INTRODUCTION
Surgical trauma and the accompanying pain are associated with postoperative alterations of the neuroendocrine, metabolic, and immune systems [
1,
2]. Proper postoperative pain management can prevent these alterations and reduce the cardiopulmonary and metabolic complications. Therefore, the use of appropriate analgesics helps improve the outcome of surgery [
3]. On the other hand, it is known that some anesthetics and analgesics, apart from their analgesic effects, themselves have immunosuppressive effects. This means that the choice of analgesic drug is important in immunocompromised patients [
4-
7].
The antiepileptic drug pregabalin (PGB) has been used extensively to treat various types of neuropathic pain, including diabetic polyneuropathy and post-herpetic neuralgia [
8]; it also reduces acute and chronic postoperative pain and postoperative opioid consumption [
9,
10]. We previously examined the effects of PGB treatment on immune reactions in chronic neuropathic mice. In that study, mice with chronic neuropathy, due to constriction of the sciatic nerve, exhibited immune upregulation and PGB treatment reversed this [
7]. However, little further is known about the immunological effects of PGB administration in acute pain or painful postoperative conditions and the advantages of perioperative PGB treatment remain controversial [
10].
The aim of the present study was to assess whether PGB treatment affects the immune reactions associated with postoperative pain. To investigate cell-mediated immune responses, we employed an incisional pain model in mice and evaluated the effects of PGB on natural killer (NK) cell activity and proliferation of splenic lymphocytes. At the same time, we assessed the antinociceptive effect of PGB using the paw withdrawal threshold (PWT) test. The mouse pain model devised by Pogatzki and Raja [
11] is a modified mouse murine version of a well-established rat model of incisional pain. Pogatzki et al. [
12] suggested that ongoing afferent input from the plantar incision wound in this model maintains sensitization of the dorsal horn neurons. As a result, the mechanical and heat hyperalgesia continue for several days which is similar with variable surgical pain in humans.
Go to :

MATERIALS AND METHODS
1. Animal use
All experiments were approved by the Institutional Animal Care and Use Committee (Incheon St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Permit Number was CIMH 2012-001). All procedures were conducted in accordance with the guidelines specified in the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals (NIH publication no. 86-23, revised 1985) as well as the Ethical Guidelines for Investigations of Experimental Pain in Conscious Animals. The experiments were performed on 50 male BALB/c mice (Orient Bio, Seoul, Korea) weighing 20 to 25 g and about 6 weeks of age. Mice were housed in groups of five per cage under standard conditions (i.e., a room temperature of 20°C to 25°C, relative humidity of 50% to 60%, and a 12/12 hr light/dark cycle, with the lights on at 08:00) and all experiments were performed at the animal laboratory between 7 am and 11 am. The mice were kept specific pathogen-free and allowed access to food and water ad libitum throughout the study. The mice were acclimatized for at least 1 week before the study.
2. Hind paw plantar incision
As an acute pain model, we used the mouse model of plantar incisional pain [
11]. Mice were anesthetized with isoflurane 2%-3% that was delivered through a nose cone. After antiseptic preparation of the left hind paw with alcohol, a No. 11 blade was used to make a 5 mm longitudinal incision on the surface of the plantar foot that traversed the skin and fascia. The underlying muscle was elevated with curved forceps. The muscle origin and insertion were left intact. The skin was closed with a single mattress suture by using a TG175-8 needle with 6-0 nylon (Ophthalmic, 1716G: Ethicon, Somerville, NJ). Mice of the control group underwent anesthesia without plantar incision. All surgeries were performed by the same person to minimize experimental variability.
3. Experimental setting and drug treatment
Mice were randomized using block randomization into one of four groups (each n = 8), as follows: saline-treated hind paw plantar incision (the incision group); PGB-treated incision (the PGB-incision group); sham controls undergoing an identical anesthesia procedure but without incision or administration of drugs (the control group); and PGB-treated controls (the PGB-control group). In the PGB-incision group, PGB in saline was administered intraperitoneally at a dose of 30 mg/kg in a volume of 0.1 mL/10 g. In the incision group, the same volume of saline was injected intraperitoneally. For the extension of analgesic effect of PGB without deep sedation, the drugs were administered divided into two doses. The first intraperitoneal injection of PGB or saline was performed 30 minutes before the plantar incision. After the surgery, mice were allowed to recover for 30 minutes before testing for postoperative mechanical thresholds. One hour after the hind paw incision, a second intraperitoneal PGB injection was performed with the same dosage. Mice were euthanized by cervical dislocation under isoflurane anesthesia 12 hours after the plantar incision, and NK cytotoxicity and lymphocyte proliferation were measured by a person who was blinded to the procedures in these animals (
Fig. 1).
