INTRODUCTION
MATERIALS AND METHODS
1. Search methods for study identification
2. Eligibility criteria
1) Inclusion criteria
Studies involving patients with post-TKA in American Society of Anesthesiologists (ASA) categories I-III, without restrictions for age, race, or nationality.
Studies involving an experimental group that underwent peripheral nerve blocks (femoral, femoral + tibial, adductor canal, and so on) and a control group that underwent epidural blocks. The primary anesthesia could be simple general, simple spinal, or general + spinal.
Randomized controlled trials with no language limitations.
Studies reporting at least one of the following indicators: postoperative complications (nausea and vomiting, hypotension, urinary retention, pruritus, and sedation), pain score, patient satisfaction, perioperative opioid dosage, and rehabilitation indices.
Studies reporting accurate and reliable data that could be transformed into binary or continuous variables to represent each index.
2) Exclusion criteria
Studies that assessed animals or corpses
TKA studies that included knee arthroscopy or hip joint operation and data on the TKA that could not be extracted independently.
Case reports, reviews, retrospective studies, or conference papers without full text.
Reports with data that could not be extracted or converted into valid data for meta-analysis.
3. Measurement index
1) Primary outcome measures
Pain score: Postoperative pain control is the most important outcome indicator. Mild pain allows limited physiotherapy, which in turn contributes to a faster recovery of knee function [15,16]. Severe postoperative pain can induce other complications, including thrombosis, infection, poor activity, and prolonged hospital stay, which increases medical costs [17]. We compared the post-TKA VAS pain score at three postoperative time slots, i.e., 0-12, 12-24, and 24-48 hours. Moreover, pain intensity assessed on a 10-mm VAS.
2) Secondary outcome measures
Complications: This is a paramount factor affecting clinical postoperative analgesia, quality of life, and acceptance of the analgesia. Common severe complications include nausea and vomiting, hypotension, urinary retention, pruritus, and sedation.
Patient satisfaction: Postoperative patient satisfaction is a major subjective index for measuring the analgesic effect.
Perioperative opioid dosage: Opioids are the main drugs used for surgical analgesia, including fentanyl, oxycodone, piritramide, and morphine. However, their serious complications are a concern for clinicians [18,19]. We analyzed the opioids used during and after surgery.
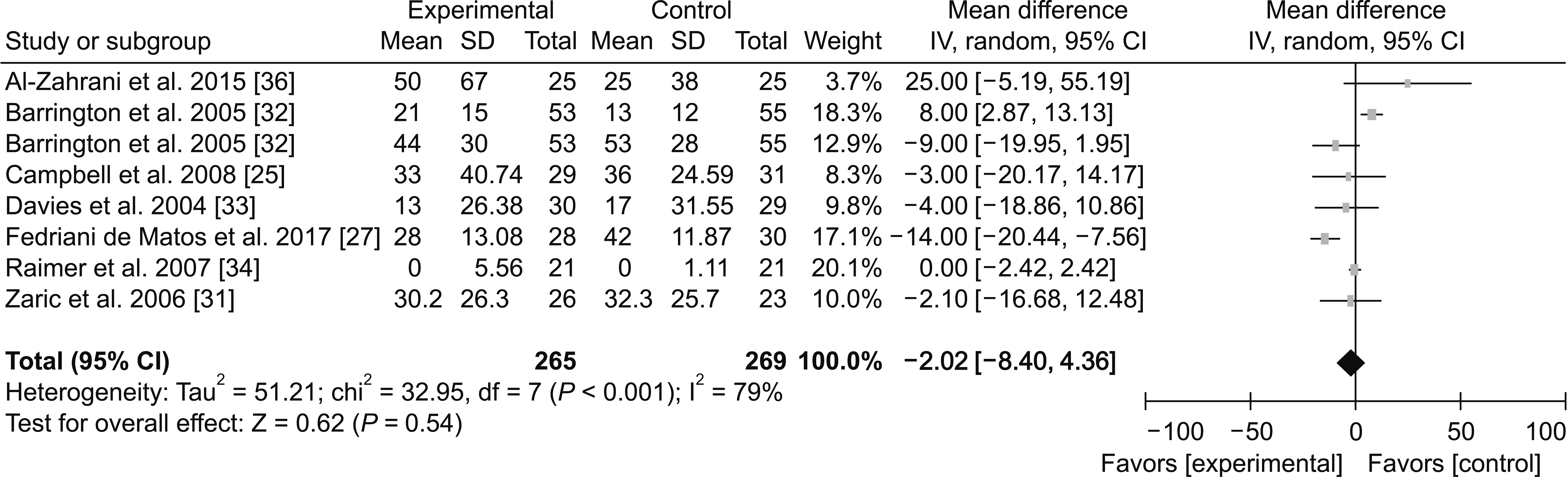
Rehabilitation indices: Good analgesic treatment allows patients who undergo TKA, to experience more active rehabilitation treatment. Mistimed functional rehabilitation could influence its eventual clinical efficacy, with some patients having to undergo a secondary release surgery. This causes a heavy burden on the patients and their families. Therefore, rehabilitation indices are major indirect indicators for the effect of analgesia. We analyzed the length of hospital stay and range of active knee flexion.
