Abstract
Background
Pillar pain may develop after carpal tunnel release surgery (CTRS). This prospective double-blinded randomized trial investigated the effectiveness of extracorporeal shock wave therapy (ESWT) in pillar pain relief and hand function improvement.
Methods
The sample consisted of 60 patients with post-CTRS pillar pain, randomized into two groups. The ESWT group (experimental) received three sessions of ESWT, while the control group received three sessions of sham ESWT, one session per week. Participants were evaluated before treatment, and three weeks, three months, and six months after treatment. The pain was assessed using the visual analogue scale (VAS). Hand functions were assessed using the Michigan hand outcomes questionnaire (MHQ).
Results
The ESWT group showed significant improvement in VAS and MHQ scores after treatment at all time points compared to the control group (P < 0.001). Before treatment, the ESWT and control groups had a VAS score of 6.8 ± 1.3 and 6.7 ± 1.0, respectively. Three weeks after treatment, they had a VAS score of 2.8 ± 1.1 and 6.1 ± 1.0, respectively. Six months after treatment, the VAS score was reduced to 1.9 ± 0.9 and 5.1 ± 1.0, respectively. The ESWT group had a MHQ score of 54.4 ± 7.7 before treatment and 73.3 ± 6.8 six months after. The control group had a MHQ score of 54.2 ± 7.1 before treatment and 57.8 ± 4.4 six months after.
Carpal tunnel syndrome (CTS) is the most prevalent peripheral mononeuropathy, characterized by pain, numbness, and hypoesthesia in the wrist caused by pressure on the median nerve [1]. Paget (1854) was the first to describe CTS in a patient who had suffered a fracture of the distal radius [2]. The general incidence of CTS is 0.1% to 0.5%. It has a bimodal distribution and is more common in women than in men and after 40 and 70 years [3-5]. Idiopathic CTS is the most common form, believed to be caused by endocrinological disorders, rheumatological diseases, tumors, traumas, anatomical variations, infections, and storage diseases. The most prominent symptom of CTS is numbness and pain at night [6]. The patient usually shakes her hand to relieve the symptoms. The symptoms in the early period begin to disturb the patient during the daytime in advanced stages. Symptoms become more severe where early diagnosis and treatment are not available. With hypoesthesia, difficulty in opsonization arises due to atrophy of the thenar eminence.
Tinel’s and Phalen’s signs are used to diagnose CTS. Typical anamnesis, physical examination, and electrophysiological testing make diagnosis much easier. Splinting, nonsteroidal anti-ınflammatory drugs (NSAIDs), vitamin B6, local corticosteroid injection, and physical therapy modalities are the primary CTS treatments [1,6]. The primary goal of conservative treatment is to release the pressure in the tunnel. Surgery is an option for those who do not respond to conservative treatment. Surgery is an etiology-focused intervention that aims to release the tunnel pressure. There are different types of surgery, such as mini-open, open, and endoscopic.
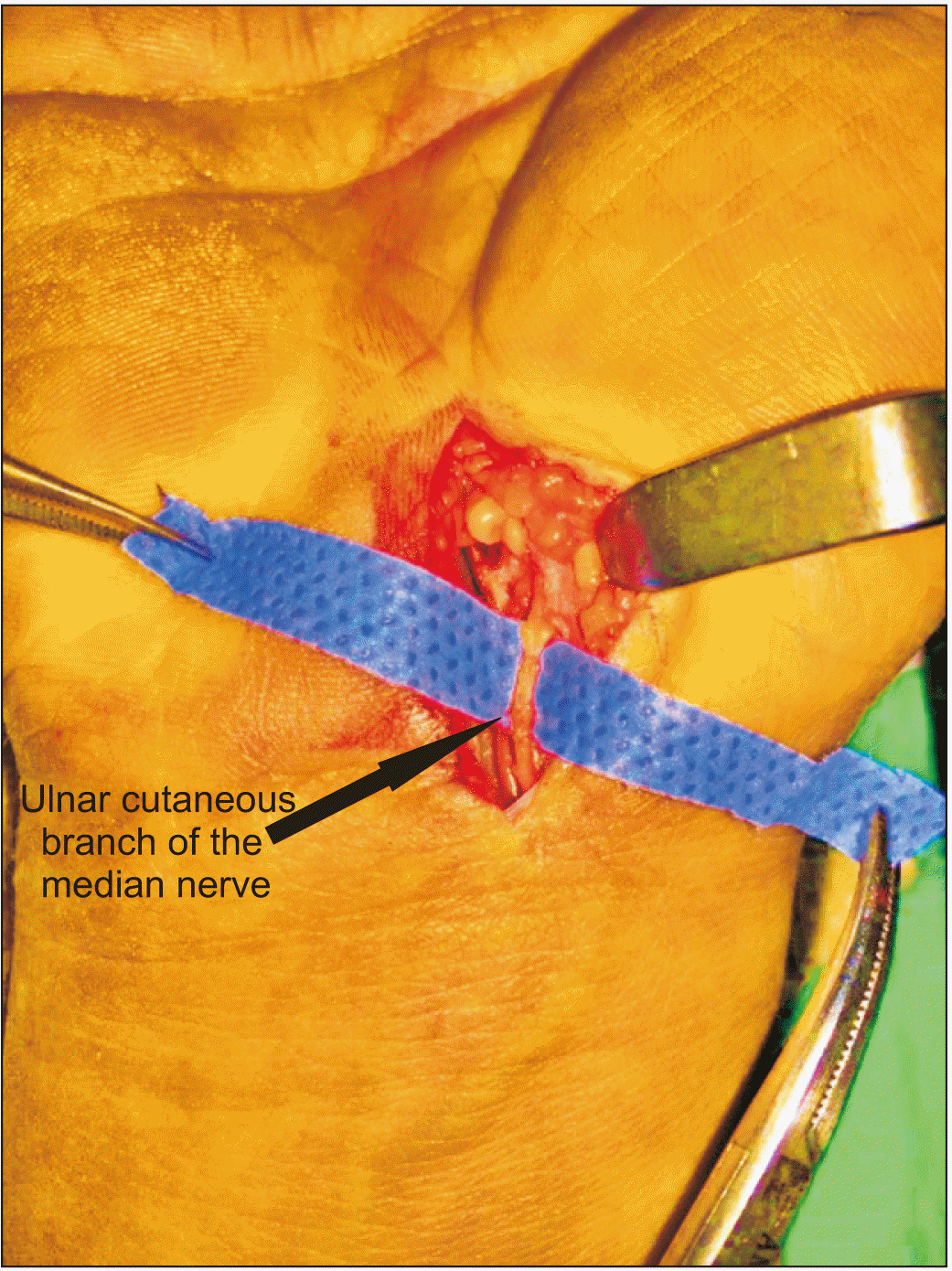
It is mostly the open surgery that causes edema and pain on the scar during the postoperative period. The pain, referred to as pillar pain, may be very severe [7], and its cause is still under investigation. However, there are some hypotheses. Some studies define it as sympathetic dystrophy [8], while others report that it is caused by skin incision, surgical experience and procedure, biomechanical changes in the carpal arch, and problems associated with flexor tendon pulley [7,9,10]. Some researchers, on the other hand, argue that it is caused by the incised ulnar cutaneous branch of the median nerve (an anatomical variant) [11] (Fig. 1).