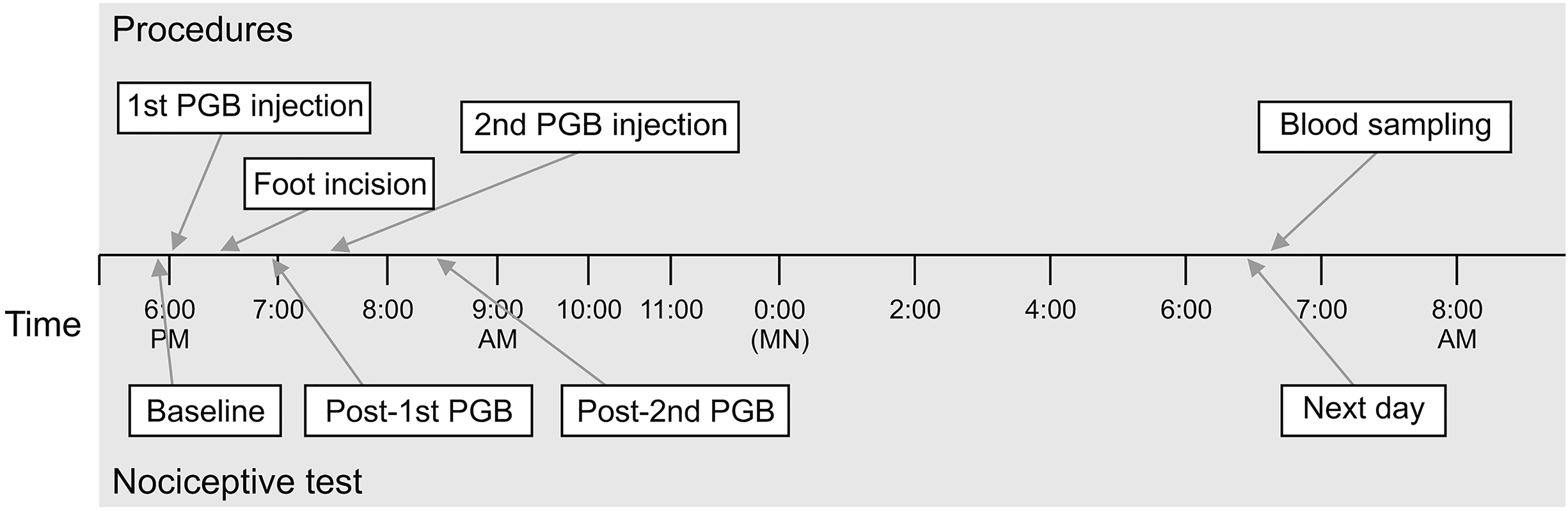 | Fig. 1Experimental procedure. At the beginning of the experiment, initial paw withdrawal threshold were assessed for mechanical nociceptive test (baseline). In the pregabalin (PGB)-incision and PGB-control groups, PGB in saline was administered intraperitoneally, at 30 mg/kg, in a volume of 0.1 mL/10 g. In the incision group, an identical volume of saline was injected intraperitoneally. Thirty minutes subsequent to the initial drug injection, plantar incision or anesthesia was applied in accordance with group. One hour following hind paw incision, a second intraperitoneal injection was performed at the same dosage. Mice were sacrificed by cervical dislocation 12 hours following plantar incision, and natural killer cytotoxicity and lymphocyte proliferation were measured. The figure shows the whole experimental process using group 3 as an example. 
