal surgery may be associated with a successful response to NCPB in patients with upper abdominal cancer-related pain. Further randomized, prospective studies are needed to validate these findings.
Go to : 
ACKNOWLEDGMENTS
The authors thank the residents and clinical fellows in the Department of Anesthesiology and Pain Medicine, Asan Medical Center for collecting data in the present study (Drs. Hanwool Park, Chan-Sik Kim, Sooin Park, and Yul Oh).
Go to : 
Notes
This work was presented in part as H-J Kwon’s MS thesis at the University of Ulsan College of Medicine (2021).
Previous presentation at conferences: This work was partly presented in the KPS 2019 Annual Meeting (the 69th Scientific Meeting of the Korean Pain Society) held in Seoul, Korea (2019).
Author contributions: Hyun-Jung Kwon: Writing/manuscript preparation; Kyunghwan Jang: Investigation; Jeong-Gil Leem: Investigation; Jin-Woo Shin: Investigation; Doo-Hwan Kim: Investigation; Seong-Soo Choi: Supervision.
Go to : 
REFERENCES
1. van den Beuken-van Everdingen MH, Hochstenbach LM, Joosten EA, Tjan-Heijnen VC, Janssen DJ. 2016; Update on prevalence of pain in patients with cancer: systematic review and meta-analysis. J Pain Symptom Manage. 51:1070–90.e9. DOI: 10.1016/j.jpainsymman.2015.12.340. PMID: 27112310.

2. de Oliveira R, dos Reis MP, Prado WA. 2004; The effects of early or late neurolytic sympathetic plexus block on the management of abdominal or pelvic cancer pain. Pain. 110:400–8. DOI: 10.1016/j.pain.2004.04.023. PMID: 15275792.

3. Si-Jie H, Wei-Jia X, Yang D, Lie Y, Feng Y, Yong-Jian J, et al. 2014; How to improve the efficacy of endoscopic ultrasound-guided celiac plexus neurolysis in pain management in patients with pancreatic cancer: analysis in a single center. Surg Laparosc Endosc Percutan Tech. 24:31–5. DOI: 10.1097/SLE.0000000000000032. PMID: 24487155. PMCID: PMC4196780.
4. Sachdev AH, Gress FG. 2018; Celiac plexus block and neurolysis: a review. Gastrointest Endosc Clin N Am. 28:579–86. DOI: 10.1016/j.giec.2018.06.004. PMID: 30241645.
5. Ischia S, Ischia A, Polati E, Finco G. 1992; Three posterior percutaneous celiac plexus block techniques. A prospective, randomized study in 61 patients with pancreatic cancer pain. Anesthesiology. 76:534–40. DOI: 10.1097/00000542-199204000-00008. PMID: 1550278.

6. Edelstein MR, Gabriel RT, Elbich JD, Wolfe LG, Sydnor MK. 2017; Pain outcomes in patients undergoing CT-guided celiac plexus neurolysis for intractable abdominal visceral pain. Am J Hosp Palliat Care. 34:111–4. DOI: 10.1177/1049909115604670. PMID: 26345319.

7. Rykowski JJ, Hilgier M. 2000; Efficacy of neurolytic celiac plexus block in varying locations of pancreatic cancer: influence on pain relief. Anesthesiology. 92:347–54. DOI: 10.1097/00000542-200002000-00014. PMID: 10691219.
8. Wong GY, Schroeder DR, Carns PE, Wilson JL, Martin DP, Kinney MO, et al. 2004; Effect of neurolytic celiac plexus block on pain relief, quality of life, and survival in patients with unresectable pancreatic cancer: a randomized controlled trial. JAMA. 291:1092–9. DOI: 10.1001/jama.291.9.1092. PMID: 14996778.

9. Eisenberg E, Carr DB, Chalmers TC. 1995; Neurolytic celiac plexus block for treatment of cancer pain: a meta-analysis. Anesth Analg. 80:290–5. DOI: 10.1213/00000539-199502000-00015. PMID: 7818115.
10. Koyyalagunta D, Engle MP, Yu J, Feng L, Novy DM. 2016; The effectiveness of alcohol versus phenol based splanchnic nerve neurolysis for the treatment of intra-abdominal cancer pain. Pain Physician. 19:281–92. DOI: 10.36076/ppj/2019.19.281. PMID: 27228515.
11. Mercadante S, Catala E, Arcuri E, Casuccio A. 2003; Celiac plexus block for pancreatic cancer pain: factors influencing pain, symptoms and quality of life. J Pain Symptom Manage. 26:1140–7. DOI: 10.1016/j.jpainsymman.2003.04.004. PMID: 14654266.

