INTRODUCTION
Pain has a negative effect on the physical and psychosocial dimensions of quality of life [
1,
2]. The history of medicine suggests novel strategies to alleviate pain in numerous diseases. Although pharmacological treatment is pivotal in pain management [
3], non-surgical and minimally-invasive procedures play a significant role, especially in pain practice [
4]. They can be rapidly conducted in an outpatient setting, lasting only a few minutes in a patient who is awake. However, in the context of medical care, it sounds paradoxical that procedural pain involving minimally-invasive procedures is intolerable beside the original pain [
5]. During the procedure, insufficient pain control may result in adverse consequences for patients, such as aborted procedures, avoidance of future therapeutic intervention, poor recovery, and psychological trauma. Skin infiltration with local anesthetics (LA) could mitigate procedure-related pain; however, it is often insufficient for patients undergoing painful procedures, leading to general or unknown anxiety during the intervention. Although intravenous (IV) sedation could be used for some painful procedures, it is not always adequate in an outpatient pain setting due to respiratory depression or hemodynamic instability [
6,
7].
Virtual reality (VR) is a non-invasive simulation that allows users to interact with a computer-generated artificial environment [
8]. Unlike augmented reality, it is a fully digital experience that can either stimulate or alter the real world [
8]. In the medical field, VR has been used to distract patients from pain during uncomfortable medical procedures, such as in patients undergoing burn and wound care [
9-
11]. It has also been a useful option to reduce pain and anxiety during orthopedic surgeries, pediatric vascular access, and dental procedures [
12-
15]. VR immersion also provided better results than IV sedation during endoscopic urologic surgery under spinal anesthesia [
16]. Combined with previous findings, VR immersion may reduce procedure-related pain in patients undergoing minimally-invasive spinal interventions. However, the role of VR immersion in an outpatient pain practice setting is seldom reported.
This study hypothesized that VR might improve procedure-related experiences as an effective adjunct to conventional skin infiltration. To this end, we investigated the role of VR immersion in alleviating procedural pain intensity and mitigation of unpleasant experiences in awake patients with chronic pain undergoing fluoroscopy-guided minimally-invasive pain interventions in a prone position.
Go to :

MATERIALS AND METHODS
1. Study design
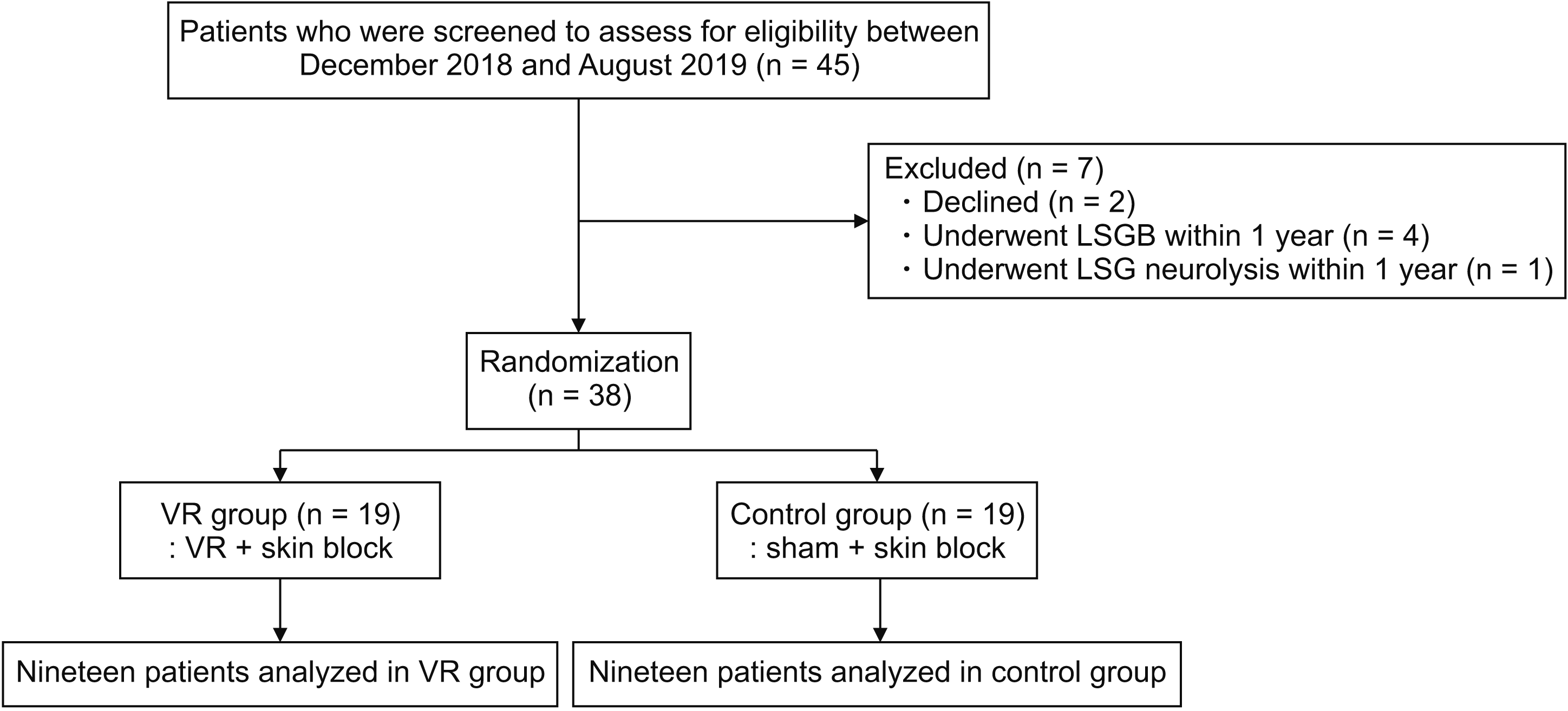
