INTRODUCTION
MAIN BODY
1. VGCCs
Table 1
Adapted from Dolphin AC. Voltage-gated calcium channel α2δ subunits: an assessment of proposed novel roles. F1000Res 2018; 7: F1000 Faculty Rev-1830 [7]. Adapted from Dolphin AC. Calcium channel auxiliary α2δ and β subunits: trafficking and one step beyond. Nat Rev Neurosci 2012; 13: 542-55 [8] with original copyright holder's permission. Adapted from Burnashev N. Calcium permeability of ligandgated channels. Cell Calcium 1998; 24: 325-32 [9] with original copyright holder's permission.
1) Main pore-forming α1 subunits of VGCCs
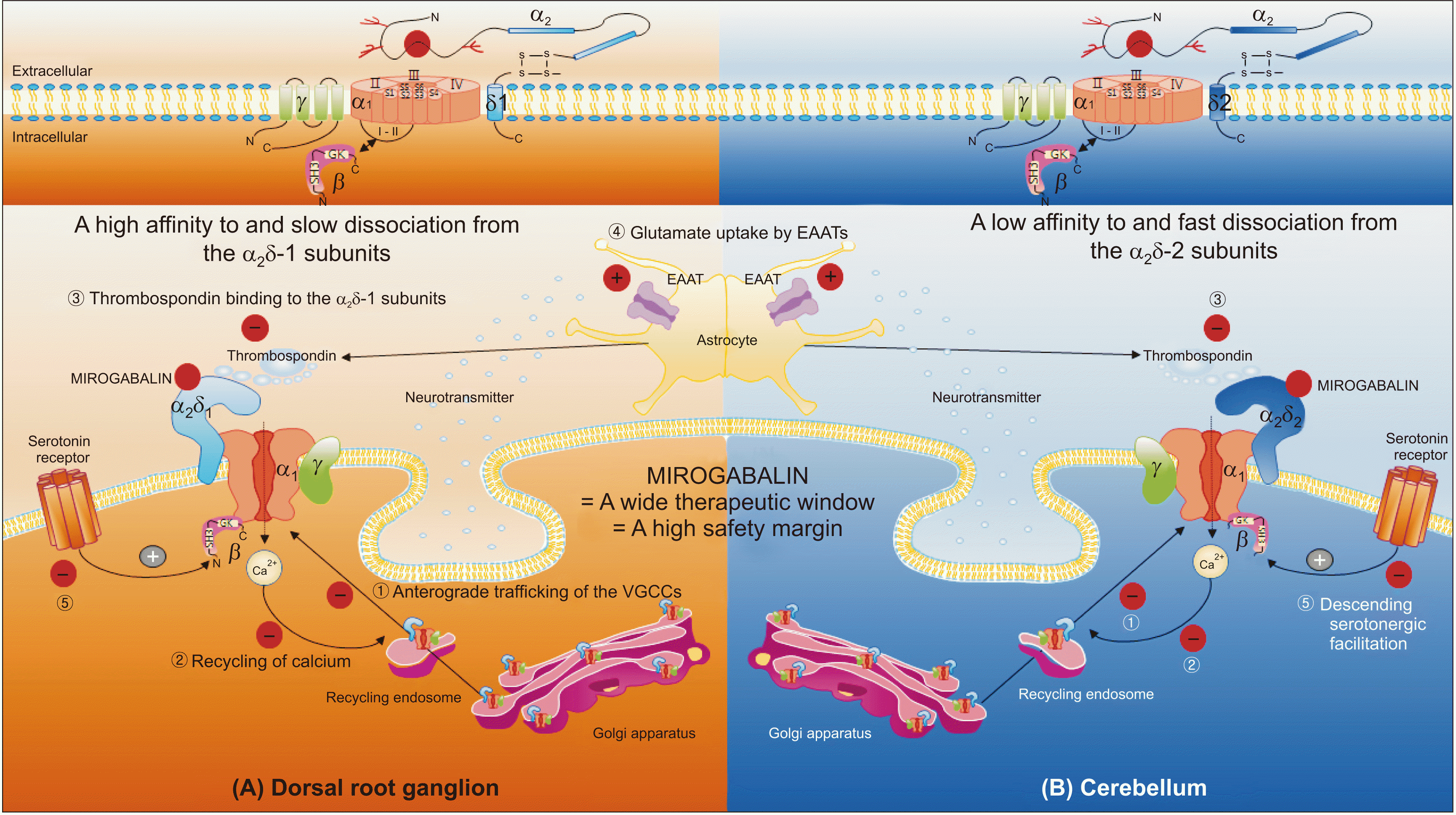 | Fig. 1A schematic illustration of the mechanisms of action for mirogabalin. Mirogabalin has a high affinity to and slow dissociation from the α2δ-1 subunits in the dorsal root ganglia (DRGs), producing greater therapeutic effects; it also has a low affinity to and fast dissociation from the α2δ-2 subunits in the cerebellum, producing lesser adverse drug reactions. In general, various mechanisms of action for gabapentinoids are suggested from inside the cell, membrane, and synapse. (1) Gabapentinoids inhibit forward (anterograde) trafficking of voltage-gated calcium channels (VGCCs) (from the endoplasmic reticulum through the Golgi complex to the cell membrane) intracellularly. Inhibition of anterograde trafficking reduces VGCCs, calcium entry, and excitatory amino acids (glutamates). (2) They inhibit the Rab-11-dependent final recycling of endosomal VGCCs intracellularly, resulting in reduced excitatory neurotransmitter release in the synapse. The small guanosine triphosphatases (GTPases, Rab) are the main regulators of intracellular membrane trafficking, from formation of transport vesicles to their fusion into the membranes. Reduced recycling of endosomal VGCCs results in reduced transmembrane VGCCs, calcium entry into the cell, and glutamate in the synapse. (3) They inhibit astrocyte-derived thrombospondin (TSP, extracellular matrix protein)-mediated excitatory synapse formation extracellularly (reduction of excitatory synaptogenesis). (4) They stimulate glutamate uptake by excitatory amino acid transporters (EAATs) extracellularly. (5) Gabapentinoids may show inhibition of descending serotonergic facilitation, stimulation of descending inhibition, anti-inflammatory effect, and influence on the affective component of pain. A magnified illustration for the transmembrane VGCC is shown in the upper left (in the DRGs) and right (in the cerebellum) quadrants. Adapted from Chincholkar M. Analgesic mechanisms of gabapentinoids and effects in experimental pain models: a narrative review. Br J Anaesth 2018; 120: 1315-34 [14]. |
2) Auxiliary α2, β, and δ subunits for modulating α1 subunits of VGCCs
(1) β subunits
(2) α2δ subunits
2. Action mechanisms of mirogabalin