4. Assessment of the methodological quality
5. Data collection
6. Statistical analysis
RESULTS
1. Search results and characteristics of the selected studies
Table 1
| Reference | Year | Country | Journal | Design |
Primary anesthesia |
Intervention | Analgesic techniques | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Peripheral nerve block | Epidural analgesia | Peripheral nerve block | Epidural analgesia | |||||||
| Adams et al. [24] | 2002 | Germany | European Journal of Anaesthesiology | RCT | GA | Continuous femoral | Continuous epidural analgesia | Single-shot femoral block: postop bolus bupivacaine 0.375% of 40 mL | Bupivacaine 0.375% bolus postop | |
| Barrington et al. [32] | 2005 | Australia | Anesthesia and Analgesia | RCT | Spinal | Continuous lumbar plexus infusion | Continuous epidural analgesia | Femoral catheter: preop bolus bupivacaine 0.25% with adrenaline 25 mL + infusion bupivacaine 0.2% with PCA bolus postop | Ropivacaine 0.2% with fentanyl infusion postop | |
| Campbell et al. [25] | 2008 | UK | European Journal of Anaesthesiology | RCT | Spinal | Continuous femoral block | Continuous epidural infusion | Infusion a mixture of levobupivacaine 0.125% and clonidine + 10 mL 0.5% levobupivacaine | 4 mL of 0.5% levobupivacaine + 4 mL 0.25% levobupivacaine | |