This prospective, exploratory randomized controlled trial was approved by the Institutional Review Board of Seoul National University Hospital (No. 1802-028-920) and registered in ClnicalTrials.gov (NCT03599479 released on 24 June 2018). This study complied with the Declaration of Helsinki and was conducted at a single pain management center between December 2018 and August 2019. Written informed consent was obtained from all participants before initiating the study.
Inclusion criteria were: (1) patients aged between 20 and 85 years; and (2) patients with at least a 3-month duration of chronic pain who were scheduled to undergo fluoroscopy-guided lumbar sympathetic ganglion block (LSGB) in an outpatient setting. In this study, LSGB was selected among various minimally-invasive pain interventions, a relatively painful procedure with moderate procedural pain [
5]. Institutional protocol requires that patients stay in the outpatient pain operation room (OR) for at least 20 minutes to confirm a temperature increase after administering LA, which is adequate to experience the VR immersion.
Exclusion criteria were: (1) patients with visual or hearing impairment; (2) patients with previous psychotic, uncontrolled anxiety, or major affective disorders; (3) patients with cognitive defects or intellectual impairment; (4) patients with a disability that could affect adverse effect assessment or interfere with study completion when enrolled; (5) patients with a recent history of LSGB within 1 year before the randomization; (6) patients contraindicated for invasive procedures (coagulopathy, skin infection on the injection site, and allergies to LA); or (7) any patients who were considered inappropriate to register in this clinical trial.
2. Randomization
Randomization was conducted in an OR before starting the procedure. In the pre-operative holding room, all participants were educated in methods to handle the device with a brief experience of VR immersion for 2 minutes in a sitting position using a Samsung Gear head-mounted display compatible with the Android platform operating on a Galaxy 7.0 device (Samsung, Seoul, Korea). After entering the OR, patients were placed in a prone position, with their neck slightly flexed, and were assisted with putting on the VR head-mounted display and headphones. A soft gel pillow and a large foam pillow supported the patient’s forehead and chest to prevent any pressure or discomfort from wearing the device (
Fig. 1). The VR immersion began with proper fitting of VR device after repositioning the patients on the procedure bed. Next, the patients were randomly assigned to a VR group or a control group (1:1), based on a group allocation number within an opaque envelope opened by a pain fellow. The number in the envelope was matched to the group in a randomization table generated by an internet-based computer program (
www.randomization.com), managed by a radio technician independent of the study. The patient was assisted with switching on the VR program in the test group or switching it off in the control group. A pain fellow was available to support patients throughout the patient’s stay in the OR.