Magnetic resonance images of patients with postoperative pillar pain show an inflammatory pattern in the thenar region. Edema in the scar tissue causes pain and redness in that region. After all, pillar pain is an inflammatory process, and therefore, its postoperative treatment also involves anti-inflammatory methods, the most common of which are NSAIDs, cold compression, and steroids. Extracorporeal shock wave therapy (ESWT) is a physical therapy modality alternative to conventional treatment. It involves the administration of high-intensity sound waves arising from sudden pressure changes to the body. Those changes result in strong waves that cause compression and tension. There are two ways in which we can explain the therapeutic effect of ESWT: (1) anesthesia of the nerve fibers through biochemical changes and (2) reduced inflammation in the soft tissues. It is believed that the release of angiogenesis-related growth factors of the mechanism of action in the soft tissues after shock wave accelerates the formation of new vessels and increases oxygenation in the environment, resulting in accelerated tissue recovery [12-14].
This prospective double-blinded randomized trial investigated the effectiveness of ESWT in pillar pain relief and hand function improvement.
The sample consisted of 60 patients admitted to an orthopedic outpatient clinic. The inclusion criteria were (1) a visual analogue scale (VAS) score of ≥ 5, (2) pillar pain after CTRS, and (3) hyperemic and edematous scar tissue. All participants underwent open mini-invasive incision and transverse carpal ligament full release surgery performed by an orthopedist.
Pillar pain was diagnosed using three clinical tests: handgrip strength, a pressure test, and a table test. In the pressure test, one of the thumbs was used to apply about two kg of pressure to the thenar and hypothenar regions. In the table test, the patient put her hands on a table’s edges and bore weight on hands [15-17]. The exclusion criteria were (1) sensory or motor neuropathy, (2) systemic inflammatory diseases, (3) a history of surgery other than CTRS or trauma/fracture in the hand and hand-wrist region, (4) local infections at the hand level, and (5) pregnancy. The study was approved by an ethics committee (Erzurum BEAH KAEK number: 2021/02-20, date: 18.01.2021). Informed consent was obtained from patients who agreed to participate.
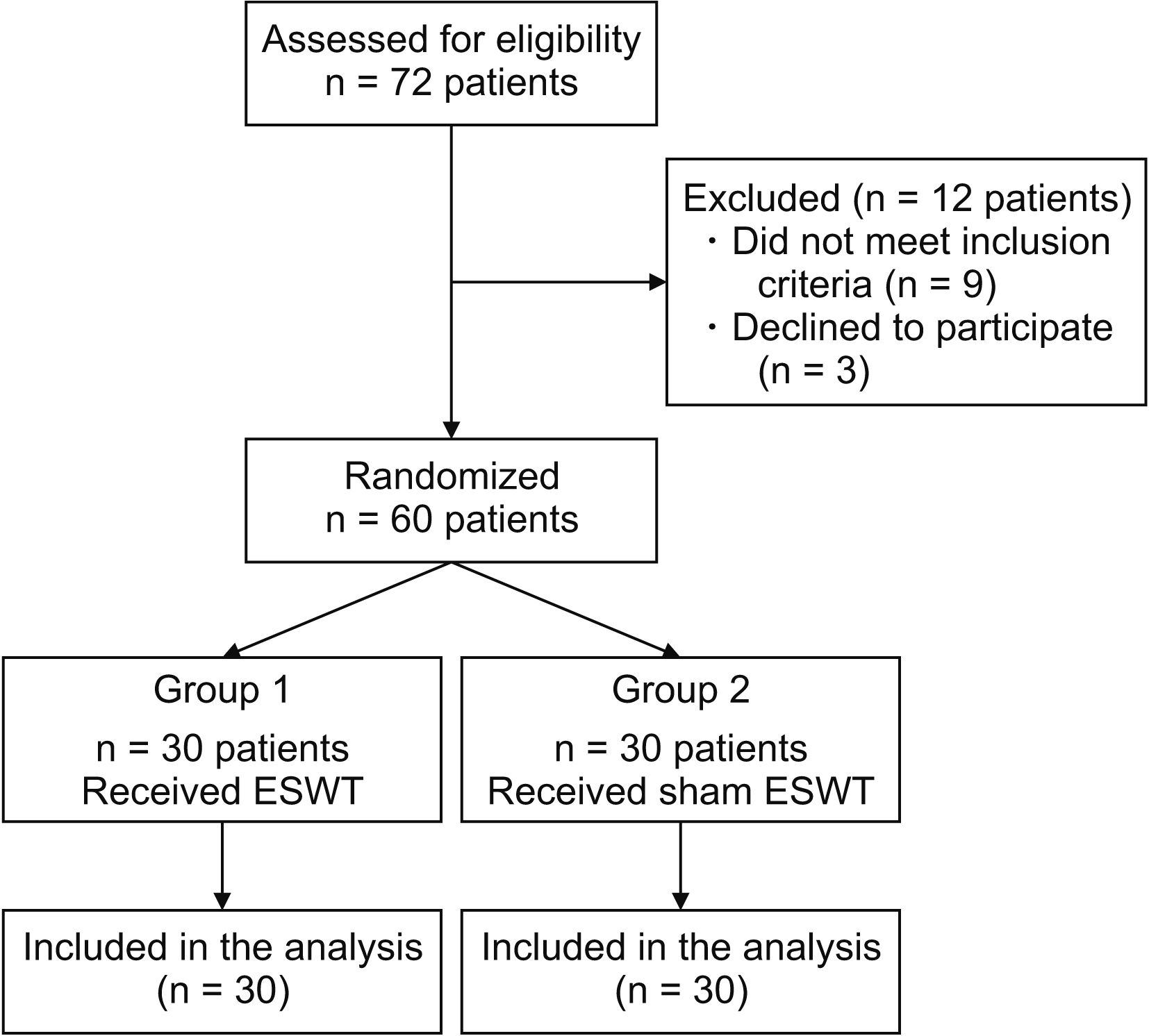
Participants were allocated into two groups (experimental, n = 30; control, n = 30) using random allocation software (Fig. 2). Age, gender, the affected hand, duration of complaints, and comorbid diseases were recorded.
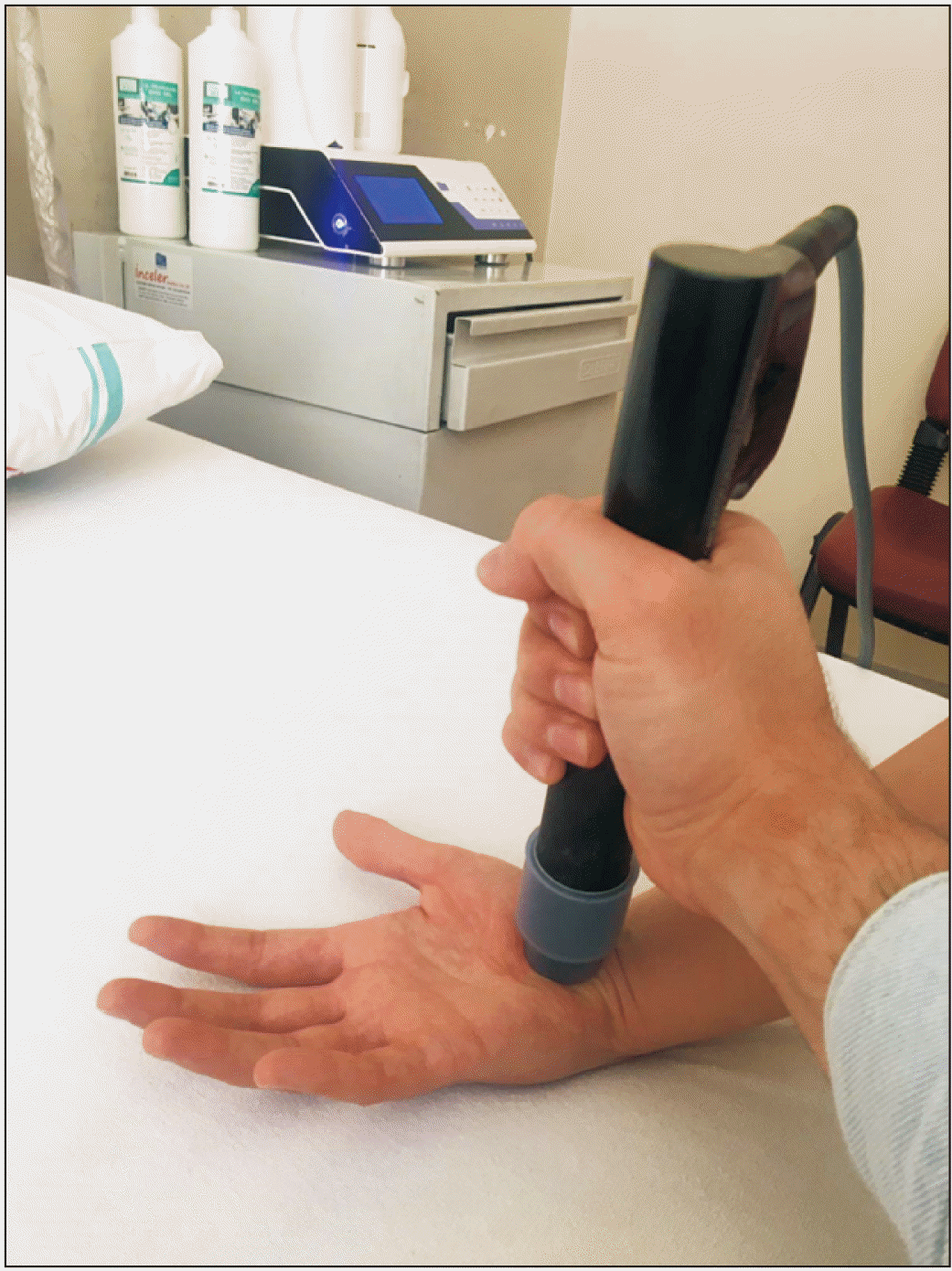
The ESWT group received three sessions of ESWT (Storz Medical AG, Tägerwilen, Switzerland), one session per week (Fig. 3). Each ESWT session involved 2,000 pulses of the focus probe at 4-bar pressure and 5 Hz frequency. The probe was applied perpendicularly to the painful, tender, edematous, and hyperemic region between the thenar and hypothenar area to treat the deep scar tissue. The first session had a very low energy flow density (0.03 mJ/mm2), which was increased in each session depending on the patient’s tolerance. The control group received three sessions of sham ESWT, one session per week. The ESWT device involved sound, as if it was powered on while no energy was applied. All participants were prescribed paracetamol three times a day in case they had pain.