| Capdevila et al. [26] | 1999 | France | Anaesthesiology | RCT | GA | Single-shot combined sciatic plus femoral (3-in-1) block | Continuous lumbar epidural analgesia | Femoral catheter: postop bolus 2% lidocaine with epinephrine 25 mL and morphine 2 mg + infusion 1% lidocaine with clonidine and morphine | Lidocaine 2% with epinephrine and morphine 2 mg to block level T10 bolus postop + infusion 1% lidocaine with clonidine and morphine | |
| Davies et al. [33] | 2004 | UK | British Journal of Anaesthesiology | RCT | GA | Continuous femoral nerve block | Continuous epidural analgesia | Single-shot femoral and sciatic blocks: preop bolus bupivacaine 0.375% 30 mL (femoral) + 25 mL (sciatic) limited to 3 mg kg-1 | Bupivacaine 0.5% bolus preop + infusion bupivacaine 0.25% postop | |
| Sakai et al. [13] | 2013 | Japan | The Journal of Arthroplasty | RCT | GA | Continuous femoral | Continuous epidural analgesia | 60-mg dose of 0.3% ropivacaine (tibial) + 60-mg dose of 0.3% ropivacaine (femoral) | 1% lidocaine 50 mg + 90-mg doses of 0.3% ropivacaine | |
