3. Li C, Yan Y, Cheng J, Xiao G, Gu J, Zhang L, et al. 2016; Toll-like receptor 4 deficiency causes reduced exploratory behavior in mice under approach-avoidance conflict. Neurosci Bull. 32:127–36. DOI:
10.1007/s12264-016-0015-z. PMID:
26898297. PMCID:
PMC5563737.

4. Oh SH, Lee HY, Ki YJ, Kim SH, Lim KJ, Jung KT. 2020; Gabexate mesilate ameliorates the neuropathic pain in a rat model by inhibition of proinflammatory cytokines and nitric oxide pathway via suppression of nuclear factor-κB. Korean J Pain. 33:30–9. DOI:
10.3344/kjp.2020.33.1.30. PMID:
31888315. PMCID:
PMC6944363.

5. Huang L, Chen C, Zhang X, Li X, Chen Z, Yang C, et al. 2018; Neuroprotective effect of curcumin against cerebral ischemia-reperfusion via mediating autophagy and inflammation. J Mol Neurosci. 64:129–39. DOI:
10.1007/s12031-017-1006-x. PMID:
29243061.

6. Xing F, Zhang W, Wen J, Bai L, Gu H, Li Z, et al. 2018; TLR4/NF-κB signaling activation in plantar tissue and dorsal root ganglion involves in the development of postoperative pain. Mol Pain. 14:1744806918807050. DOI:
10.1177/1744806918807050. PMID:
30270727. PMCID:
PMC6196615.

7. Zhang H, Li Y, de Carvalho-Barbosa M, Kavelaars A, Heijnen CJ, Albrecht PJ, et al. 2016; Dorsal root ganglion infiltration by macrophages contributes to paclitaxel chemotherapy-induced peripheral neuropathy. J Pain. 17:775–86. DOI:
10.1016/j.jpain.2016.02.011. PMID:
26979998. PMCID:
PMC4939513.
8. Liu P, Yuan HB, Zhao S, Liu FF, Jiang YQ, Guo YX, et al. 2018; Activation of GABAB receptor suppresses diabetic neuropathic pain through toll-like receptor 4 signaling pathway in the spinal dorsal horn. Mediators Inflamm. 2018:6016272. DOI:
10.1155/2018/6016272. PMID:
30647535. PMCID:
PMC6311757.

9. Tanga FY, Nutile-McMenemy N, DeLeo JA. 2005; The CNS role of Toll-like receptor 4 in innate neuroimmunity and painful neuropathy. Proc Natl Acad Sci U S A. 102:5856–61. DOI:
10.1073/pnas.0501634102. PMID:
15809417. PMCID:
PMC556308.

13. Moser VA, Uchoa MF, Pike CJ. 2018; TLR4 inhibitor TAK-242 attenuates the adverse neural effects of diet-induced obesity. J Neuroinflammation. 15:306. DOI:
10.1186/s12974-018-1340-0. PMID:
30396359. PMCID:
PMC6217784.

14. Yang J, Xie MX, Hu L, Wang XF, Mai JZ, Li YY, et al. 2018; Upregulation of N-type calcium channels in the soma of uninjured dorsal root ganglion neurons contributes to neuropathic pain by increasing neuronal excitability following peripheral nerve injury. Brain Behav Immun. 71:52–65. DOI:
10.1016/j.bbi.2018.04.016. PMID:
29709527.

15. Zang Y, He XH, Xin WJ, Pang RP, Wei XH, Zhou LJ, et al. 2010; Inhibition of NF-kappaB prevents mechanical allodynia induced by spinal ventral root transection and suppresses the re-expression of Nav1.3 in DRG neurons in vivo and in vitro. Brain Res. 1363:151–8. DOI:
10.1016/j.brainres.2010.09.048. PMID:
20858468.