 | Fig. 1Photographs of a person wearing the virtual reality device in a prone position to undergo the lumbar sympathetic ganglion block. 
|
Patients in the VR group experienced a 30-minute VR immersion (NUVO program by Oncomfort SA, Wavre, Belgium). The three-dimensional VR software consists of a seashore view with Korean language narrations designed to induce relaxation (
Fig. 2). The program was developed initially as VR hypnosis to manage anxiety and pain during anxiety-provoking moments of treatment with portable immersive 360° audio and video. During the VR immersion, the patient could feel relaxed, virtually sitting in front of the seashore. The patient could then move around and look at the scene from other views, enhancing physical and emotional comfort while listening to narrations designed to induce relaxation. The first author (EKK) corresponded with the program developers to translate the original English version into the Korean language. The VR experience started right after the group allocation, in order to create effective immersion in the hypnotic program. Patients in the VR group were free to request discontinuation of the VR immersion at any time. In the control group, patients were asked to wear the VR head-mounted display and headphones, but the program was switched off while undergoing the procedure. During the procedure, the performing physician was blinded to which patients were in which group. The VR device was finally removed when the patient moved to the recovery room. Both groups could request removing their device at any time if they felt discomfort wearing the VR head-mount display and headphones.
 | Fig. 2Images of NUVO program by Oncomfort SA, Wavre, Belgium. 
|
3. Procedures
After entering the OR, patients were held in a prone position with a pillow under the lower abdomen, and draped in a sterile fashion. Other cushions were used to support the forehead and chest in patients wearing the VR head-mounted display in a prone position. All patients were administered an IV infusion of lactated Ringer’s solution and monitored via pulse oximetry, electrocardiogram (EKG), and blood pressure measurements throughout the procedure. In addition, temperature probes were tightly attached to both soles using transparent patches (TegadermTM, 3M Health Care, St. Paul, MN) before covering the patient’s body to confirm the temperature increase in the ipsilateral lower extremity after the LSGB. After sterilizing the skin around the puncture sites, the body was covered by a sterile surgical drape. The procedure was performed under fluoroscopic guidance (OEC 9800 series; GE OEC Medical Systems, Salt Lake City, UT) by a single pain physician (JYM) who had at least 10 years of experience to minimize inter-physician variation during the intervention. The pain physician was blinded to the patients’ group allocation during the procedure and throughout the study. Skin infiltration with LA (2-3 mL of 1% lidocaine) was performed in both groups. At least 3 minutes after skin infiltration, a 21-gauge 15 cm Chiba needle (Cook Inc., Bloomington, IN) was advanced at the L3 vertebral level under fluoroscopy-guided oblique projection. If a patient complained of moderate-to-severe procedure-related pain while advancing the needle, additional LA was injected via the Chiba needle. The total amount of LA was less than 5 mL, including skin infiltration, for procedural analgesia. When the needle reached the proper target site (anterolateral border of the L3 vertebral body), 1-2 mL of contrast agent was injected to confirm adequate spread around the target, followed by injection of 8 mL of 0.25% levobupivacaine. After removing the needle, temperature changes in the ipsilateral and contralateral soles were recorded for 20 minutes in the OR. The patients were then transferred to the recovery room.
4. Data collection
The primary outcome was the patient-reported procedural pain, which was measured using the 11-point numerical rating scale (NRS) on a scale of 0 (no pain) to 10 (the most severe pain imaginable). A research nurse recorded the pain scores immediately after the patients arrived in the recovery room.