Pre- and post-treatment scores were assessed by an orthopedist blinded to the group assignment. The participants were randomized into the groups by a physical medicine and rehabilitation specialist. ESWT programs were determined and then applied by an experienced physiotherapist.
The pain was assessed using the VAS, while hand functions were assessed using the Michigan hand outcomes questionnaire (MHQ) before treatment, and three weeks, three months, and six months after the treatment. The VAS is a measure of self-reported health status scored on a scale of “0 = no pain” to “10 = unbearable pain.”
The MHQ is a 57-item patient-reported outcome measure in hand surgery. The scale assesses six domains: overall hand function, activities of daily living, pain, work performance, aesthetics, and satisfaction with hand function. Each item is scored on a scale of 1 to 5. A domain score ranges from 0 (worst) to 100 (best), with higher scores indicating better hand function. However, the total score of a “pain domain” is reversed (“0 = best” to “100 = worst”), and therefore, a higher score in that domain indicates greater pain. The scoring method of the scale was determined by its developers [18]. The scale was adapted to Turkish by Öksüz et al. [19].
A power analysis was performed using G*Power (version 3.1.9.4; University of Kiel, Kiel, Germany). The result showed that a sample size of 60 was large enough to detect between-group differences (power of 80%, α = 0.05, effect size = 0.65). The data were analyzed using the IBM SPSS Statistics for Windows, version 25.0 (IBM Co., Armonk, NY) at a significance level of 0.05. Between-group differences were analyzed using the Mann–Whitney U and Student’s t-tests. The Wilcoxon signed-rank test was used to analyze participants’ pre- and post-treatment scores. The qualitative data were analyzed using Pearson’s chi-square, Fisher’s exact, Fisher–Freeman–Halton, and Yates’ continuity correction tests. The descriptive data were analyzed using numbers (n) and percentages (%). A chi-square (χ2) test was used for numerical data. Logistic regression and correlation tests were performed.
The sample consisted of 60 patients (50 women, 10 men). The ESWT group (n = 30) received ESWT, whereas the control group (n = 30) received sham ESWT. Participants had a mean age of 51.8 ± 11.6 years (min: 20, max: 69) and a mean body mass index of 25.9 ± 3.7 (min: 20, max: 40). The time between when participants developed pillar pain and when they were first seen by healthcare professionals for treatment was 9.6 ± 10.0 months (min: 3, max: 60). Thirty-eight patients (63.3%) had pillar pain in the right upper limb, whereas the remainder (36.7%) in the left upper limb. Thirty-eight patients (63.3%) had no comorbidity. The others had diabetes mellitus (n = 11, 18.3%), hypothyroidism (n = 5, 8.3%), rheumatoid arthritis (n = 3, 5.0%), or both diabetes mellitus and hypothyroidism (n = 3, 5.0%) (Table 1).
Before the treatment, the ESWT and control groups had a mean VAS score of 6.8 ± 1.3 (min: 5, max: 10) and 6.7 ± 1.0 (min: 5, max: 9), respectively. The results indicated no significant difference in VAS scores between the groups before the treatment (P = 0.757). Six months after the treatment, the ESWT and control groups had a mean VAS score of 1.9 ± 0.9 (min: 1, max: 5) and 5.1 ± 1.0 (min: 4, max: 8), respectively. The results indicated a significant difference in VAS scores between the groups six months after the treatment (P < 0.001) (Table 2).
