INTRODUCTION
Chemotherapy is one of the main treatments for cancer, but it also affects normal cells along with the cancer cells, causing various side effects. Disorders of the nervous system are the second most common side-effect [
1]. Chemotherapy-induced peripheral neuropathy (CIPN) is a disorder associated with exercise, sensation, and autonomic nerves, caused by damage to the peripheral nerve after anti-cancer drug treatment, which eventually leads to various symptoms such as muscle weakness, decreased sensation, and pain, disrupting daily life [
2-
4]. The pain associated with CIPN is distinguished from cancer pain caused by direct invasion of nerve tissues and pressure on the nerves or passage through the bone. It occurs in approximately 40% of patients undergoing complex chemotherapy. Anti-cancer drugs associated with CIPN include platinum, vinca alkaloids, bortezomib, and taxanes [
5].
Pharmacological and non-pharmacological therapies have been used to treat patients with CIPN. Pharmacological therapy consists of calcium and magnesium, vitamin E, glutathione, amifostin, gabapentin, carbamazepine, amythriptyline, and narcotic painkillers. Non-pharmacological therapies include transcutaneous nerve stimulation (TENS), relaxation therapy, exercise, physical therapy, and massage; however, the effect of non-pharmacological therapies in cancer patients has yet to be investigated comprehensively because of its limited use [
6,
7].
The direct cause of peripheral neuropathy is attributed to peripheral nerve injury and micro-sized blood vessels triggered by the use of neurotoxic anti-cancer drugs. The pathophysiological mechanism involves a direct damage to the peripheral nerve, induced by exposure to toxic anti-cancer drugs. It is also mediated by nerve damage induced by damage to the associated peripheral micro-sized blood vessels and decreased microvascular reactivity, due to defective endothelial cells [
8]. The symptoms include pain and edema. Thermal therapy is used to alleviate pain and edema, and entails stimulation of blood flow with hot temperature for symptom relief [
9,
10].
Microvascular reactivity is elicited by sustained and consistent blood volume under stress in specific regions of the body. Cold temperature induces vasoconstriction of skin and subcutaneous regions, whereas hot conditions promote vasodilation. Under these external thermal conditions, blood distribution in the body is coordinated secondarily with the maintenance of individual body function. Recovery from endothelial damage and impaired vascular reactivity is attributed to repeated and consecutive vasoconstriction and vasodilation in the micro-vessels, because vessels may undergo stress preconditioning due to change of blood flow mediated via vasoconstrictive and vasodilatory effects [
11-
13]. Physical exercise is known to prevent the progression of vascular disease [
14]. We expected that vasoconstriction and vasodilation via bidirectional thermal stimulation could maintain or recover vascular reactivity by contraction and relaxation of peripheral blood vessels in the peripheral tissues.
Red blood cell (RBC) scintigraphy is used for the quantitative assessment of blood distribution by labeling RBCs with radioactive isotopes. It is used to clinically perform assessments of vascular reactivity and diagnose Raynaud syndrome, which is caused by desecrated vascular reactivity. Both qualitative and quantitative assessments are possible in proceeding scintigraphical analysis [
15-
19].
In this study, we acted two new theories. One was that stress RBC scintigraphy was performed and the data was quantitatively analyzed and evaluated under the assumption that one of the mechanisms behind the symptoms of CIPN patients could be similar to Raynaud’s syndrome. The other was proposing thermal therapy from the perspective of peripheral vascular or tissue exercise as a way to improve the symptoms of CIPN patients, and the introduction of bidirectional thermal therapy rather than cold or hot unidirectional thermal therapy. In addition, the research conducted a subjective assessment of the degree and characteristics of CIPN symptoms through questionnaire testing. We assumed that CIPN symptoms would be related to vascular reactivity and confirmed this correlation through comparative evaluation of questionnaire tests and RBC scintigraphy results.