The secondary outcomes included patient-reported anxiety before and after the procedure and patient-reported satisfaction with procedural pain control. The patient-reported anxiety involving the procedure was assessed using the 5-point Likert scale (1, not at all anxious; 2, a little anxious; 3, moderately anxious; 4, very anxious; and 5, extremely anxious) [
17]. It was based on two questions: 1) “How anxious do you feel about your upcoming procedure right now?”, which was asked before entering the OR, and 2) “Please imagine that you are supposed to undergo the same procedure right now. How anxious would you feel about the procedure at this moment?”, which was asked in the recovery room 30 minutes after the procedure. At the same time, patient-reported satisfaction with control of procedure-related pain was also evaluated using a 5-point Likert scale (1, dissatisfied; 2, less satisfied; 3, satisfied; 4, very satisfied; and 5, completely satisfied).
In the OR, the number of additive LA requirements (except for skin block) and any interruption of communication during the VR application were recorded. The total procedural time (min) was recorded, starting with the acquisition of the first radiographic image to the removal of the Chiba needle. The total VR time (min) was recorded, specifically starting from putting on the VR device in the OR to its removal. In the recovery room, subjective perception of stay (min) was evaluated by asking: “How much time were you supposed to stay in the OR?” Also, the patients in the VR group were asked about their subjective feedback on VR use and whether they would prefer VR immersion in their next pain procedures.
Safety evaluation was conducted via regular monitoring of any adverse events throughout the procedure. Pre- and post-procedural hemodynamic variables were compared using an average of 3 repeated measurements before the skin infiltration and before wearing off the VR device. Additionally, the intraoperative hemodynamic parameters were measured at regular intervals in the presence of bradycardia (heart rate < 40 beats/min), hypotension (decrease in mean arterial pressure < 55 mmHg or up to a 30% decrement from baseline), oxygen desaturation (SpO2 < 90%), or provocation of arrhythmia on the EKG. Adverse effects due to VR immersion, such as dizziness, seizure, headache, or muscle twitching, were monitored until discharge.
Baseline demographic and clinical data included age, sex, body mass index, comorbidities (hypertension, diabetes mellitus, asthma, cardiovascular diseases, and cerebrovascular disease), comorbid neuropsychiatric disorders (depression and anxiety), the presence of motion sickness, educational level (< high school, high school, > high school), duration of computer use (< 1 year, 1-5 years, > 5 years), diagnosis of pain, pain duration (month), baseline pain intensity (average level and the worst pain during the last week) based on an 11-point NRS pain score, and the use of strong opioids (morphine, oxycodone, hydromorphone, or fentanyl). Previous VR experience was evaluated dichotomously (yes or no). The patients’ Hamilton Anxiety Rating (HAM-A) score was measured before the procedure. The HAM-A score is based on 14 individually rated items with the total score ranging from 0 to 54: a score of 14 or less indicates mild anxiety, a score ranging from 15 to 23 represents mild to moderate anxiety, and a score of 24 to 30 suggests moderate to severe anxiety [
18].
5. Statistical analysis and sample size justification
In the absence of a preliminary study investigating the relevance of VR immersion to procedure-related pain scores in pain practice, we adopted a conventional strategy based on the differences in procedural pain between the two groups, suggested by Dworkin et al. [
19], which was assumed to represent a significant decrease in the 11-point NRS pain score of 2.0 under clinical settings. It was comparable to a previous VR study of pain control during dental procedures, which reported that the procedural pain was 1.8/10.0 for those using VR and 4.0/10.0 for the control group [
20]. We determined that 17 patients per group were necessary, assuming a standard deviation (SD) of 2.1 based on a previous study involving LSGB [
5], with a type 1 error of 0.05 and a type 2 error of 0.2. Allowing for 10% attrition, a total of 38 randomized patients were required for this study (G*Power 3.1.9.2).
All statistical analyses were performed using SPSS version 22.0 (IBM Co., Armonk, NY). The intention-to-treat approach was used for data analysis. To analyze continuous variables such as the primary endpoint (procedure-related NRS pain scores), the normality distribution was determined with the Kolmogorov–Smirnov test and an independent t-test was used to compare the normally distributed variables. The Mann–Whitney U-test was used to compare continuous variables without normal distribution. A chi-square test or Fisher’s exact test was used to analyze all categorical data. Lastly, we calculated the Spearman correlation coefficients (ρ) to measure the correlation between the procedure-related NRS pain scores for the baseline and procedural variables. Data were presented as the mean ± SD or the number (%). All P values are two-sided, and P values less than 0.05 were considered to indicate statistical significance.