|
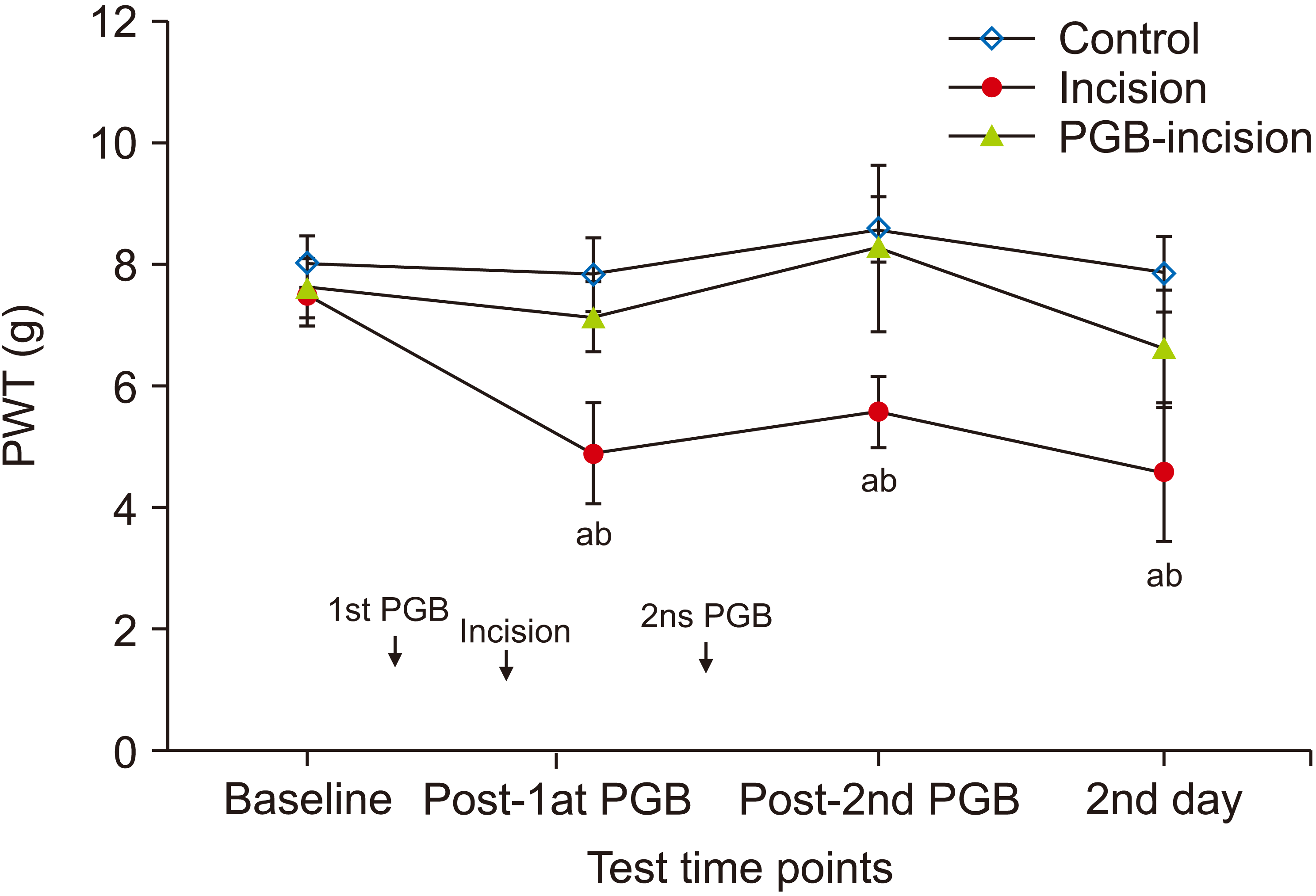
4. Nociceptive testing
To determine the mechanical allodynia caused by the incision and the analgesic effect of preabalin on the pain, the mechanical nociceptive thresholds of the incision group and the PGB-incision group were compared with the control group. Mechanical nociceptive thresholds were assessed by PWT 30 minutes before plantar incision, 1 hour after the first and second PGB administrations, and 12 hours after surgery (the second day). The tests were performed by an investigator who was blinded to the drug or procedure that the mice had received. A dynamic plantar aesthesiometer (Ugo Basile, Gemonio, Italy) was used to measure the mechanical thresholds. Animals were placed individually in a test cage with a wire-mesh floor. A rigid-tipped filament was applied to the skin of the mid-plantar area of the hind paw. The filament was exerted with an increasing force, starting below the threshold of detection and increasing to 5 g in 20 seconds, until the animal removed its paw. The PWT was expressed in grams (g). All measurements were performed in triplicate on the same hind paw at 30 second intervals. The mechanical threshold was defined as the mean value.
5. Cell preparation
Mice were euthanized by cervical dislocation under deep isoflurane anesthesia (3%-4%) and the spleen was aseptically excised and stored in tissue culture medium (RPMI 1640; Gibco BRL, Grand Island, NY). A single-cell suspension was prepared by using a 5 mL syringe plunger to pass spleen tissue in fresh wash medium through a 70 µm mesh strainer (BD Falcon, San Diego, CA). The splenocytes were suspended in complete RPMI medium (RPMI 1640 supplemented with 10% fetal bovine serum, 100 U/mL penicillin, 100 µg/mL streptomycin, and 2 mM L-glutamine). Cell density and viability were estimated with a hemocytometer. Trypan blue exclusion was performed to identify the dead cells.