To explain in detail, the patients’ hands were exposed to cycles of cooling and warming stimuli using a cyclic therapeutic device during the RBC scintigraphy. Cyclic stimuli induce vasodilation and vasoconstriction consecutively. We hypothesized that these cyclic stimuli enhance the microcirculation in the peripheral tissue. We defined cyclic cooling and warming therapy by setting the highest and the lowest temperatures, and employing exposure to repetitive stimuli to raise and lower the target temperatures. Fingertips carry a higher number of capillaries than other areas of the fingers, and experience greater variations in temperature. Most of the actual symptoms of CIPN patients, such as tingling sensations and pain, are felt at the fingertips. Therefore, the vascular reactivity to thermal stimuli can be measured via analysis of the fingertips, using RBC scintigraphy.
This study was conducted to evaluate the usefulness of cyclic thermal therapy and RBC scintigraphy in patients with CIPN in investigator-initiated clinical trials (IIT). In this study, we measured the symptom improvement following therapy and observed the changes in the blood volume using RBC scintigraphy of the fingertips.
Go to :

MATERIALS AND METHODS
1. Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee, and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
2. Informed consent
Informed consent approved by the institution was obtained for this study. The informed consent number is CBNUHLNMD_IRB_2017P001 (ver.2.4).
3. Study design
This multi-center study was conducted to investigate the benefit of cyclic thermal therapy in patients treated with CIPN from October 2017 to June 2019 at the Departments of Hemato-oncology and Nuclear Medicine of two university hospitals. The research protocol was approved by the Korean Ministry of Food and Drug Safety (No.788), Jeonbuk National University Hospital Institutional Review Board (IRB) and Keimyeong University Dongsan Hospital IRB (approval no. CUH201708022, and DSMC201811005, respectively). The study was performed in accordance with relevant guidelines/regulations, and informed consent was obtained from all participants and/or their legal guardians. This study was designed as a non-randomized, single intervention.
The subjects were screened when they visited the hospital a total of 12 times. During visit 1 (day 0), the European Organization for Research and Treatment of Cancer–Quality of Life Questionnaire–Chemotherapy-Induced Peripheral Neuropathy (EORTC-QLQ-CIPN 20) questionnaire and
99mTc-labeled RBC scintigraphy were used to obtain patient data before treatment. During visits 2 to 11 (day 1 to 10), subjects underwent cyclic therapy. During visit 12 (day 11), the EORTC-QLQ-CIPN 20 and RBC scintigraphy were used again to obtain patient data after treatment (
Table 1).
Table 1
Observations and schedule
|
Contents |
Screening |
Visit 1 |
Treatment
(Visit 2-11) |
Visit 12 |
|
History |
● |
|
|
|
|
Agreement |
● |
|
|
|
|
Vital sign |
● |
● |
|
● |
|
Physical examination |
● |
● |
|
● |
|
EORTC-QLQ-CIPN 20 |
|
● |
|
● |
|
RBC scintigraphy |
|
● |
|
● |
|
Subject number |
● |
|
|
|
|
Abnormal reaction |
|
|
● |
● |
|
Treatment |
|
|
● |
|

4. Participants
This study was an IIT. A total of 15 patients were recruited by the research manager after agreeing to interview the subjects (10 males and 5 females) in the outpatient clinic of each hospital Department of Hemato-oncology, based on specific criteria. The average age of the patients was 62.8 ± 12.1 years. Three of the 15 patients were diagnosed with colorectal cancer, while 12 others were diagnosed with stomach cancer, and the chemotherapy duration (CD) varied between patients. The vital signs of all the patients were stable.
The inclusion criteria were: 1) adults above 19 years of age; 2) patients diagnosed with stomach cancer, lung cancer, colon cancer, multiple myoma or lymphoma and treated with neurotoxic anti-cancer drugs such as oxalipatin, paclitaxel, docetaxel, thalidomide, vortexib, and vincristine; and 3) patients complaining of peripheral neuropathy (National Cancer Institute-Common Terminology Criteria for Adverse Events-class I and higher).