Before the treatment, the ESWT and control groups had a mean MHQ score of 54.4 ± 7.7 (min: 36.3, max: 67.0) and 54.2 ± 7.1 (min: 43.3, max: 69.5), respectively. The results indicated no significant difference in MHQ scores between the groups before the treatment (P = 0.934). Six months after the treatment, the groups had a mean MHQ score of 73.3 ± 6.8 (min: 52.7, max: 83.7) and 57.8 ± 4.4 (min: 47.7, max: 65.2), respectively. The results indicated a significant difference in MHQ scores between the groups six months after the treatment (P < 0.001) (Table 3).
CTS is a neuropathy that is usually treated surgically. Pillar pain is one of the complications of CTRS, adversely affecting functional hand use, and thus, activities of daily living. Pillar pain should be treated because, otherwise, it would result in delayed recovery and delayed return to work. It can be treated with restricted hand use, splints, symptomatic superficial hot/cold compression, or massage. However, there is a limited body of research on those treatment techniques [20]. Earlier studies have shown that ESWT reduces the cutaneous nerve fibers and inflammatory cytokines, and increases the levels of nitric oxide and calcitonin gene-related peptide (a substance P-like neuropeptide) which is released from nociceptor type-C nerve fibers and causes inflammation due to vasodilation [21-23]. Our results show that ESWT is an effective method in the treatment of pillar pain.
Open, mini-open, and endoscopic methods are different types of transverse carpal ligament release surgery performed to relieve the pressure on the median nerve to treat CTS. However, there is still an ongoing debate as to which one is the best method. Conventional open tunnel release involves an incision proximally extending to the flexor skin fold of the wrist, resulting in a full and safe release of the nerve. Incisions are more invasive than other surgical techniques, causing cosmetic concerns and wound site problems. However, the primary objective of CTRS is to eliminate complaints, not cosmetic concerns. Patients’ cosmetic concerns lead orthopedic surgeons to mini-open and endoscopic techniques to get the same result with smaller incisions. However, mini-open tunnel release makes surgeons feel unconfident as they try to release the tunnel without seeing it clearly, sometimes resulting in an inadequately released tunnel [24].
In this study, all of the participants were patients who experienced pillar pain after open surgery. Open surgery is a method with proven success. Open surgery rarely results in an inadequate release, but most open surgery patients suffer pillar pain in the postoperative period. Its cause is still under investigation, but there are some hypotheses in the literature. According to some studies, it is caused by skin incision, surgical experience and procedure, biomechanical changes in the carpal arch, and problems associated with flexor tendon pulley. Some researchers argue that cutting the ulnar cutaneous branch of the median nerve (an anatomical variant) is the cause of pillar pain (Fig. 1) [24].