12. Yoon DM, Yoon KB, Baek IC, Ko SH, Kim SH. 2018; Predictors of analgesic efficacy of neurolytic celiac plexus block in patients with unresectable pancreatic cancer: the importance of timing. Support Care Cancer. 26:2023–30. DOI: 10.1007/s00520-018-4043-2. PMID: 29344736.

13. De Cicco M, Matovic M, Bortolussi R, Coran F, Fantin D, Fabiani F, et al. 2001; Celiac plexus block: injectate spread and pain relief in patients with regional anatomic distortions. Anesthesiology. 94:561–5. DOI: 10.1097/00000542-200104000-00006. PMID: 11379673.
14. Wyse JM, Carone M, Paquin SC, Usatii M, Sahai AV. 2011; Randomized, double-blind, controlled trial of early endoscopic ultrasound-guided celiac plexus neurolysis to prevent pain progression in patients with newly diagnosed, painful, inoperable pancreatic cancer. J Clin Oncol. 29:3541–6. DOI: 10.1200/JCO.2010.32.2750. PMID: 21844506.
15. Luz LP, Al-Haddad MA, DeWitt JA. 2014; EUS-guided celiac plexus interventions in pancreatic cancer pain: an update and controversies for the endosonographer. Endosc Ultrasound. 3:213–20. DOI: 10.4103/2303-9027.144515. PMID: 25485268. PMCID: PMC4247528.

16. Lu F, Dong J, Tang Y, Huang H, Liu H, Song L, et al. 2018; Bilateral vs. unilateral endoscopic ultrasound-guided celiac plexus neurolysis for abdominal pain management in patients with pancreatic malignancy: a systematic review and meta-analysis. Support Care Cancer. 26:353–9. DOI: 10.1007/s00520-017-3888-0. PMID: 28956176.

17. Yoon WJ, Oh Y, Yoo C, Jang S, Cho SS, Suh JH, et al. 2020; EUS-guided versus percutaneous celiac neurolysis for the management of intractable pain due to unresectable pancreatic cancer: a randomized clinical trial. J Clin Med. 9:1666. DOI: 10.3390/jcm9061666. PMID: 32492883. PMCID: PMC7356927.

18. Kong YG, Shin JW, Leem JG, Suh JH. 2013; Computed tomography (CT) simulated fluoroscopy-guided transdiscal approach in transcrural celiac plexus block. Korean J Pain. 26:396–400. DOI: 10.3344/kjp.2013.26.4.396. PMID: 24156008. PMCID: PMC3800714.

19. Vissers KC, Besse K, Wagemans M, Zuurmond W, Giezeman MJ, Lataster A, et al. 2011; 23. Pain in patients with cancer. Pain Pract. 11:453–75. DOI: 10.1111/j.1533-2500.2011.00473.x. PMID: 21679293.
20. Kambadakone A, Thabet A, Gervais DA, Mueller PR, Arellano RS. 2011; CT-guided celiac plexus neurolysis: a review of anatomy, indications, technique, and tips for successful treatment. Radiographics. 31:1599–621. DOI: 10.1148/rg.316115526. PMID: 21997984.

21. Kahan M, Mailis-Gagnon A, Wilson L, ivastava A Sr. 2011; Canadian guideline for safe and effective use of opioids for chronic noncancer pain: clinical summary for family physicians. Part 1: general population. Can Fam Physician. 57:1257–66. PMID: 22084455. PMCID: PMC3215602.
22. Dworkin RH, Turk DC, Wyrwich KW, Beaton D, Cleeland CS, Farrar JT, et al. 2008; Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain. 9:105–21. DOI: 10.1016/j.jpain.2007.09.005. PMID: 18055266.
23. Hameed M, Hameed H, Erdek M. 2010; Pain management in pancreatic cancer. Cancers (Basel). 3:43–60. DOI: 10.3390/cancers3010043. PMID: 24212605. PMCID: PMC3756348.

24. Nagels W, Pease N, Bekkering G, Cools F, Dobbels P. 2013; Celiac plexus neurolysis for abdominal cancer pain: a systematic review. Pain Med. 14:1140–63. DOI: 10.1111/pme.12176. PMID: 23802777.