16. Huang Y, Zang Y, Zhou L, Gui W, Liu X, Zhong Y. 2014; The role of TNF-alpha/NF-kappa B pathway on the up-regulation of voltage-gated sodium channel Nav1.7 in DRG neurons of rats with diabetic neuropathy. Neurochem Int. 75:112–9. DOI:
10.1016/j.neuint.2014.05.012. PMID:
24893330.

17. Huang Y, Li Y, Zhong X, Hu Y, Liu P, Zhao Y, et al. 2017; Src-family kinases activation in spinal microglia contributes to central sensitization and chronic pain after lumbar disc herniation. Mol Pain. 13:1744806917733637. DOI:
10.1177/1744806917733637. PMID:
28952414. PMCID:
PMC5624351.

18. Kaneuchi Y, Sekiguchi M, Kameda T, Kobayashi Y, Konno SI. 2019; Temporal and spatial changes of μ-opioid receptors in the brain, spinal cord and dorsal root ganglion in a rat lumbar disc herniation model. Spine (Phila Pa 1976). 44:85–95. DOI:
10.1097/BRS.0000000000002776. PMID:
30005035.

19. Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. 1994; Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 53:55–63. DOI:
10.1016/0165-0270(94)90144-9. PMID:
7990513.

20. Hargreaves K, Dubner R, Brown F, Flores C, Joris J. 1988; A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 32:77–88. DOI:
10.1016/0304-3959(88)90026-7. PMID:
3340425.

22. Gadjradj PS, Arts MP, van Tulder MW, Rietdijk WJR, Peul WC, Harhangi BS. 2017; Management of symptomatic lumbar disk herniation: an international perspective. Spine (Phila Pa 1976). 42:1826–34. DOI:
10.1097/BRS.0000000000002294. PMID:
28632645.
23. Andrade P, Hoogland G, Garcia MA, Steinbusch HW, Daemen MA, Visser-Vandewalle V. 2013; Elevated IL-1β and IL-6 levels in lumbar herniated discs in patients with sciatic pain. Eur Spine J. 22:714–20. DOI:
10.1007/s00586-012-2502-x. PMID:
23014739. PMCID:
PMC3631043.

24. Zhong Y, Huang Y, Hu Y, Xu M, Zhu L, Deng Z. 2019; SFKs/p38 pathway is involved in radicular pain by promoting spinal expression of pro-inflammatory cytokines in a rat model of lumbar disc herniation. Spine (Phila Pa 1976). 44:E1112–21. DOI:
10.1097/BRS.0000000000003076. PMID:
31261268.

25. Albrecht DS, Ahmed SU, Kettner NW, Borra RJH, Cohen-Adad J, Deng H, et al. 2018; Neuroinflammation of the spinal cord and nerve roots in chronic radicular pain patients. Pain. 159:968–77. DOI:
10.1097/j.pain.0000000000001171. PMID:
29419657. PMCID:
PMC5908728.

26. Yousefi N, Sotoodehnejadnematalahi F, Heshmati-Fakhr N, Sayyah M, Hoseini M, Ghassemi S, et al. 2019; Prestimulation of microglia through TLR4 pathway promotes interferon beta expression in a rat model of Alzheimer's disease. J Mol Neurosci. 67:495–503. DOI:
10.1007/s12031-018-1249-1. PMID:
30610591.

27. Xiang HF, Cao DH, Yang YQ, Wang HQ, Zhu LJ, Ruan BH, et al. 2014; Isoflurane protects against injury caused by deprivation of oxygen and glucose in microglia through regulation of the Toll-like receptor 4 pathway. J Mol Neurosci. 54:664–70. DOI:
10.1007/s12031-014-0373-9. PMID:
25012594.

28. Zusso M, Lunardi V, Franceschini D, Pagetta A, Lo R, Stifani S, et al. 2019; Ciprofloxacin and levofloxacin attenuate microglia inflammatory response via TLR4/NF-kB pathway. J Neuroinflammation. 16:148. DOI:
10.1186/s12974-019-1538-9. PMID:
31319868. PMCID:
PMC6637517.