The exclusion criteria were: 1) sensory abnormalities due to central nervous system diseases or conditions such as stroke, cerebral hemorrhage, spinal cord compression syndrome, and spinal stenosis; 2) possible peripheral neuropathy associated with diabetes or alcohol-dependent disease; 3) treatment with neurotoxic drugs other than the anti-cancer drug mentioned in the selection criteria; 4) a diagnosis of peripheral vascular disease; 5) mental illness, which might prevent comprehension of the questionnaires and studies, due to limited or delayed intelligence or expression; 6) voluntary refusal of consent to participate in a clinical trial; 7) possible pregnancy or breastfeeding; 8) suppressed immunity; 9) critical condition; 10) vulnerable environment; and 11) drug addiction or alcoholism.
5. Treatment
In this study, all patients were treated with a device (healing-touch, HT-001; KAI Biotech, Jeonju, Korea) that generated warm and cool stimuli. The device consisted of thermoelectric elements capable of generating hot and cold energy according to the direction of current, a cooling system comprising a fan and heat sink, and a control system that set the target temperature, operating time, and the number of repetitions. The treatment protocol is shown in
Fig. 1. All patients were treated with 10 cycles at each visit. Each cycle lasted a total of 6 minutes and 30 seconds, including 3 minutes and 30 seconds of cooling and 3 minutes of warming. The duration and temperature of stimulation were set according to the safety limits of the patients [
20]. After five cycles of treatment, patients rested for 5 minutes. Treatment was performed for a total of 1 hour and 10 minutes at each visit. The target temperature of cooling was set at 15°C and the target temperature of warming was 41°C. A thermal stimulation ranging between 15° and 41° was repeatedly delivered to the patient’s hands.
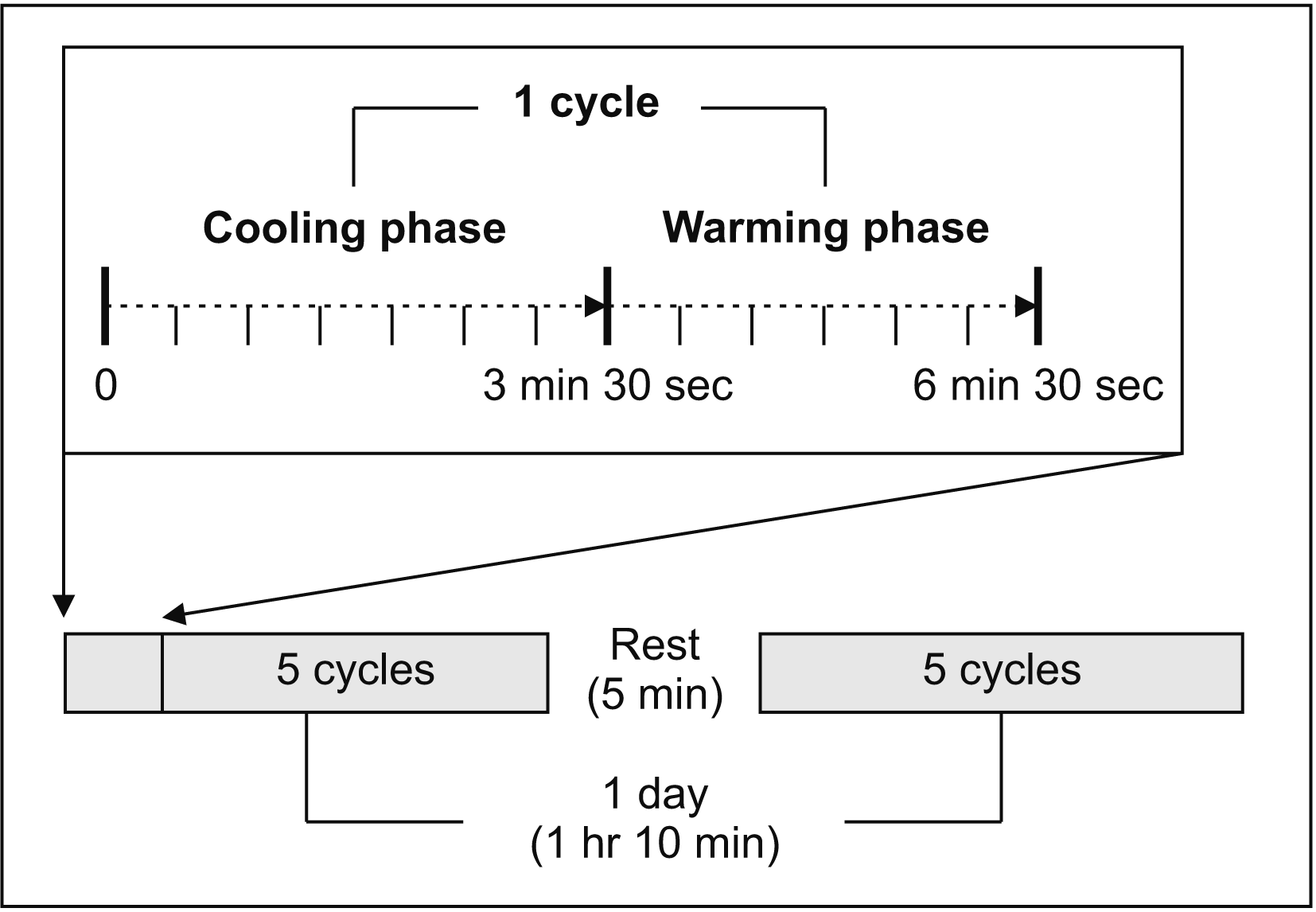 | Fig. 1Scheme of the treatment protocol. One cycle was lasted a total of 6 minutes and 30 seconds, including 3 minutes and 30 seconds of cooling phase and 3 minutes of warming phase. Total of 10 cycles was performed. Patients rested for 5 minutes after five cycles of treat. The treatment was performed for a total of 1 hour and 10 minutes at 1 day. 
|
6. Assessment
The subjects’ blood volume was assessed via RBC scintigraphy before and after treatment in response to the EORTC-QLQ-CIPN 20 questionnaires.
1) EORTC-QLQ-CIPN 20