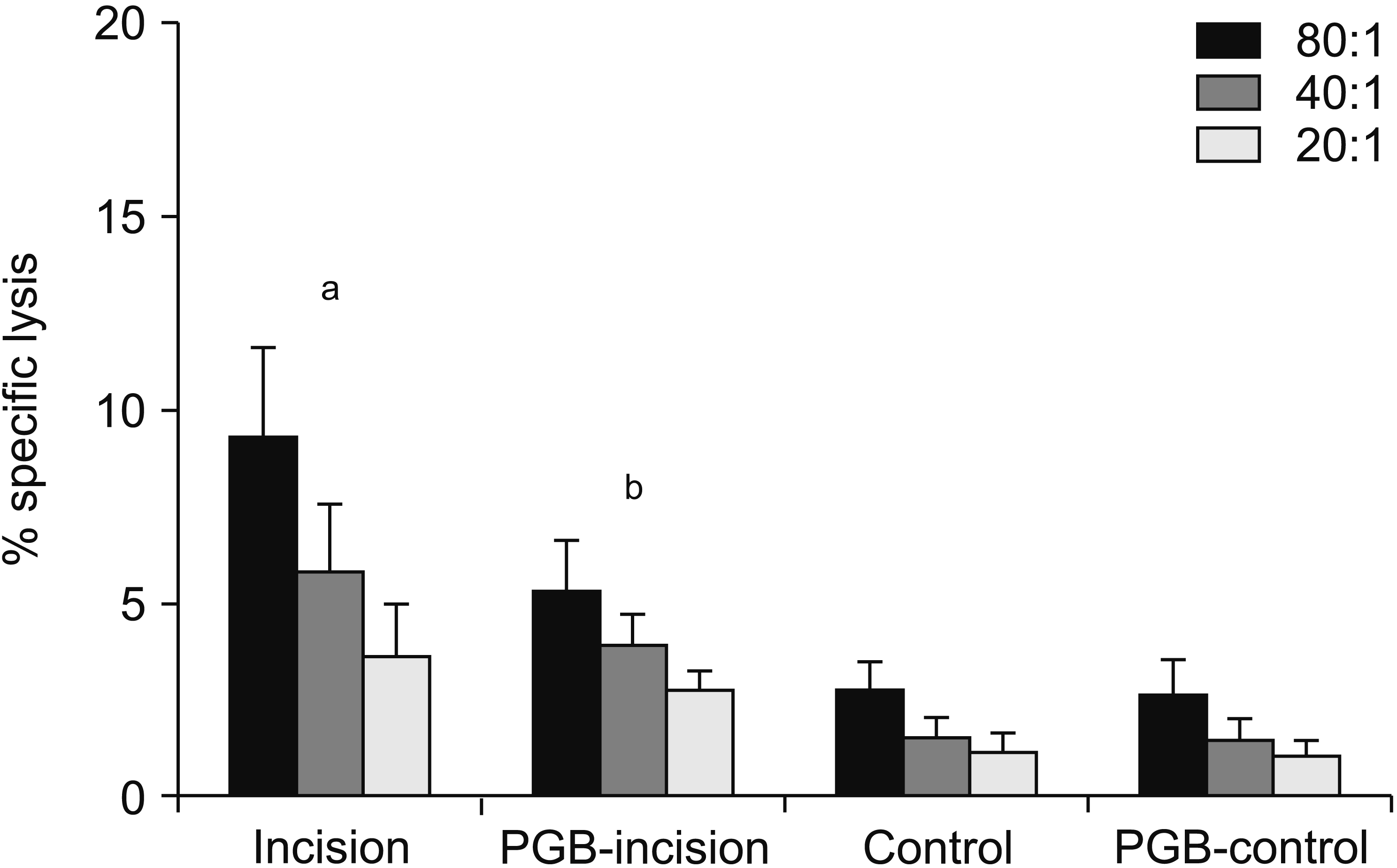
6. NK cell cytotoxicity assay
A lactate dehydrogenase (LDH) assay with YAC-1 (mouse lymphoma cells) targets was conducted to determine the effects of incisional pain and of PGB treatment on the tumoricidal activity of NK cells from the spleen of each mouse. Target YAC-1 lymphoma cells were grown in RPMI 1640 medium supplemented with antibiotics and fetal bovine serum. Effector splenocyte suspensions were prepared from each mouse, and various numbers of effector cells were added to the wells of a microtiter plate containing 1 × 104 target YAC-1 cells in 100 µL to achieve final effector to target cell ratios of 80:1, 40:1, and 20:1. The plates were incubated for 4 hours at 37°C in 5% CO2 at 90% humidity. The cells were then removed by centrifugation, and the supernatants were collected and transferred to a flat-bottom plate. After 100 µL of freshly prepared LDH detection mixture was added to each well, the plate was incubated at room temperature for 30 minutes to permit color development. The absorbance in each well was measured at 490 nm with a plate reader, and the percentage of specific LDH release was determined according to the following equation:
where LDHexperimental = LDH release from coculture of effector cells and target cells; LDHeffector cells = LDH release from effector cells cultured on their own; LDHspontaneous = LDH release from YAC-1 cells cultured on their own (low control); and LDHmaximal = LDH release when YAC-1 cells were lysed in Triton X-100 (high control).