| Shanthanna et al. [30] | 2012 | Canada | Indian Journal of Anaesthesia | RCT | GA and spinal | Continuous femoral | Continuous epidural analgesia | Femoral catheters: 12 mL bolus of 0.125% bupivacaine mixed with 2 mcg/mL fentanyl + 0.125% bupivacaine with fentanyl postop | 25 mcg fentanyl + 12 mL bolus of 0.125% bupivacaine mixed with 2 mcg/mL fentanyl + 0.125% bupivacaine with fentanyl postop | |
| Singelyn et al. [2] | 1998 | Belgium | Anesthesia and Analgesia | RCT | GA | Continuous femoral and sciatic nerve blocks | Continuous epidural infusion | 37 mL of 0.25% bupivacaine with epinephrine + continuous infusion of 0.125% bupivacaine with sufentanil 0.1 µg/mL and clonidine 1 µg/mL postop | 3 mL of 0.25% bupivacaine with epinephrine + 10 µg of sufentanil bolus preop + infusion bupivacaine 0.125% with sufentanil and clonidine postop | |
| Zaric et al. [31] | 2006 | Denmark | Anesthesia and Analgesia | RCT | GA | Femoral | Epidural catheter analgesia | Femoral and sciatic catheters: preop bolus ropivacaine 0.75% 30 mL each catheter + infusion ropivacaine 0.2% with sufentanil (femoral) and ropivacaine 0.05% (sciatic) postop | Ropivacaine 0.75% boluses to block level T10 preop + infusion ropivacaine 0.25% with sufentanil postop | |