EORTC-QLQ-CIPN 20 questionnaires are used to measure the quality of life in patients with CIPN [
21,
22]. A total of 20 questions (
Table 2) were included in each questionnaire and all patients responded before and after treatment. The total score, the score related to the upper limb, the scores closely related to the upper limb, and the scores unrelated to the upper limbs were calculated and used as a result.
Table 2
EORTC-QLQ-CIPN 20 questionnaires
|
1 |
Tingling fingers of hands? |
|
2 |
Tingling toes or feet? |
|
3 |
Numbness in fingers of hands? |
|
4 |
Numbness in toes of feet? |
|
5 |
Aching or burning pain in fingers or hands? |
|
6 |
Aching or burning pain in toes or feet? |
|
7 |
Cramps in hands? |
|
8 |
Cramps in feet? |
|
9 |
Trouble standing or walking? |
|
10 |
Trouble distinguishing hot and cold water? |
|
11 |
Trouble holding a pen making writing difficult? |
|
12 |
Trouble handling small objects (e.g., buttoning a blouse)? |
|
13 |
Trouble opening jar/bottle due to loss of strength in hands? |
|
14 |
Trouble walking because your feet come down to hard? |
|
15 |
Trouble walking stairs of standing up from a chair due to weakness in legs? |
|
16 |
Dizziness after standing up? |
|
17 |
Blurry vision? |
|
18 |
Trouble hearing? |
|
19 |
Only for those driving cars: Trouble driving due to use of pedals? |
|
20 |
Only for males: Trouble getting or maintaining an erection? |

2) RBC scintigraphy
RBC scintigraphy, a test procedure used in nuclear medicine, is designed for quantitative assessment of blood movement and blood volume by labeling RBC in the blood with the radioactive isotope 99mTc. First, the pyrophosphates are injected intravenously, and about 30 minutes later, 5 mL of the patient’s blood is collected in a 10 mL syringe containing citrate-phosphate-dextrose-adenine 0.8 mL and 15 mCi of 99mTcO4 and incubated at room temperature for about 10 minutes. After 5 to 10 minutes following injection of the incubated blood, the images at rest were acquired using a gamma camera for 2 minutes. After the rest image was acquired, the patient placed his or her hands on the device and the stress image was acquired using a gamma camera for 390 seconds. During the stress image acquisition, the device was operated under the same treatment cycle (15°C to 41°C, 390 seconds). The gamma camera was set up to obtain the images at the rate of 1 frame per 3 seconds for a total of 40 resting images and 130 stress images. A set of rest images and three sets of stress images were obtained before and after treatment.