Only two previous studies were conducted to determine the effectiveness of ESWT in the treatment of post-CTRS pillar pain. Romeo et al. [25] administered ESWT to 40 patients with pillar pain, at least six months after CTRS surgery. The treatment consisted of three sessions (2,800 beats) applied once a week. They reported a significant improvement in all patients four months after the treatment. The mean VAS score of the patients was 6.1 at the baseline which was after 0.4 four months. They also reported a significant reduction in redness and swelling in the surgical scar tissue [25]. Similarly, we observed that ESWT treatment significantly reduced VAS scores in patients with pillar pain. The improvement we detected in pain levels twenty days after the treatment had been reported by Romeo et al. [25] forty days after treatment. This result suggests that ESWT is much more quick-acting than previously thought. Additionaly, the present study revealed that pain relief and hand functions continued to improve throughout a six month follow-up period.
Haghighat et al. [26] also randomized 40 pillar pain patients into two groups (ESWT and control) and evaluated them before, and one and three months after treatment. The ESWT group had a mean VAS score of 5.9 before treatment, which came down to 1.8 three months after treatment. The control group had a mean VAS score of 6.1 before sham treatment, which was 3.6 three months after sham treatment. The ESWT group had a mean MHQ score of 37.5 before treatment, which was 75.4 three months after treatment. The control group had a mean MHQ score of 40.7 before sham treatment, which rose to 63.7 three months after sham treatment. While there was a more significant decrease in VAS scores in the ESWT group, the researchers reported no significant difference in the increase in hand functions between the two groups, in contrast to our results [26]. We observed that the ESWT group had a more significant reduction in pain levels, and the improvement in hand functions was greater than the control group. After all, pain affects hand functions adversely, and therefore, we think that ESWT, which has such an effect on pain, should have a significant effect on hand functions as well. Our results also showed that ESWT is a quick-acting and long-lasting treatment option in patients with pillar pain.
This study had five strengths: (1) a power analysis, (2) a control group, (3) a large sample size, (4) a double-blinded randomized design, and (5) follow-ups three weeks, and three and six months after treatment. We think that this study contributes to the literature as it is the first to assess the long-term effectiveness of ESWT on pillar pain, for the treatment of which there is little data available.
CTS, and hence, CTRS are common. The right surgical techniques and postop care management are critical for clinicians. Therefore, future studies should recruit larger samples to better understand the etiology of pillar pain and the effectiveness of ESWT in its management. Pillar pain is a common complication of CTRS. This prospective double-blinded randomized trial investigated the effectiveness of ESWT in pillar pain and hand functions. The results show that ESWT helps reduce pillar pain and improve hand functions. It is a safe and non-invasive method that can be used to treat pillar pain, which is a dreaded postoperative complication for patients and surgeons.
Notes
REFERENCES
1. Slater RR Jr. 1999; Carpal tunnel syndrome: current concepts. J South Orthop Assoc. 8:203–13. PMID: 12132866.
2. Pfeffer GB, Gelberman RH, Boyes JH, Rydevik B. 1988; The history of carpal tunnel syndrome. J Hand Surg Br. 13:28–34. DOI: 10.1016/0266-7681(88)90046-0. PMID: 3283274.