29. Kouli A, Horne CB, Williams-Gray CH. 2019; Toll-like receptors and their therapeutic potential in Parkinson's disease and α-synucleinopathies. Brain Behav Immun. 81:41–51. DOI:
10.1016/j.bbi.2019.06.042. PMID:
31271873.

30. Azam S, Jakaria M, Kim IS, Kim J, Haque ME, Choi DK. 2019; Regulation of toll-like receptor (TLR) signaling pathway by polyphenols in the treatment of age-linked neurodegenerative diseases: focus on TLR4 signaling. Front Immunol. 10:1000. DOI:
10.3389/fimmu.2019.01000. PMID:
31134076. PMCID:
PMC6522942.

31. Balducci C, Frasca A, Zotti M, La Vitola P, Mhillaj E, Grigoli E, et al. 2017; Toll-like receptor 4-dependent glial cell activation mediates the impairment in memory establishment induced by β-amyloid oligomers in an acute mouse model of Alzheimer's disease. Brain Behav Immun. 60:188–97. DOI:
10.1016/j.bbi.2016.10.012. PMID:
27751869.

32. Wang Y, Xu J, Liu Y, Li Z, Li X. 2018; TLR4-NF-κB signal involved in depressive-like behaviors and cytokine expression of frontal cortex and hippocampus in stressed C57BL/6 and ob/ob mice. Neural Plast. 2018:7254016. DOI:
10.1155/2018/7254016. PMID:
29765402. PMCID:
PMC5885403.

33. Woller SA, Ravula SB, Tucci FC, Beaton G, Corr M, Isseroff RR, et al. 2016; Systemic TAK-242 prevents intrathecal LPS evoked hyperalgesia in male, but not female mice and prevents delayed allodynia following intraplantar formalin in both male and female mice: the role of TLR4 in the evolution of a persistent pain state. Brain Behav Immun. 56:271–80. DOI:
10.1016/j.bbi.2016.03.026. PMID:
27044335. PMCID:
PMC4917460.

34. Su M, Ran Y, He Z, Zhang M, Hu G, Tang W, et al. 2018; Inhibition of toll-like receptor 4 alleviates hyperalgesia induced by acute dural inflammation in experimental migraine. Mol Pain. 14:1744806918754612. DOI:
10.1177/1744806918754612. PMID:
29310498. PMCID:
PMC5805005.
36. Cho HK, Ahn SH, Kim SY, Choi MJ, Hwang SJ, Cho YW. 2015; Changes in the expressions of Iba1 and calcitonin gene-related peptide in adjacent lumbar spinal segments after lumbar disc herniation in a rat model. J Korean Med Sci. 30:1902–10. DOI:
10.3346/jkms.2015.30.12.1902. PMID:
26713069. PMCID:
PMC4689838.

37. Zhong Y, Huang YL, Hu YM, Zhu LR, Zhao YS. 2018; Puerarin alleviate radicular pain from lumbar disc herniation by inhibiting ERK-dependent spinal microglia activation. Neuropeptides. 72:30–7. DOI:
10.1016/j.npep.2018.10.001. PMID:
30466510.

38. Saghaei E, Abbaszadeh F, Naseri K, Ghorbanpoor S, Afhami M, Haeri A, et al. 2013; Estradiol attenuates spinal cord injury-induced pain by suppressing microglial activation in thalamic VPL nuclei of rats. Neurosci Res. 75:316–23. DOI:
10.1016/j.neures.2013.01.010. PMID:
23419864.

39. Gruber-Schoffnegger D, Drdla-Schutting R, Hönigsperger C, Wunderbaldinger G, Gassner M, Sandkühler J. 2013; Induction of thermal hyperalgesia and synaptic long-term potentiation in the spinal cord lamina I by TNF-α and IL-1β is mediated by glial cells. J Neurosci. 33:6540–51. DOI:
10.1523/JNEUROSCI.5087-12.2013. PMID:
23575851. PMCID:
PMC6619063.