25. Polati E, Finco G, Gottin L, Bassi C, Pederzoli P, Ischia S. 1998; Prospective randomized double-blind trial of neurolytic coeliac plexus block in patients with pancreatic cancer. Br J Surg. 85:199–201. DOI: 10.1046/j.1365-2168.1998.00563.x. PMID: 9501815.

26. Kawamata M, Ishitani K, Ishikawa K, Sasaki H, Ota K, Omote K, et al. 1996; Comparison between celiac plexus block and morphine treatment on quality of life in patients with pancreatic cancer pain. Pain. 64:597–602. DOI: 10.1016/0304-3959(95)00189-1. PMID: 8783327.

27. Amr YM, Makharita MY. 2013; Comparative study between 2 protocols for management of severe pain in patients with unresectable pancreatic cancer: one-year follow-up. Clin J Pain. 29:807–13. DOI: 10.1097/AJP.0b013e3182757673. PMID: 23917696.
28. Erdek MA, Halpert DE, González Fernández M, Cohen SP. 2010; Assessment of celiac plexus block and neurolysis outcomes and technique in the management of refractory visceral cancer pain. Pain Med. 11:92–100. DOI: 10.1111/j.1526-4637.2009.00756.x. PMID: 20002595.

29. Bahn BM, Erdek MA. 2013; Celiac plexus block and neurolysis for pancreatic cancer. Curr Pain Headache Rep. 17:310. DOI: 10.1007/s11916-012-0310-y. PMID: 23299904.

30. Titton RL, Lucey BC, Gervais DA, Boland GW, Mueller PR. 2002; Celiac plexus block: a palliative tool underused by radiologists. AJR Am J Roentgenol. 179:633–6. DOI: 10.2214/ajr.179.3.1790633. PMID: 12185033.

31. Mercadante S, Nicosia F. 1998; Celiac plexus block: a reappraisal. Reg Anesth Pain Med. 23:37–48. DOI: 10.1097/00115550-199823010-00009. PMID: 9552777.

32. Kim SH, Hwang JH. 2015; Preoperative glycosylated haemoglobin as a predictor of postoperative analgesic requirements in diabetic patients: a prospective observational study. Eur J Anaesthesiol. 32:705–11. DOI: 10.1097/EJA.0000000000000282. PMID: 26086279.
33. Choi SS, Lee JH, Kim D, Kim HK, Lee S, Song KJ, et al. 2016; Effectiveness and factors associated with epidural decompression and adhesiolysis using a balloon-inflatable catheter in chronic lumbar spinal stenosis: 1-year follow-up. Pain Med. 17:476–87. DOI: 10.1093/pm/pnv018. PMID: 26814254.

34. Borsook D, Kussman BD, George E, Becerra LR, Burke DW. 2013; Surgically induced neuropathic pain: understanding the perioperative process. Ann Surg. 257:403–12. DOI: 10.1097/SLA.0b013e3182701a7b. PMID: 23059501. PMCID: PMC3546123.
35. Sweetser S. 2019; Abdominal wall pain: a common clinical problem. Mayo Clin Proc. 94:347–55. DOI: 10.1016/j.mayocp.2018.04.031. PMID: 30711130.

36. Akhan O, Ozmen MN, Basgun N, Akinci D, Oguz O, Koroglu M, et al. 2004; Long-term results of celiac Ganglia block: correlation of grade of tumoral invasion and pain relief. AJR Am J Roentgenol. 182:891–6. DOI: 10.2214/ajr.182.4.1820891. PMID: 15039160.
37. Iwata K, Yasuda I, Enya M, Mukai T, Nakashima M, Doi S, et al. 2011; Predictive factors for pain relief after endoscopic ultrasound-guided celiac plexus neurolysis. Dig Endosc. 23:140–5. DOI: 10.1111/j.1443-1661.2010.01046.x. PMID: 21429019.

38. Levy MJ, Topazian M, Keeney G, Clain JE, Gleeson F, Rajan E, et al. 2006; Preoperative diagnosis of extrapancreatic neural invasion in pancreatic cancer. Clin Gastroenterol Hepatol. 4:1479–82. DOI: 10.1016/j.cgh.2006.08.012. PMID: 17101297.

Go to : 




 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download