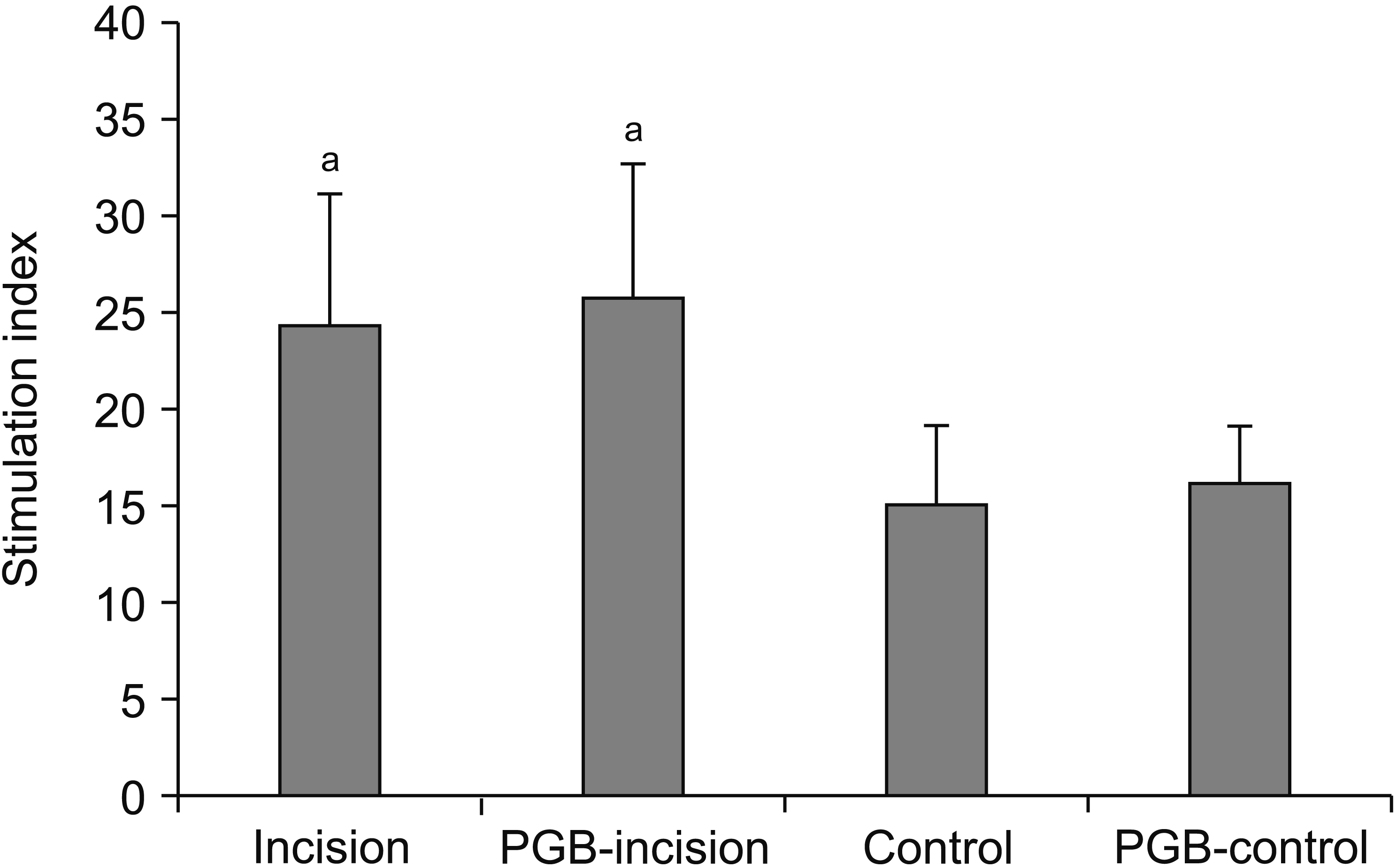
7. Splenic lymphocyte proliferation activity assay
The proliferative response of isolated splenocytes to phytohemagglutinin (PHA) was measured using a bromodeoxyuridine (BrdU) detection ELISA kit (Roche Molecular Biochemicals, Mannheim, Germany). The splenocytes from each mouse were aliquoted into 96-well micro plates at 2 × 105 cells/well (100 µL/well) and cultured at 37°C in 5% CO2 at 90% humidity in the presence of PHA for 2 days; 5 µg PHA was added at the start of incubation and then again 24 hours later. For pulse labeling, 10 µL BrdU was added to each well (10 µM final concentration) and the cells were incubated for another 18 hours. The labeling medium was removed using a needle, the cells were air-dried, and 200 µL FixDenant (Roche Diagnostics, Indianapolis, IN) agent was added to each well to fix the cells and denature the genomic DNA, thereby exposing the incorporated BrdU to immunodetection. After removing the fixative, an anti-BrdU antibody (clone BMG 6H8, Fab fragment) was added to each well and the plate was incubated at room temperature for 90 minutes. After washing with phosphate-buffered saline and adding substrate (tetramethylbenzidine), the plate was incubated at 25°C for 20 minutes. The absorbance at 370 nm was then measured by using an automated plate reader. Proliferation was determined as the stimulation index (SI), calculated as follows:
where OD = optical density.