7. Quantitative data analysis
RBC scintigraphy was used for quantitative data analysis on a dedicated workstation PMOD 3.7 (PMOD Technologies, Zurich, Switzerland). When the gamma camera images were acquired, a standard source was used for each patient. The standard source was prepared with 99mTc pertechnetate, which is the same radioisotope injected into the patients. The radioactivity of injection and the standard source of 99mTc, injection time, and image acquisition times were recorded for decay correction during the quantitative analysis.
The RBC scintigraphic data consisted of 3 sets which were composed of 130 frames. Ten consecutive frames of data were selected as one data point; 13 data points were obtained for each set and a total of 39 data points were obtained. A region of interest (ROI) was drawn on each fingertip, and the ROI count was calculated.
Fig. 2 presents the RBC image acquired and ROIs drawn on the fingertips and on the standard source. The quantitative analysis is represented by the following equation.
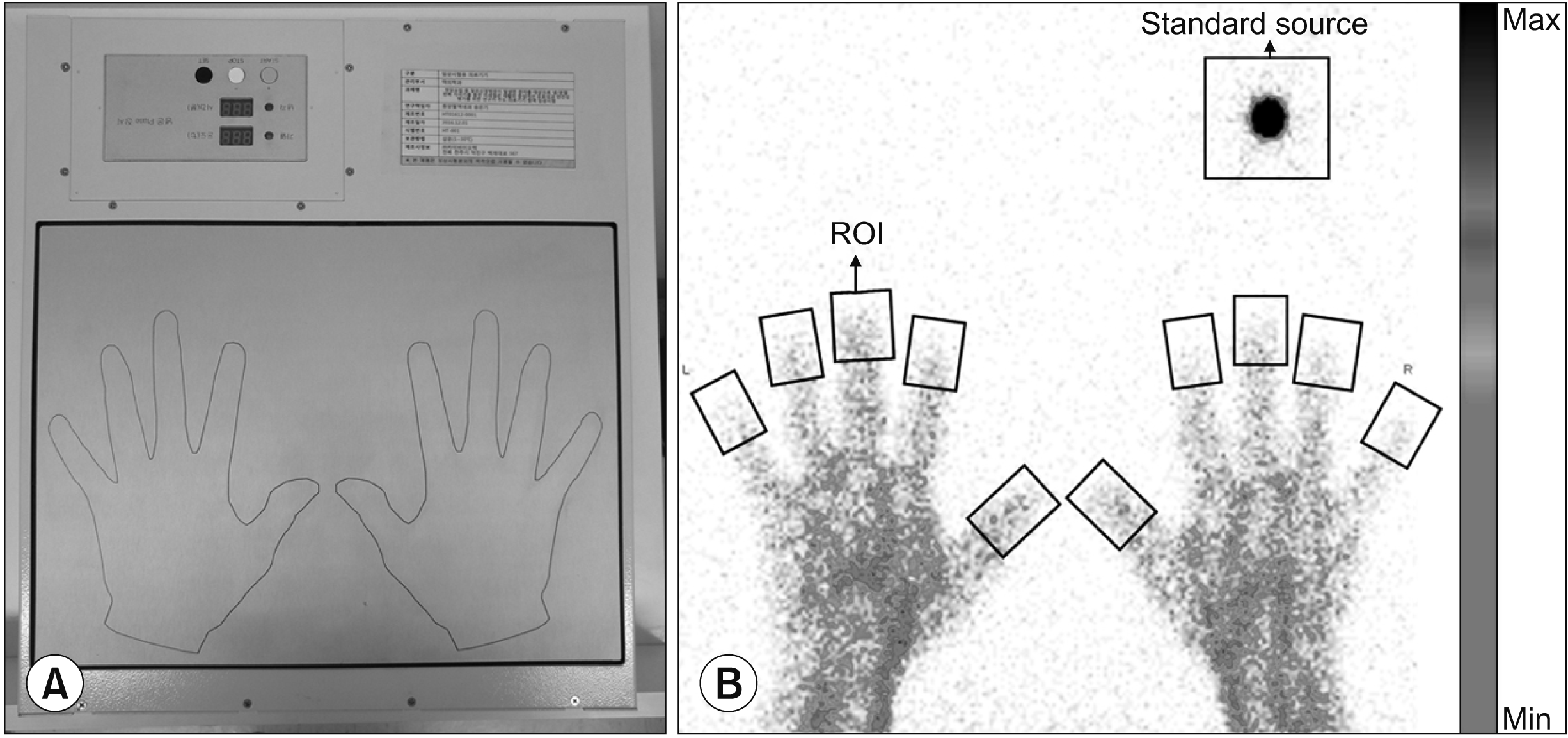 | Fig. 2Representative images of the method of treating the patient and red blood cell scintigraphy analysis. (A) Picture of the device. The patient puts his or her hands on the device. The hand position was printed on the device. (B) The standard source which is made by 99mTc was located in the upper right side. The patients placed both hands on the cyclic therapy device. The ROIs were drawn on each distal phalanx. ROI: region of interest, Min: minimum, Max: maximum. 
|
A simple comparison of 99mTc uptake on the fingertip may not be accurate because the count or radioactivity of 99mTc in each patient’s fingertips differs according to the total injected dose. The ratio of fingertip radioactivity of the total 99mTc injected dose was calculated and used as a result.
Because the blood volume at the fingertips in each hand represents a tiny fraction of the whole blood in a human, all comparisons were performed in the fingertips, and the blood volume results were expressed as common constants, 1 × e-3 in T and 1 × e-5 in H.
8. Statistical analysis