3. Atroshi I, Gummesson C, Johnsson R, Ornstein E, Ranstam J, Rosén I. 1999; Prevalence of carpal tunnel syndrome in a general population. JAMA. 282:153–8. DOI: 10.1001/jama.282.2.153. PMID: 10411196.

4. Tanaka S, Wild DK, Seligman PJ, Behrens V, Cameron L, Putz-Anderson V. 1994; The US prevalence of self-reported carpal tunnel syndrome: 1988 National Health Interview Survey data. Am J Public Health. 84:1846–8. DOI: 10.2105/AJPH.84.11.1846. PMID: 7977933. PMCID: PMC1615207.

5. Stevens JC. 1997; AAEM minimonograph #26: the electrodiagnosis of carpal tunnel syndrome. American Association of Electrodiagnostic Medicine. Muscle Nerve. 20:1477–86. DOI: 10.1002/(SICI)1097-4598(199712)20:12<1477::AID-MUS1>3.0.CO;2-5. PMID: 9390659.

6. Posch J, Marcotte D. 1976; Carpal tunnel syndrome: an analysis of 1,201 cases. Orthop Rev. 5:25–35.
7. Gerritsen AA, Uitdehaag BM, van Geldere D, Scholten RJ, de Vet HC, Bouter LM. 2001; Systematic review of randomized clinical trials of surgical treatment for carpal tunnel syndrome. Br J Surg. 88:1285–95. DOI: 10.1046/j.0007-1323.2001.01858.x. PMID: 11578281.