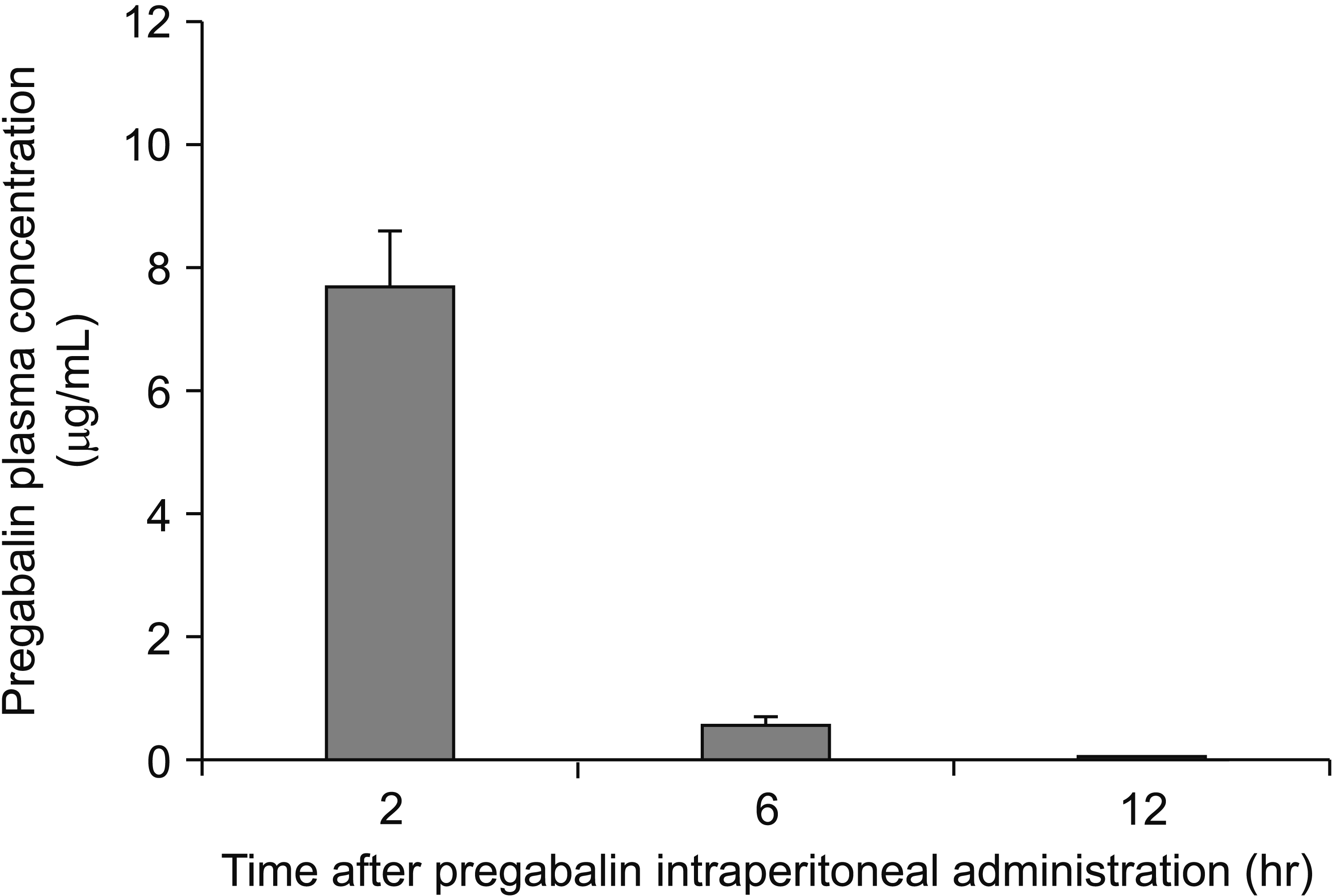
8. Measurement of the plasma concentration of PGB
To determine whether the remaining plasma PGB that was not metabolized affected immune response (
i.e., NK cytotoxicity and lymphocyte proliferation), plasma concentrations of PGB were measured in 3 separate groups of mice (n = 6). Mice were subjected to intraperitoneal injection of 30 mg/kg PGB, anesthesia, plantar incision, and a second injection of PGB 90 minutes after the first, as described above (
Fig. 1). Plasma samples were obtained from the orbital sinus of each mouse 2, 6, or 12 hours (depending on the assigned group) after the first PGB injection and placed in a tube with sodium heparin. Each sample was centrifuged and the plasma was harvested and stored at –80°C until the analysis was performed. Plasma PGB concentrations were measured by validated high-performance liquid chromatography/mass spectrometry (HPLC; 1260 solvent delivery system/6460 tandem mass spectrometer, Agilent, Santa Clara, CA).