The collected data were statistically analyzed using the IBM SPSS 25.0 program (IBM Co., Armonk, NY). The subjects’ scores in response to the EORTC questionnaire, and blood volume measurements were calculated and expressed as mean ± standard deviation. The collected data were subjected to statistical analysis via a paired t-test. The relationship between the duration of chemotherapy and the treatment effects was subjected to correlation analysis (Spearman’s rho). It was considered statistically significant when the P value was below 0.05.
Go to :

DISCUSSION
Currently, CIPN patients are treated via pharmacological and non-pharmacological approaches; however, it is not based on any theoretical background, because of the paucity of studies involving cancer patients [
6,
7]. This clinical study evaluated the usefulness of cyclic thermal therapy for patients with CIPN. The advantages of cyclic thermal therapy may be mediated via induction of vasoconstriction and vasodilation, decreased edema, and altered blood flow [
23]. We investigated the relationship between symptom relief and blood volume at the fingertips calculated quantitatively using RBC scintigraphy. RBC scintigraphy is appropriate for the measurement of blood flow and blood flow changes occurring in peripheral tissue compared to other diagnostic methods.
In this study, 15 CIPN patients were exposed to cyclic thermal therapy for a total of 10 times at each visit to ameliorate CIPN symptoms via possible improvement in blood circulation. To monitor the changes in CIPN symptoms, the EORTC-QLQ-CIPN 20 was administered before and after treatment, and RBC scintigraphy was performed before and after treatment to determine the changes in blood volume. Although the number of participants was 15, there were 6 independent data sets from the cooling and warming phase in pre- and post-treatment for each patient, and each data set was composed of numerous resultant corresponding values for each of the acquisition time points. It was judged that sufficient comparison would be possible with the data set.
In general, the items in the EORTC-QLQ-CIPN 20, which is used to diagnose CIPN, were divided into three types: sensory symptoms and defects, motor, and autonomic scales [
24]. In this study, the items listed in the questionnaires were used to calculate the total scores, the scores related to the upper limbs, the scores closely related to the upper limbs, and the scores excluding the upper limbs, because the patients’ upper limbs were treated directly.