40. Chirila AM, Brown TE, Bishop RA, Bellono NW, Pucci FG, Kauer JA. 2014; Long-term potentiation of glycinergic synapses triggered by interleukin 1β. Proc Natl Acad Sci U S A. 111:8263–8. DOI:
10.1073/pnas.1401013111. PMID:
24830427. PMCID:
PMC4050559.

41. Zhong Y, Zhou LJ, Ren WJ, Xin WJ, Li YY, Zhang T, et al. 2010; The direction of synaptic plasticity mediated by C-fibers in spinal dorsal horn is decided by Src-family kinases in microglia: the role of tumor necrosis factor-alpha. Brain Behav Immun. 24:874–80. DOI:
10.1016/j.bbi.2010.01.007. PMID:
20116424.
42. Shen KF, Zhu HQ, Wei XH, Wang J, Li YY, Pang RP, et al. 2013; Interleukin-10 down-regulates voltage gated sodium channels in rat dorsal root ganglion neurons. Exp Neurol. 247:466–75. DOI:
10.1016/j.expneurol.2013.01.018. PMID:
23357618.

43. Wang K, Bao JP, Yang S, Hong X, Liu L, Xie XH, et al. 2016; A cohort study comparing the serum levels of pro- or anti-inflammatory cytokines in patients with lumbar radicular pain and healthy subjects. Eur Spine J. 25:1428–34. DOI:
10.1007/s00586-015-4349-4. PMID:
26684469.

44. Kraychete DC, Sakata RK, Issy AM, Bacellar O, Santos-Jesus R, Carvalho EM. 2010; Serum cytokine levels in patients with chronic low back pain due to herniated disc: analytical cross-sectional study. Sao Paulo Med J. 128:259–62. DOI:
10.1590/S1516-31802010000500003. PMID:
21181064.

45. Pedersen LM, Schistad E, Jacobsen LM, Røe C, Gjerstad J. 2015; Serum levels of the pro-inflammatory interleukins 6 (IL-6) and -8 (IL-8) in patients with lumbar radicular pain due to disc herniation: a 12-month prospective study. Brain Behav Immun. 46:132–6. DOI:
10.1016/j.bbi.2015.01.008. PMID:
25653193.

46. Liu ZH, Miao GS, Wang JN, Yang CX, Fu ZJ, Sun T. 2016; Resolvin D1 inhibits mechanical hypersensitivity in sciatica by modulating the expression of nuclear factor-κB, phospho-extracellular signal-regulated kinase, and pro- and antiinflammatory cytokines in the spinal cord and dorsal root ganglion. Anesthesiology. 124:934–44. DOI:
10.1097/ALN.0000000000001010. PMID:
26808633.

47. Sasaki N, Kikuchi S, Konno S, Sekiguchi M, Watanabe K. 2007; Anti-TNF-alpha antibody reduces pain-behavioral changes induced by epidural application of nucleus pulposus in a rat model depending on the timing of administration. Spine (Phila Pa 1976). 32:413–6. DOI:
10.1097/01.brs.0000255097.18246.bc. PMID:
17304130.
48. Olmarker K, Nutu M, Størkson R. 2003; Changes in spontaneous behavior in rats exposed to experimental disc herniation are blocked by selective TNF-alpha inhibition. Spine (Phila Pa 1976). 28:1635–41. DOI:
10.1097/01.BRS.0000083162.35476.FF. PMID:
12897484.

49. Rothman SM, Winkelstein BA. 2010; Cytokine antagonism reduces pain and modulates spinal astrocytic reactivity after cervical nerve root compression. Ann Biomed Eng. 38:2563–76. DOI:
10.1007/s10439-010-0012-8. PMID:
20309734.