1) Conventional gabapentinoids
2) Comparison of binding and dissociation kinetics of gabapentin, pregabalin, and mirogabalin for the α2δ-1 and α2δ-2 subunits
3. Review articles, clinical trials, case reports, and animal studies
Table 2
| Classification | Author | Title | Journal | Contents |
|---|---|---|---|---|
| Review articles (8) | Deeks | Mirogabalin: first global approval. | Drugs 2019; 79: 463-8. [2] | Mirogabalin is suitable for the treatment of peripheral neuropathic pain, including DPNP and PHN. |
| Erratum | Drugs 2019; 79: 469. [3] | |||
| Javed et al. | Mirogabalin and emerging therapies for diabetic neuropathy. | J Pain Res 2018; 11: 1559-66. [19] | Mirogabalin is expected to become the 4th US FDA-approved therapy for DPNP, following pregabalin, duloxetine, and tapentadol. | |
| Calandre et al. |
Alpha2delta ligands, gabapentin, pregabalin and mirogabalin: a review of their clinical pharmacology and therapeutic use. Erratum |
Expert Rev Neurother 2016; 16: 1263-77. [21] | Mirogabalin shows a promising result for DPNP with ADRs, including fatigue, dizziness, sedation, somnolence, ataxia, weight gain, and edema. | |
| Domon et al. | Binding characteristics and analgesic effects of mirogabalin, a novel ligand for the α2δ subunit of voltage-gated calcium channels. | J Pharmacol Exp Ther 2018; 365: 573-82. [6] | Mirogabalin shows potent and selective binding affinities for human and rat α2δ subunits, and slower dissociation rates for the α2δ-1 subunit than the α2δ-2 subunit. It shows potent and long-lasting analgesic effects in rat models of neuropathic pain, and wider safety margins for side effects in the central nervous system, due to its unique binding characteristics. | |
| Burgess et al. | Mirogabalin besylate in the treatment of neuropathic pain. | Drugs Today (Barc) 2020; 56: 135-49. [23] | Mirogabalin has a potent pain-modulating effect with a uniquely high affinity and prolonged dissociation rate for the α2δ-1 subunit of voltage-gated calcium channels on the dorsal root ganglion. | |
| Merante | The mirogabalin ALDAY phase 3 program in pain associated with fibromyalgia: the lessons learned. | Curr Med Res Opin 2020; 36: 661-6. [22] | The negative outcome of the mirogabalin ALDAY phase 3 clinical program resulted from the absence of a selection criteria for FM, a lack of regulatory study guidance, and a need for previous a dose ranging study in FM. | |
| Alcántara Montero et al. | Emerging therapies in clinical development and new contributions for neuropathic pain. | Rev Esp Anestesiol Reanim 2019; 66: 324-34. [18] | This review focuses on new pharmacological treatments for neuropathic pain for which clinical data are already available, including older and known drugs with new data on their anti-neuropathic activity. | |