9. Statistical analysis
Data are reported as means ± standard deviation. All results were analyzed using Sigma-Stat version 3.1 (SPSS Inc., Chicago, IL). Two-way repeated-measures analysis of variance (ANOVA; two-factor repletion) with pairwise multiple-comparisons (Holm-Sidak method) was used to analyze the results of the behavioral experiment. Two-way ANOVA and Bonferroni t-tests were performed to evaluate differences in NK cytotoxicity. One-way ANOVA and multiple comparison tests (Bonferroni t-tests) were used to evaluate differences in the splenocyte proliferative response of the different groups. The Kruskal–Wallis one-way ANOVA by ranks test was applied to compare the serum PGB concentrations at different time points. A value of P < 0.05 was taken to indicate statistical significance.
Go to :

DISCUSSION
The present study showed that immediately after plantar incision, mechanical allodynia developed. This response was maintained until the termination of the experiment 12 hours after the incision was made. The incision increased NK cell activity and splenocyte proliferation. Perioperative PGB treatment attenuated this incision-induced NK cell activation to some degree. However, it did not influence the incision-induced splenocyte proliferation at all.
PGB is increasingly being seen as a useful adjunct in the multimodal management of postoperative pain. This is supported by the study of Field et al. [
9], who showed, with a rat model of post-incisional pain, that preoperative administration of PGB may reduce the tissue damage-induced hyperexcitability of the dorsal horn neurons, thereby reducing both acute postoperative pain and the development of postoperative chronic pain. Moreover, a meta-analysis of clinical trials showed that perioperative PGB administration effectively reduces postoperative opioid consumption, especially after major surgery that induces severe pain [
10,
13,
14]. Notably, it has been reported that depending on the type of pain, the analgesic dose of PGB varies from 10 to > 200 mg/kg [
15]. Behavior observation studies in animals may be affected by sedation or decreased motor function caused by the administered drug, but previous studies have shown that PGB did not cause severe sedation or gait abnormalities at the analgesic dose [
16].
In our study, 30 mg/kg PGB was administered twice (30 min before surgery and 1 hr after surgery); we observed that the pain of the mice was effectively blocked despite the fact that the pain stimulus was ongoing. PGB-treated mice did not exhibit severe sedation. Moreover, during our preliminary experiment, we observed that the PWT of the PGB-control group was not different from the PWT of the control group. These results imply that the therapeutic dose of PGB in mice does not inhibit PWT in the painless state, which is consistent with a previous study by Takeuchi et al. [
17].
Several studies have shown that severe or chronic pain in the postoperative period can have both immunostimulatory and immunosuppressive effects. Sacerdote et al. [
18] showed that abdominal surgery for uterine cancer suppresses mitogen-induced T cell proliferation. Sharify et al. [
19] found that mice given heat shocks exhibited upregulation of NK cell activity and splenocyte proliferation. Similarly, our previous study showed that when mice were subjected to chronic constriction injury of the sciatic nerve, their NK cell activity and splenocyte proliferation both rose [
7]. Our present study observed similar effects from pain: we observed that plantar incision in mice significantly upregulated NK cell cytotoxic activity and mitogen-induced splenocyte proliferation. These findings probably reflect the fact that the immune and nervous systems are known to interact [
20,
21]. These immunomodulatory effects of pain can be harmful, especially if the pain is prolonged and severe, because it could limit the ability of the patient to resist infection [
22]. Consequently, it is important to properly manage severe postoperative pain.