| Chelly et al. [35] | 2001 | USA | The Journal of Arthroplasty | RCT | GA | Lumbar plexus + sciatic blocks | Epidural anesthesia | 15 mL of 0.75% ropivacaine + 15 mL of 1.5% mepivacaine + 0.2% ropivacaine postop | Epidural catheter: 20 mL of a mixture containing 2% lidocaine and 0.5% bupivacaine | |
| Horasanli et al. [28] | 2010 | Turkey | Clinics | RCT | GA | Continuous femoral infusion | Epidural analgesia | 3 mL of 2% lidocaine + 30 mL of 0.375% ropivacaine + 20 mL of 0.375% ropivacaine | 2 mL of 2% lidocaine + 3 mL of 2% lidocaine with epinephrine + 15 mL of 0.75% epidural ropivacaine | |
| Kim et al. [29] | 2012 | Korea | Korean Journal of Anesthesiology | RCT | Spinal | Femoral/sciatic nerve block | Combined spinal epidural nerve block | 20 mL of 1.5% mepivacaine + 5 mL of 1.5% mepivacaine + 20 mL of 1.5% mepivacaine | 1.3 mL of 0.5% hyperbaric bupivacaine + 10 mL of 0.75% ropivacaine + fentanyl, ketorolac, ramosetron postop | |
| Raimer et al. [34] | 2007 | Germany | Acta Orthopaedica | RCT | Spinal | Continuous psoas compartment and sciatic analgesia | Epidural analgesia | 25 mL of 0.75% ropivacaine + 25 mL 1% prilocaine (sciatic block) | 0.5% bupivacaine | |