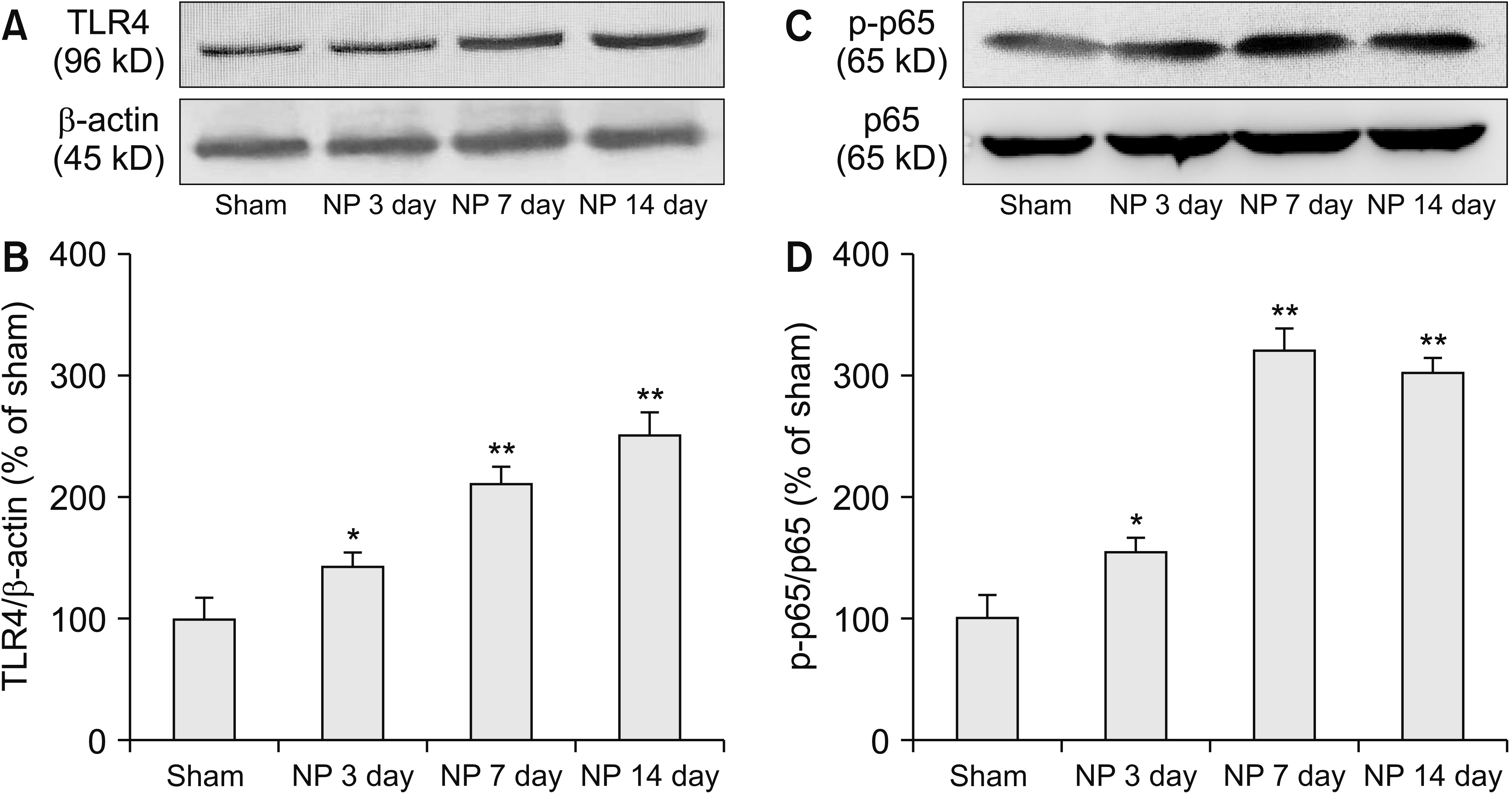





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download