The total questionnaire score was reduced from 41.6 ± 12.8 before treatment to 32.7 ± 10.7 points after treatment (P = 0.002). The scores related to upper limbs (question numbers 1, 3, 5, 7, 10, 11, 12, and 13) were reduced from 17.7 ± 6.4 before treatment to 13.7 ± 4.4 after treatment (P = 0.002). The scores closely related to the upper limbs (question numbers 1, 3, 5, and 7) were reduced form 8.6 ± 3.2 before treatment to 6.7 ± 2.2 after treatment (P = 0.006). The results also showed that the scores excluding upper limbs were reduced from 23.9 ± 7.0 to 18.9 ± 6.8 (P = 0.002). The results suggest a possible attenuation of CIPN symptoms involving the upper limbs by improving the blood circulation in both hands during the cyclic thermal therapy. In addition, interestingly, the scores excluding the upper limbs were also reduced after treatment. The results, in conjunction with the results from the upper extremities, suggest that circulation was improved in the vessels of the peripheral tissues following cyclic therapy, and this effect was widespread throughout the body. Finally, in patients with CIPN, the whole-body symptoms were mitigated.
In this study, a contact thermal stimulation device was used to provide cyclical thermal therapy. Several types of drugs, including pain killers, are being used to treat symptoms such as pain and edema in CIPN patients. However, because drug treatment alone does not have much effect on patients’ symptoms, it is accompanied by thermal physical therapy which is uses cold or hot packs. An alternative hydrotherapy, infrequently used in clinics, is a method of dipping the body in tanks containing cold and hot water. But in this study, we applied a cyclic thermal therapy that periodically and repeatedly provided hot and cold temperatures during a relatively short period.
As a preclinical study, we had already confirmed that this alternative cyclic thermal therapy has the effect of inflammatory inhibition, swelling reduction, and vascular permeability reduction in a trimellitic anhydride-induced acute contact hypersensitivity mouse model [
25]. These results are thought to be one of the mechanisms for interpreting clinical results from CIPN patients, and one of the appropriate ways to prove them is RBC scintigraphy which can be evaluated by quantification of blood flow volumes.
RBC scintigraphy was used to assess the blood volume at rest and the changes in blood volume, reflected by stress in any parts of body, including both hands, and quantitative analysis in the ROI, here in the fingertips. RBC scintigraphy can provide objective, repetitive, and quantitative information compared to other modalities in the peripheral tissues. The fingertip area was selected for analysis due to the abundant microvascular and nerve distribution in this area. Most patients’ symptoms involve the microvascular and nervous systems, whereas large vessels act as mere conduits for blood.
The results of RBC scintigraphy suggested that the blood volume was reduced from 29.4 ± 9.23 T before to 25.8 ± 7.89 T after treatment (P < 0.001). Based on stress type, the blood volume in the cooling phase was decreased from 29.6 ± 9.38 T before treatment to 26.0 ± 8.05 T after treatment (P < 0.001). Also, it was decreased from 28.7 ± 9.17 T before treatment to 25.2 ± 7.50 T after treatment during the warming phase (P < 0.001).
The blood volume and blood volume variance in patients was decreased following cyclic thermal therapy due to effective circulation and the reduction of interstitial edema via gradual and consecutive vasoconstriction and vasodilation of peripheral blood vessels. Most CIPN patients, even with varying severity, have swollen hands, weak hands, and numbness. Vascular damage occurs due to chemotherapy, and the resulting increase in capillary permeability triggers edema [
26], and peripheral blood stasis, which may interfere with effective circulation. The decrease in blood volume after cyclic thermal therapy is attributed to a reduction in edema and blood stasis following restoration of the vascular reactivity of damaged blood vessels. The circulation may have increased via a reduction of blood stasis, which resulted in the restoration of micro-circulation in the whole body as well as both upper extremities, thereby mitigating the symptoms of CIPN.
Of all the variations in blood volume during the whole study period, the blood volume in the cooling and warming phases was significantly decreased after treatment compared with pre-treatment values, as shown in
Table 4. The results show that vascular constriction and relaxation, upon exposure to the stimuli, were stabilized, thereby maintaining the reactivity of the vessels. Vascular reactivity can be said to be a stable response of blood vessels to causes of change, and maintaining vascular reactivity means that the organs or peripheral tissues of our body are stable against external changes. Therefore, it can be interpreted that blood flow is recovering from vascular reactivity that does not show much difference in each of the periods after treatment.