| Al-Zahrani et al. [36] | 2015 | Saudi Arabia | The Journal of Arthroplasty | RCT | GA | Continuous femoral nerve block with single shot sciatic nerve block | Continuous epidural infusion | Femoral nerve catheter: preop 15 mL of 0.25% bupivacaine; 10 mL 0.25% bupivacaine + 0.2% bupivacaine | Epidural catheter: preop 10 mL of 0.25% bupivacaine + 50 mcg fentanyl; 0.0625% bupivacaine + fentanyl | |
| Fedriani de Matos et al. [27] | 2017 | Spain | Rev Esp Anestesiol Reanim | RCT | Spinal | Continuous femoral | Continuous epidural block | 15 mL of levobupivacaine 0.25% + levobupivacaine 0.125% postop | 3 mL bupivacaine 0.25% with adrenalin + levobupivacaine 0.125% postop | |
| Kayupov et al. [37] | 2018 | USA | The Journal of Arthroplasty | RCT | GA and spinal | Continuous adductor canal blocks | Epidural analgesia | Spinal + CACB: 3 mL 1.5% + lidocaine with epinephrine, 0.2% ropivacaine postop; | Epidural catheter: 0.1% bupivacaine postop | |
| General + CACB: 30 mL 0.5% ropivacaine with epinephrine, ropivacaine postop | ||||||||||
Table 2
| Reference | Total | CG | TG | Mean age (yr)a | Sex (M/F)a | ASA (I/II/III)a | BMI (kg/m2)a |
|---|---|---|---|---|---|---|---|
| Adams et al. [24] | 42 | 21 | 21 | 69 vs.70 | 7/14 vs. 5/16 | NR | NR |