8. van Beek EJ, Kuijer PM, Büller HR, Brandjes DP, Bossuyt PM, ten Cate JW. 1997; The clinical course of patients with suspected pulmonary embolism. Arch Intern Med. 157:2593–8. DOI: 10.1001/archinte.1997.00440430075009. PMID: 9531228.

9. Tomaino MM, Plakseychuk A. 1998; Identification and preservation of palmar cutaneous nerves during open carpal tunnel release. J Hand Surg Br. 23:607–8. DOI: 10.1016/S0266-7681(98)80011-9. PMID: 9821603.

10. Brooks JJ, Schiller JR, Allen SD, Akelman E. 2003; Biomechanical and anatomical consequences of carpal tunnel release. Clin Biomech (Bristol, Avon). 18:685–93. DOI: 10.1016/S0268-0033(03)00052-4. PMID: 12957554.

11. Akhtar S, Arenas Prat J, Sinha S. 2005; Neuropraxia of the palmar cutaneous branch of the ulnar nerve during carpal tunnel decompression. Ann R Coll Surg Engl. 87:W1–2. DOI: 10.1308/147870805X42635. PMID: 16395819. PMCID: PMC1963910.

12. Speed C. 2014; A systematic review of shockwave therapies in soft tissue conditions: focusing on the evidence. Br J Sports Med. 48:1538–42. DOI: 10.1136/bjsports-2012-091961. PMID: 23918444.