Go to :

DISCUSSION
We conducted an exploratory randomized controlled trial to evaluate the effectiveness of VR immersion combined with conventional skin infiltration compared to skin infiltration alone. To the best of our knowledge, this study was the first one to assess the feasibility of VR in patients in a prone position while performing a painful procedure. The procedure-related pain during LSGB was significantly lower in the VR group (3.7 ± 1.4 in the 11-pointed NRS score) than in the control group (5.5 ± 1.7 in the 11-point NRS score; P = 0.002), without any severe adverse events associated with the VR immersion during the procedure. Fewer patients required additive LA in the VR group. Although satisfaction with procedural pain control did not differ between the groups, post-procedural anxiety was lower, and patients’ subjective perception of their stay in the OR was shorter in the VR group than in the control group. Almost two-thirds of patients (63.2%) wanted to undergo VR immersion again in their following pain procedure.
Despite their minimal invasiveness, pain interventions, including LSGB, are still painful and distressing for patients with chronic pain [
5]. Pain physicians have attempted to alleviate procedure-related pain and anxiety during their interventions. VR is one of the emerging options for rapid implementation in various clinical settings, ranging from managing acute procedure-related pain to rehabilitating chronic pain conditions [
8,
9]. It has been used in needle-related procedures, anesthesia administration, and other painful interventions, including dressing changes for burn wounds [
10-
16]. In recent studies, VR was suggested to positively modulate chronic intractable pain [
21], and was self-administered at home to manage chronic low back pain during COVID-19 [
22]. However, few studies have reported the use of VR in reducing acute procedure-related pain of the elderly in outpatient pain practice. Presumably, there have been several limitations in adopting VR in outpatient pain settings. First, most fluoroscopy-guided procedures in pain practice require patients to be in the prone position, which may hinder the comfortable use of the current VR device. Second, pain physicians may want to ensure instant patient response during the procedure. Accordingly, VR immersion may impede the interaction between patients and their physicians. Besides, many studies investigated the use of VR for procedural pain targets in pediatric cases, adolescents, or young adults rather than the elderly [
9,
23]; however, most of the subjects in pain practice are elderly, as in our study, which may discourage the clinical application of VR in routine practice. Recently, Brown et al. [
24] reported lower anxiety scores in patients with VR immersion than the controls at their outpatient spine center visits. However, in their study, patients experienced VR in a sitting position, but not in a prone position, ahead of the spine procedures [
24]. Therefore, our study firstly showed the benefit of VR in procedure-related pain control for elderly patients undergoing minimally-invasive pain procedures in a prone position in outpatient pain practice. Although three patients in our study felt discomfort wearing the VR headset in a prone position, it was bearable. We suggest that lighter and more user-friendly VR hardware can extend the use of VR technology in various medical environments.
The types of VR for reducing procedural pain differ according to the subject’s environment, ranging from non-immersive to fully-immersive approaches [
9]. In previous studies, fully immersive VR distraction games were often used to relieve acute procedural pain, resulting in mostly successful outcomes [
9]. Such VR distraction games might draw the patients’ attention away from adverse stimuli by exposing them to rich sensory stimuli, creating a realistic experience [
25,
26]. Instead of VR distraction games, an immersive VR program commercially developed for medical hypnosis was used in our study, resulting in reduced procedural pain and anxiety. VR hypnosis has rarely been studied in procedure-related pain control compared to VR distraction games [
27]. Previously, Konstantatos et al. [
28] used an 18-minute VR self-hypnosis program in burn patients undergoing awake dressings changes. However, their results showed increased pain after dressing in the VR hypnosis group, which suggested that a single session of VR hypnosis for burn patients undergoing an 18-minute dressing was not enough to reduce procedural pain. Although we used a VR hypnosis program, the pain-evoking procedure of LSGB lasted only about 6 minutes (shown in
Table 2). Although our patients might not have been fully immersed in VR hypnosis during the first 6 minutes, they could experience it for the remaining 20 minutes without painful external stimuli, which probably contributed to differences between our study and Konstantatos’s. Because there are numerous pain procedures with different durations and levels of invasiveness, further studies are necessary to identify the types of VR, which effectively attenuate procedural pain and anxiety in patients.