However, this approach also has its pitfalls because some analgesic drugs can themselves significantly alter immune responses: several studies in humans and animals show that opiates such as morphine suppress NK cell activity, T cell responses in delayed type hypersensitivity, cytotoxic T cell activity, T cell proliferation to mitogens, phagocytosis, and chemotaxis [
18,
20,
23]. Moreover, in the absence of pain, mice treated with tramadol show upregulation of NK cell cytotoxicity and mitogen-induced splenocyte proliferation [
6], while Lewis rats treated with morphine exhibit marked downregulation of these responses [
24]. Notably, however, our present and previous [
7] studies both showed that PGB treatment had no effect on the NK cell cytotoxicity or mitogen-induced splenocyte proliferation of control (non-incised) mice. These discrepancies may reflect the varying effects of different analgesics.
Several studies show that analgesics can also alter the immune changes induced by pain [
4-
6]. In particular, our previous study showed that when mice with chronic constriction of the sciatic nerve were given twice-daily postoperative 30 mg/kg PGB treatment starting 2 days after surgery, their pain-upregulated NK cell activity and splenocyte proliferation 7 days after surgery were both significantly depressed [
7]. Our present observations in our incision model differ somewhat: while pain increased both of these immune responses and PGB largely (but not completely) reversed the effect of pain on NK cell activity, PGB treatment had no effect on mitogen-induced splenocyte proliferation 12 hours after surgery. These differences may reflect the fact that our present and previous [
7] studies employed different pain models and that the immune responses were observed at different times (12 hr and 7 days after surgery, respectively).
These observations led us to hypothesize that PGB has a positive immunomodulatory effect, namely, it significantly suppresses the immunostimulatory effects of pain on NK cell activity and, depending on the type of pain, lymphocyte proliferation. This is important because patients with chronic neuropathic pain often require long-term use of PGB, and PGB is commonly prescribed as a supplement to opioids, which have immunosuppressive effects [
10]: it is possible that PGB may ameliorate the pain-induced alterations in immune functions, although this requires further research. These findings together also suggest that the immunomodulatory effects of pain and analgesics depend on the nature and duration of the stressor that is applied, the immunological variables that are being investigated, and the analgesic that is used [
19].
To determine whether the immune responses we observed 12 hours after PGB injection was directly affected by the PGB in the plasma, we examined the changes in PGB plasma concentrations 2, 6, and 12 hours after the intraperitoneal injection of this analgesic. The blood PGB concentration was close to zero at the 12 hours timepoint. Moreover, our previous
in vitro study showed that 3 µg/mL PGB does not decrease NK cell cytotoxic activity [
7]. Thus, it seems that the immunomodulatory effects of PGB on NK cell activity are not a direct consequence of the drug remaining in plasma; rather, it may be a pharmacodynamic consequence of the analgesic effect of the drug during the early postoperative period.
How pain activates NK cell functions is not clear. However, because lymphocytes express variable hormone receptors, it has been suggested that hormones mediate stress-induced changes in immune function. In particular, catecholamines seem to play an important role in the endocrine-neural-immune network [
25,
26]. Interestingly, a study by Takeuchi et al. [
17] showed that intra-cisternal administration of catecholaminergic neurotoxin 6-hydroxydopamine hydrobromide to decrease the central noradrenalin attenuated the analgesic effect of PGB. How PGB reverses the upregulatory effect of pain on NK cell activity is unclear. However, it is thought that the analgesic effects of PGB involve a supraspinal mechanism that affects the locus coeruleus neurons that associate with the descending noradrenergic inhibitory system and spinal adrenergic receptors [
17,
27].
Thus, this supraspinal mechanism may also mediate the immunomodulatory effect of PGB. Our study provides the framework for further investigations on the immunomodulatory effects of PGB. Future studies will aim to identify the multiple factors that affect immune responses to pain and PGB. These studies will include other types of pain, its effects on other immune responses, and other PGB treatment regimens. How PGB interacts with other analgesics in terms of its immunomodulatory effects should be assessed. In summary, foot incision in mice not only generated mechanical allodynia, it also increased NK cell cytotoxic activity and lymphocyte proliferation. Perioperative PGB treatment largely prevented the pain-induced increase in NK cell activity. However, these effects of PGB on NK cell activity were not evident in the absence of incisional pain.
In conclusion, this study suggests that PGB may be able to modulate the immune response that accompanies tissue injury and postoperative pain. As a drug used to treat chronic pain patients, these immunomodulatory effects are of great clinical importance and deserve further study.
Go to :






 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download