The relationship between CD and symptom improvement is presented in
Table 5. The CD was not related to changes in average blood volume and variance but was correlated with changes in the total scores of the questionnaire. These results demonstrate an uplifting effect on patients under prolonged chemotherapy and may be related to psychological support associated with cyclic thermal therapy as well as objective changes in circulation. The results also suggest that chemotherapy induces peripheral microangiopathy in the peripheral tissue as well as peripheral neural injury.
There has been no study qualitatively evaluating the blood volume in the peripheral tissue and fingertips. In this study, we introduced RBC scintigraphy to evaluate blood volume and its change by cyclic thermal stimulation, and to demonstrate vascular instability and validate a therapeutic effect on subjective symptoms and objective changes in vascular reactivity. In our other study using cyclic thermal stimulation RBC scintigraphy for stroke patients, we found that peripheral vasoreactivity and neuropathy were related [
27]. Cyclic thermal stimulation could show the status of peripheral vasoreactivity during about 30 minutes of stimulation. In this study, we applied cyclic thermal therapy repetitively for 10 days to patients with CIPN and observed changes in vasoreactivity and symptoms. We tried to see whether repeated application of this stimulation could have a therapeutic effect. After analyzing the results of the RBC scintigraphy and EORTC-QLQ-CIPN 20 scores, we confirmed that vasoreactivity was strengthened and EORTC-QLQ-CIPN 20 scores were lowered after applying cyclic thermal therapy. When the studies were combined, the CIPN symptoms were alleviated by increasing vasoreactivity using cyclic thermal therapy, which means that cyclic thermal therapy can be used as a treatment for CIPN.
The limitations of this study are that when cyclic thermal therapy was applied to patients with CIPN, it was not compared with the results of applying cold or warm alone, and that the number of participants was set at 15 based on a calculation of sample size in an investigator-initiated trial. Because the pre- and post-treatment RBC scintigraphic quantitative data created 39 data point for each patient, we could make a sufficient comparison. Most patients suffered a lot from chemotherapy before participating in this study, and could not use the existing method because they had had experienced no particular effect from existing treatments (include thermal therapy). The research before and after the application of this treatment will confirm its effectiveness. Second, there is a limit in not being able to work with other methods besides RBC scintigraphy to measure peripheral vasoreactivity. In addition to the results of the survey on neuropathy treatment, except for the questionnaire result, further study is needed on neurological tests. Further research using this treatment in the clinical setting is planned. Third, in this study, no research has been done on how long the therapeutic effects last. Further research on the relationship between treatment duration and treatment effectiveness needs to be continued. Since this study was the first research of its kind to be attempted, it was arbitrarily set up as 10 times for 2 weeks considering the ease of application and the duration of treatment effects for patients.
In future studies, we will apply RBC scintigraphy to conduct a comparative study of each of the therapeutic tools. Also, although it is difficult to conduct studies with patients, it is necessary to investigate additional pathophysiological mechanisms underlying chemotherapy-induced neuropathy and microangiopathy at least in animals. Further studies including the use of different evaluation tools are needed to address these limitations.
In conclusion, patients diagnosed with CIPN were surveyed using questionnaires and RBC scintigraphy to investigate the severity of CIPN-causing symptoms and peripheral blood volume before and after exposure to cyclic thermal therapy. The CIPN symptoms and blood stasis, possibly brought about by chemotherapy-induced edema, were improved, and the vascular reactivity was stabilized. Therefore, we suggest that cyclic thermal therapy is useful in ameliorating CIPN symptoms by improving blood circulation. Additionally, RBC scintigraphy can be used to objectively evaluate blood volume under specific stress conditions as well as at rest in peripheral tissues. It is expected that the application of RBC scintigraphy can be expanded by utilizing blood volume information to visualize and objectively analyze the vascular response to stimuli.
Go to :







 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download