| Barrington et al. [32] | 108 | 55 | 53 | 71 ± 9 vs. 69 ± 10 | 25/30 vs. 26/27 | NR | 31 ± 5.2 vs. 33 ± 6.1 |
| Campbell et al. [25] | 60 | 31 | 29 | 70 ± 8.4 vs. 72 ± 9.9 | 14/17 vs. 14/15 | 2.2 ± 0.6 vs. 2.2 ± 0.6 | 30.8 ± 4.8 vs. 29.1 ± 4.6 |
| Capdevila et al. [26] | 37 | 17 | 20 | 51 ± 15 vs. 54 ± 17 | 10/7 vs. 8/12 | ASA I or II | NR |
| Davies et al. [33] | 60 | 30 | 30 | 73.1 ± 9.0 vs. 72.3 ± 9.5 | 13/17 vs. 19/11 | NR | NR |
| Sakai et al. [13] | 60 | 30 | 30 | 73 (53-86) vs. 72 (48-84) | 4/26 vs. 4/26 | 1/26/3 vs. 2/24/4 | 24.8 (16.4-33.3) vs. 24.8 (19.1-37.0) |
| Shanthanna et al. [30] | 38 | 19 | 19 | 63.6 ± 5.0 vs. 63.5 ± 5.0 | 11/8 vs. 10/9 | NR | NR |
| Singelyn et al. [2] | 30 | 15 | 15 | NR | NR | ASA II or III | NR |
| Zaric et al. [31] | 49 | 23 | 26 | 67 ± 6 vs. 66 ± 7 | 12/11 vs. 11/15 | 3/20/0 vs. 4/17/5/0 | NR |
| Chelly et al. [35] | 59 | 30 | 29 | 70 (65-74) vs. 66 (60-74) | NR | NR | NR |
| Horasanli et al. [28] | 76 | 39 | 37 | 54.0 ± 16.9 vs. 51.3 ± 14.9 | 27/12 vs. 24/13 | 14/29/0 vs. 14/23/0 | NR |
| Kim et al. [29] | 80 | 40 | 40 | 67.4 ± 1.3 vs. 71.8 ± 1.3 | 4/36 vs. 3/37 | 1/38/0 vs. 2/36/2 | NR |