Although VR immersion reduced procedural pain with a statistical significance, the difference in the 11-point NRS pain scores between the VR and control groups was only 1.6 points, which did not meet our preset level that was a reduction of 2/10 or more in the 11-point NRS pain score. Nonetheless, the IMMPACT (Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials) guideline stated that a smaller difference, of less than 2/10 between groups, might indicate a significant difference [
19]. Besides the procedural pain reduction, VR immersion alleviated post-procedural anxiety in our study, similar to other VR studies [
8,
9]. In contrast to the former VR study [
16], satisfaction with procedural pain control was not different between the VR and the control groups in the present study. We assume that this may be related to the interactive use of additive LA in conscious patients in both groups, responding to patients’ requirements during the procedure. VR technology is affordable, safer, and more user-friendly than IV sedation in busy outpatient pain settings. Therefore, there is no reason not to embrace this modality in pain practice.
Beyond the acute procedural pain control, VR technology is available for chronic pain management, such as phantom limb pain, complex regional pain syndrome, and fibromyalgia, suggesting its potential neuromodulatory effect [
8,
27,
29,
30]. Although it was not shown in our results, when we compared the NRS pain scores in patients’ follow-up visits, patients’ average and worst pain intensity were not different between the groups at one month. We presume that a single session of VR intervention was not adequate to deduce the intensity of chronic pain, in contrast to previous studies that demonstrated the impact of multiple VR sessions [
29-
31]. The use of VR in conjunction with conventional chronic pain management warrants further investigation, incorporating different types of VR, interactivity, embodiment, and duration or frequencies of VR intervention according to the target goals of use in pain medicine.
The current study has several limitations. First, our study was a single-center trial with a small sample size, which might limit its external validity. The effect of VR intervention may differ in various clinical settings. Additional studies are needed to spread VR technology in other minimally invasive pain procedures, such as relatively simple (
e.g., single epidural injection) or complex procedures (
e.g., multi-level heat radiofrequency neurodestruction). Second, although we used sham intervention in the control group wearing the VR device, patients and physicians could not be blinded. Add-on design (VR + LA injection
vs. LA injection standalone) without blinding entails a placebo effect. To investigate that effect, it might be better to include a 3rd group not wearing the VR device, undergoing the procedure in the usual way in pain practice. Third, we measured patients’ anxiety using the HAM-A score at baseline and a single 5-pointed Likert scale before and after the procedures. The Beck Anxiety Inventory may facilitate the evaluation of changes in procedure-related anxiety in detail [
32]. Fourth, most of our patients were naïve to the VR application. The results could potentially differ if our participants were familiar with VR technology or the particular VR program. Finally, regarding the VR applications, we used the commercial VR hypnosis program rather than VR distraction games. Hypnosis would require some time of induction before the onset of the effect; however, we did not consider it in this study. Further, it is necessary to explore whether different VR programs work differently and investigate the types of VR that are the most effective to control procedural pain in the elderly during minimally invasive pain interventions.
In conclusion, the procedure-related pain during LSGB, and anxiety after the procedure, were significantly lower in patients exposed to VR, along with skin infiltration, than in those with skin infiltration alone in outpatient pain practice. No severe adverse events were associated with the VR immersion during the procedure. Although patients’ degree of satisfaction with their procedural pain control was similar, the additive requirements of LA during the procedure were fewer in patients exposed to VR immersion. This study suggests that VR immersion can be safely used as a novel adjunct to conventional skin block for managing procedural pain and anxiety in elderly patients undergoing fluoroscopy-guided spine procedures in a prone position.
Go to :





 PDF
PDF Citation
Citation Print
Print





 XML Download
XML Download