13. Furia JP. 2006; High-energy extracorporeal shock wave therapy as a treatment for insertional Achilles tendinopathy. Am J Sports Med. 34:733–40. DOI: 10.1177/0363546505281810. PMID: 16627628.

14. Conforti G, Capone L, Corra S. 2014; Intradermal therapy (mesotherapy) for the treatment of acute pain in carpal tunnel syndrome: a preliminary study. Korean J Pain. 27:49–53. DOI: 10.3344/kjp.2014.27.1.49. PMID: 24478901. PMCID: PMC3903801.

15. Yung PS, Hung LK, Tong CW, Ho PC. 2005; Carpal tunnel release with a limited palmar incision: clinical results and pillar pain at 18 months follow-up. Hand Surg. 10:29–35. DOI: 10.1142/S0218810405002413. PMID: 16106498.

16. Wilson KM. 1994; Double incision open technique for carpal tunnel release: an alternative to endoscopic release. J Hand Surg Am. 19:907–12. DOI: 10.1016/0363-5023(94)90088-4. PMID: 7876487.

17. Boya H, Özcan Ö, Öztekin HH. 2008; Long-term complications of open carpal tunnel release. Muscle Nerve. 38:1443–6. DOI: 10.1002/mus.21068. PMID: 18949783.

18. Chung KC, Pillsbury MS, Walters MR, Hayward RA. 1998; Reliability and validity testing of the Michigan Hand Outcomes Questionnaire. J Hand Surg Am. 23:575–87. DOI: 10.1016/S0363-5023(98)80042-7. PMID: 9708370.

19. Öksüz Ç, Akel BS, Oskay D, Leblebicioğlu G, Hayran KM. 2011; Cross-cultural adaptation, validation, and reliability process of the Michigan Hand Outcomes Questionnaire in a Turkish population. J Hand Surg Am. 36:486–92. DOI: 10.1016/j.jhsa.2010.11.016. PMID: 21295925.

20. Ludlow KS, Merla JL, Cox JA, Hurst LN. 1997; Pillar pain as a postoperative complication of carpal tunnel release: a review of the literature. J Hand Ther. 10:277–82. DOI: 10.1016/S0894-1130(97)80042-7. PMID: 9399176.
21. Ogden JA, Alvarez RG, Levitt R, Marlow M. 2001; Shock wave therapy (Orthotripsy) in musculoskeletal disorders. Clin Orthop Relat Res. 387:22–40. DOI: 10.1097/00003086-200106000-00005. PMID: 11400888.

22. Mariotto S, Cavalieri E, Amelio E, Ciampa AR, de Prati AC, Marlinghaus E, et al. 2005; Extracorporeal shock waves: from lithotripsy to anti-inflammatory action by NO production. Nitric Oxide. 12:89–96. DOI: 10.1016/j.niox.2004.12.005. PMID: 15740982.

23. Takahashi N, Wada Y, Ohtori S, Saisu T, Moriya H. 2003; Application of shock waves to rat skin decreases calcitonin gene-related peptide immunoreactivity in dorsal root ganglion neurons. Auton Neurosci. 107:81–4. DOI: 10.1016/S1566-0702(03)00134-6. PMID: 12963418.

24. Tetik C, Erol B. 2002; Comparison of the alternative methods used in the surgical treatment of carpal tunnel syndrome. Artroplasti Artroskopik Cerrahi. 13:5–9. Turkish.
25. Romeo P, d'Agostino MC, Lazzerini A, Sansone VC. 2011; Extracorporeal shock wave therapy in pillar pain after carpal tunnel release: a preliminary study. Ultrasound Med Biol. 37:1603–8. DOI: 10.1016/j.ultrasmedbio.2011.07.002. PMID: 21856074.

26. Haghighat S, Zarezadeh A, Khosrawi S, Oreizi A. 2019; Extracorporeal shockwave therapy in pillar pain after carpal tunnel release: a prospective randomized controlled trial. Adv Biomed Res. 8:31. DOI: 10.4103/abr.abr_86_18. PMID: 31214549. PMCID: PMC6521616.

Table 1
Demographic and clinical data of the patients
Table 2
Comparison of VAS scores between groups
Table 3
Comparison of MHQ scores between groups




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download