| Raimer et al. [34] | 42 | 21 | 21 | 64 (61-73) vs. 69 (61-75) | 7/14 vs. 10/11 | 2/12/7 vs. 2/15/4 | 33 (28-35) vs. 30 (26-34) |
| Al-Zahrani et al. [36] | 50 | 25 | 25 | 60 ± 8.5 vs. 62 ± 7.5 | 7/18 vs. 8/17 | 2/23/0 vs. 6/19/0 | 33 ± 5 vs. 33 ± 5 |
| Fedriani de Matos et al. [27] | 58 | 30 | 28 | 68.6 ± 6.5 vs. 68.0 ± 8.2 | 70%/30% 32%/68% | 0%/80%/20% vs. 4%/89%/7% | 33.5 ± 8.5 vs. 30.8 ± 5.0 |
| Kayupov et al. [37] | 132 | 44 | 41/47 | 64/63/60 | 45%/55% 46%/54% 59%/41% | NR | 30/31/31 |
2. Methodology evaluation
3. Clinical outcomes
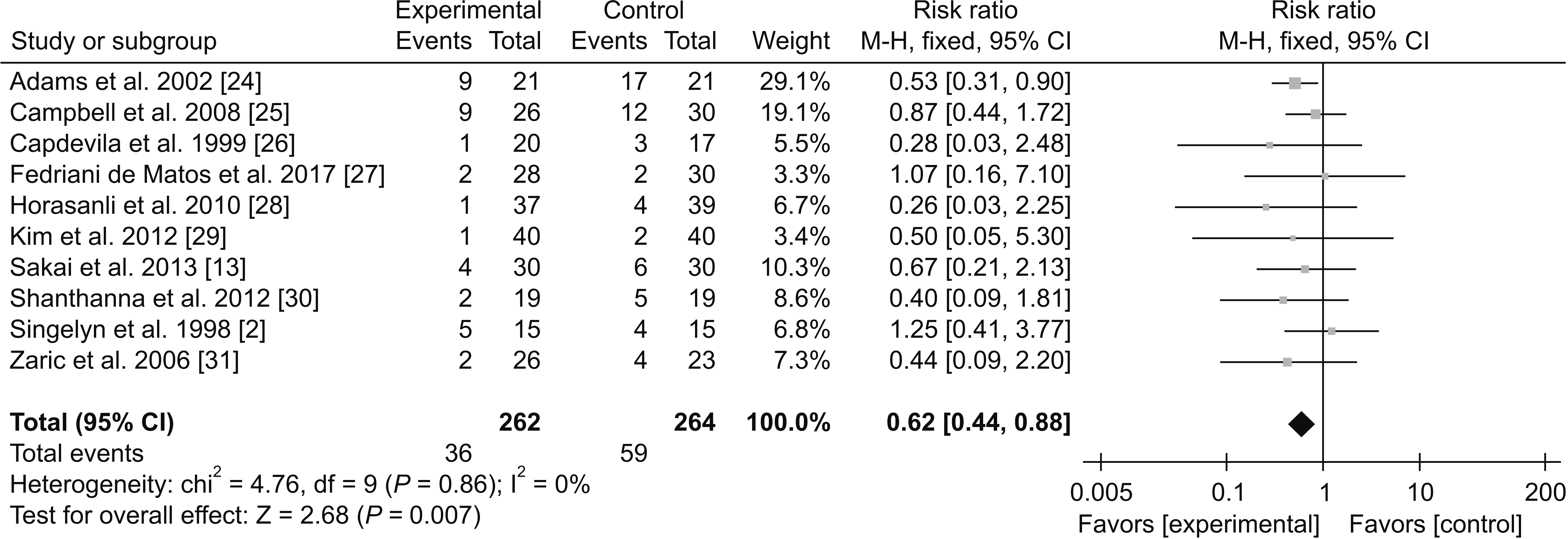
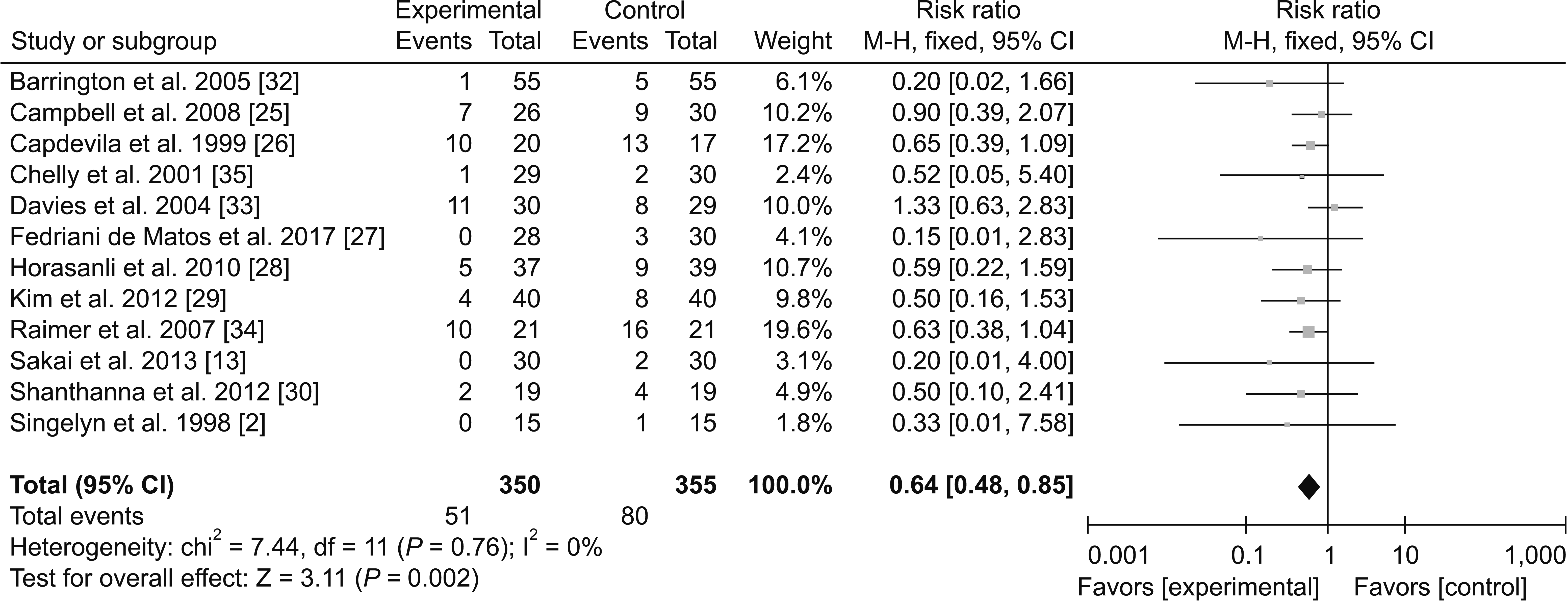
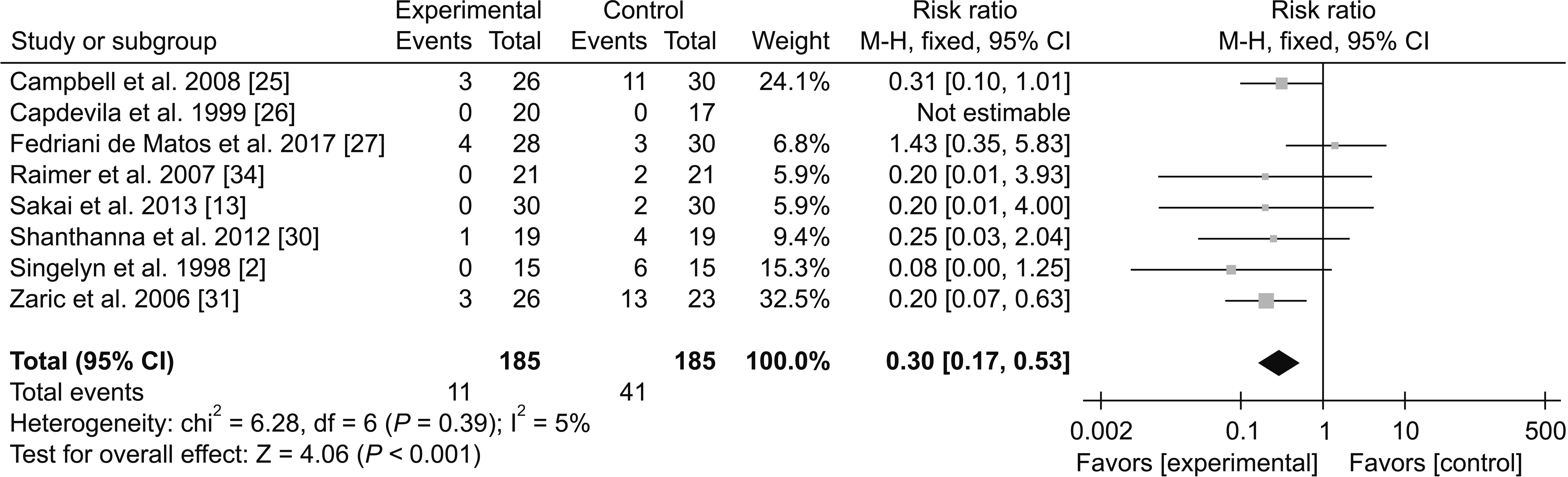
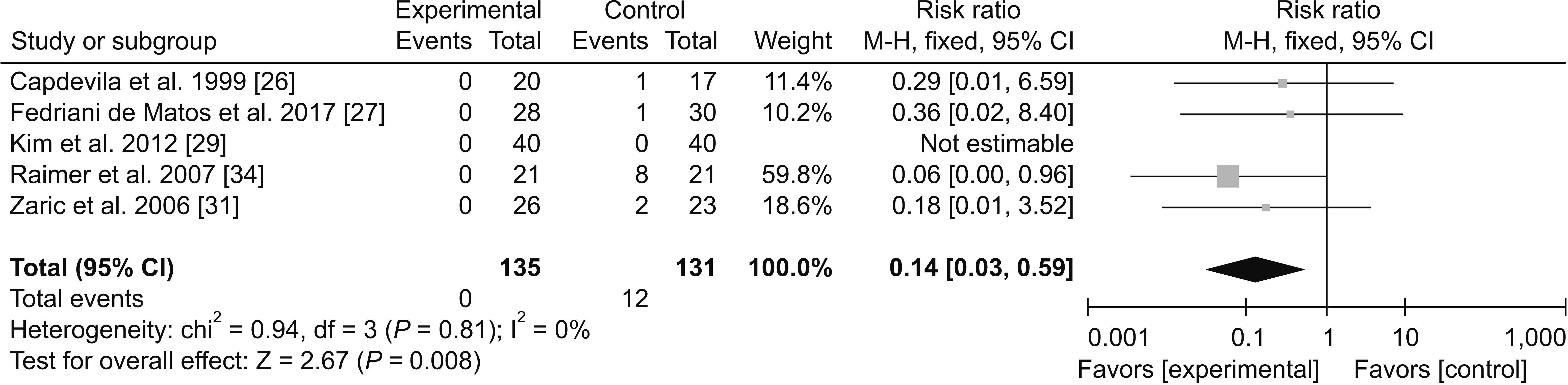
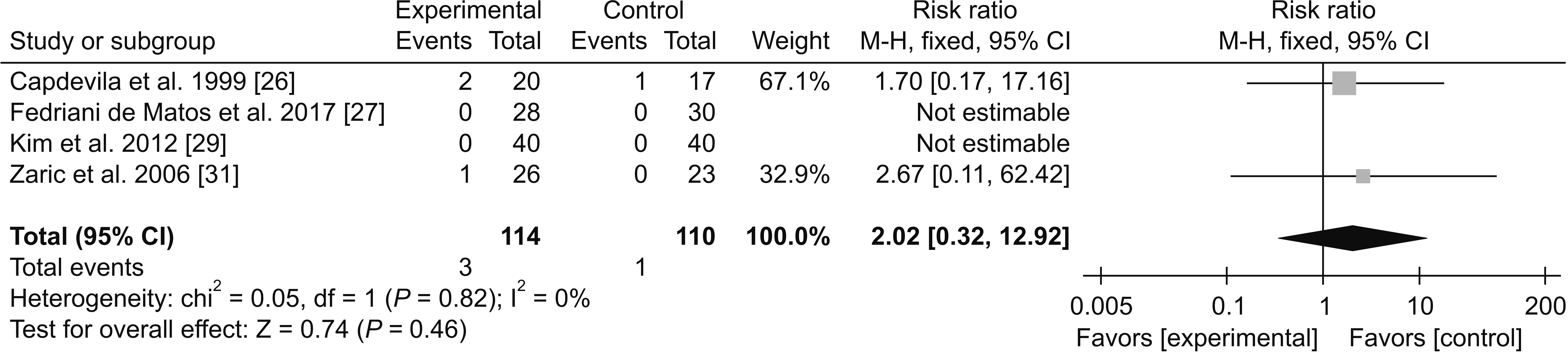
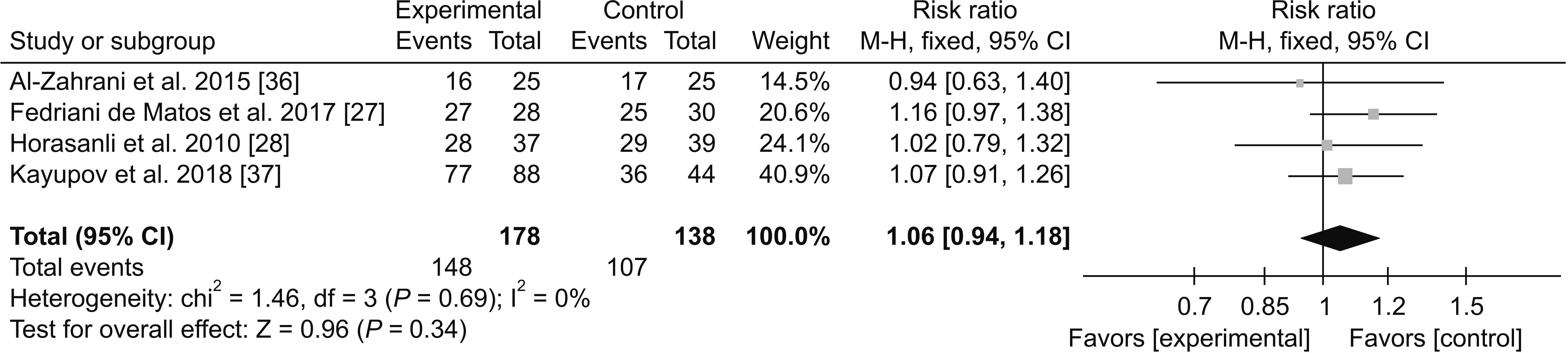
1) Adverse effects
(1) Nausea and vomiting
(2) Hypotension
(3) Urinary retention
(4) Pruritus
(5) Sedation
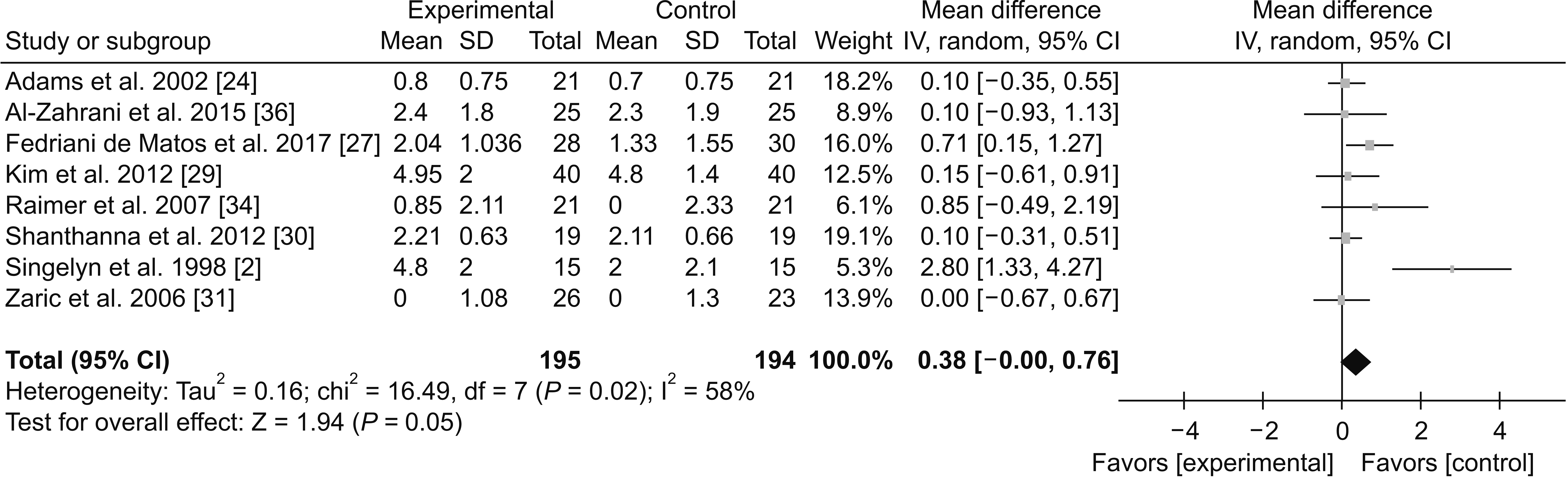
2) VAS score
(1) 0-12 hours
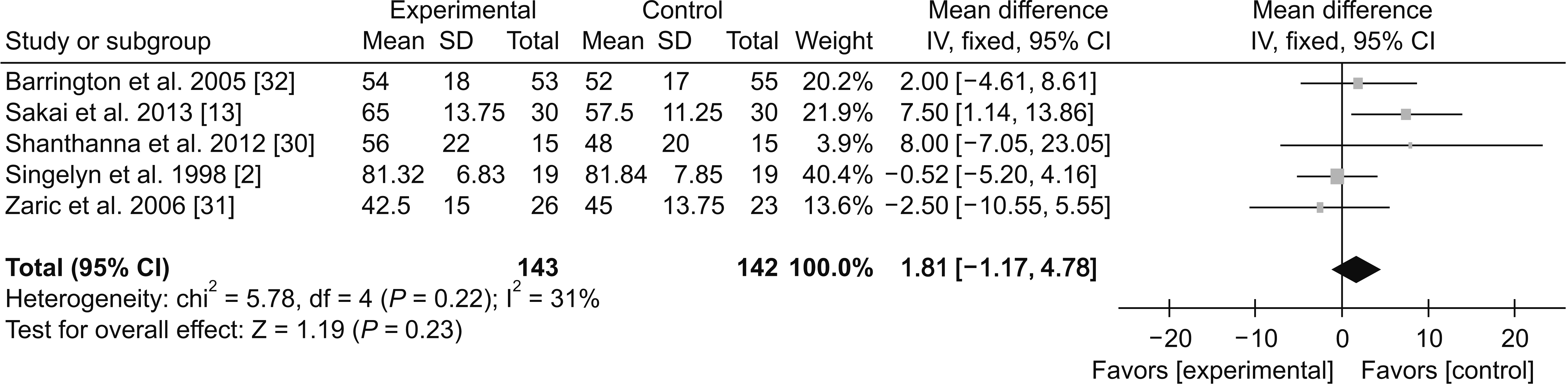
(2) 12-24 hours
Fig. 9
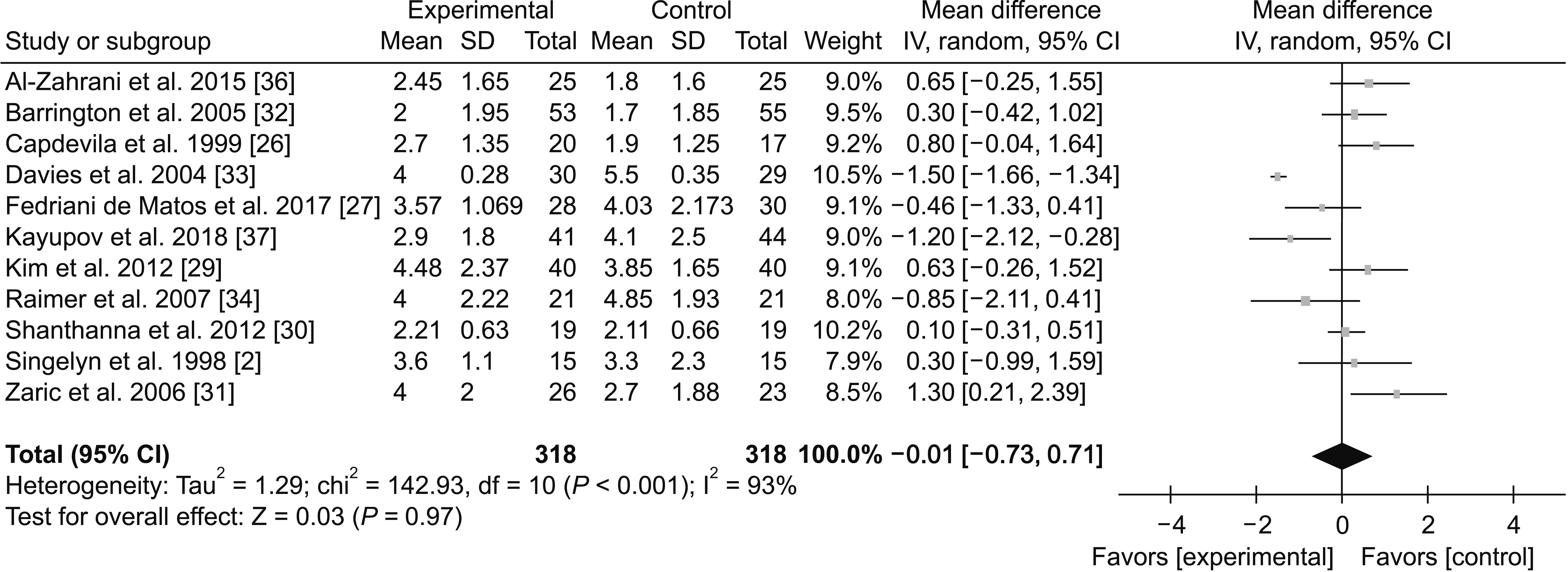
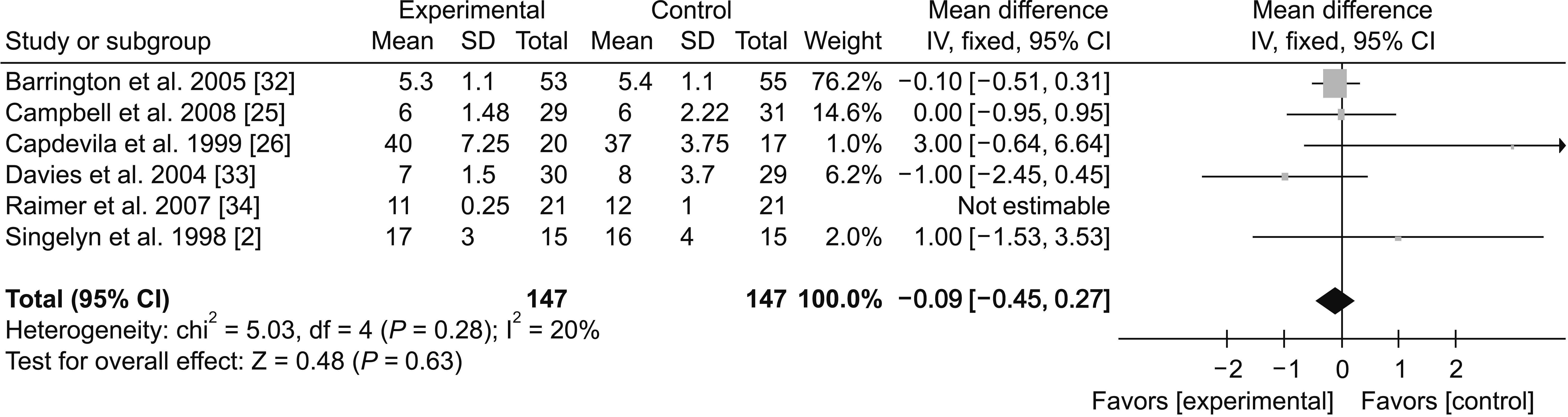
(3) 24-48 hours
Fig. 10
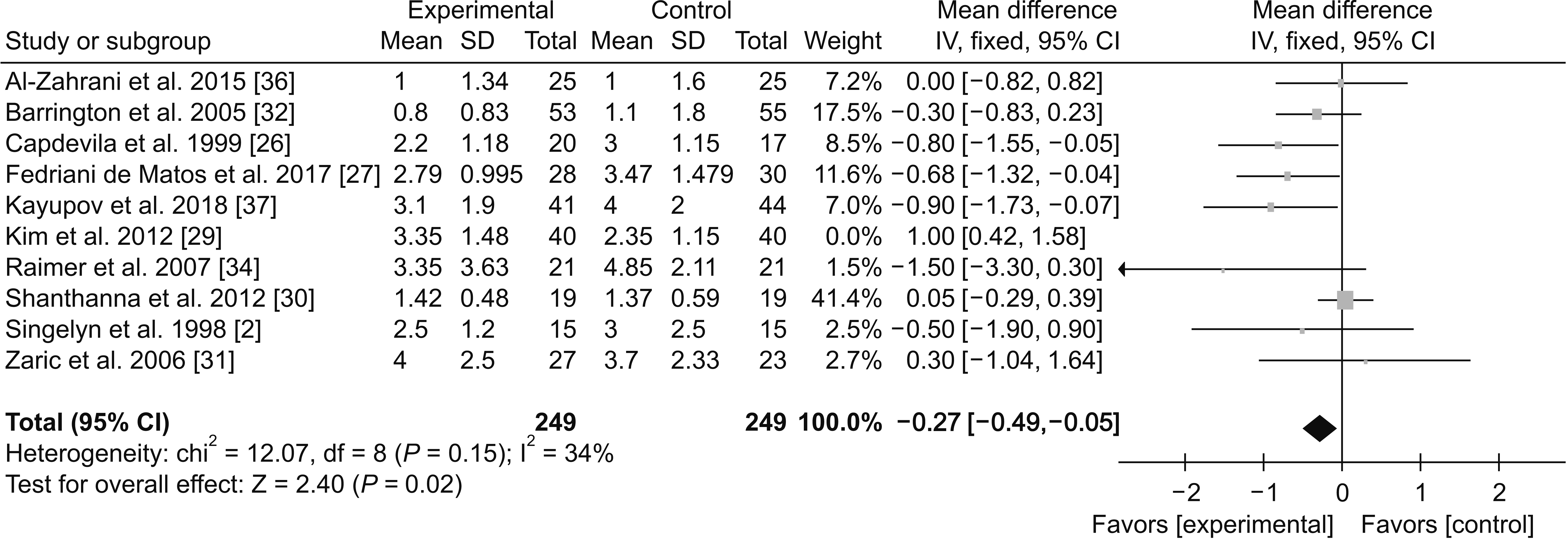




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download