| Alyoubi et al. | Efficacy and safety of mirogabalin treatment in patients with diabetic peripheral neuropathic pain: a systematic review and meta-analysis of randomised controlled trials. | Int J Clin Pract 2020. doi: 10.1111/ijcp.13744. [20] | In patients with DPNP, mirogabalin treatment was superior to a placebo and pregabalin in decreasing the average daily pain score over time. Besides, mirogabalin was largely safe and associated with some manageable adverse events. | |
| Clinical Trials (22) | Baba et al. | Mirogabalin in Japanese patients with renal impairment and pain associated with diabetic peripheral neuropathy or post-herpetic neuralgia: a phase III, open-label, 14-week study. | J Pain Res 2020; 13: 1811-21. [37] | Mirogabalin was well tolerated and significantly reduced pain levels when used to treat DPNP/PHN at a fixed dose of 7.5 mg once or twice daily in 35 patients with renal impairment. |
| Kato et al. | Long-term safety and efficacy of mirogabalin in Asian patients with postherpetic neuralgia: results from an open-label extension of a multicenter randomized, double-blind, placebo-controlled trial. | Medicine (Baltimore) 2020; 99: e21976. [26] | A flexible dose of 10 or 15 mg of mirogabalin twice a day was shown to be effective and well tolerated for treating PHN in an Asian multicenter, randomized, double-blind, placebo-controlled, 14-week study. | |
| Kim et al. | Therapeutic effect of mirogabalin on peripheral neuropathic pain due to lumbar spine disease. | Asian Spine J 2020. doi: 10.31616/asj.2020.0136. [36] | Mirogabalin (maximal dose: 30 mg/d) improved leg symptoms, low back pain, and sleep disturbance in patients with lumbar spine disease. | |
| Dow et al. | Effect of coadministration of metformin with mirogabalin: results from a phase 1, randomized, open-label, drug-drug interaction study. | Int J Clin Pharmacol Ther 2018; 56: 451-8. [41] | Coadministration of mirogabalin 15 mg and metformin 850 mg is well tolerated in healthy subjects with no evidence of a drug-drug interaction. | |
| Baba et al. | Mirogabalin for the treatment of diabetic peripheral neuropathic pain: a randomized, double-blind, placebo-controlled phase III study in Asian patients. | J Diabetes Investig 2019; 10: 1299-306. [29] | Mirogabalin 30 mg/d showed significant pain relief (vs. placebo) in Asian DPNP patients. All doses of mirogabalin tested were well tolerated. | |
| Kato et al. | Mirogabalin for the management of postherpetic neuralgia: a randomized, double-blind, placebo-controlled phase 3 study in Asian patients. | Pain 2019; 160: 1175-85. [27] | Mirogabalin was superior to a placebo in all groups (15, 20, and 30 mg/d) for relieving PHN. | |
| Erratum | Pain 2019; 160: 1905. | |||
| Mendell et al. | Abuse potential of mirogabalin in recreational polydrug users. | Ther Adv Drug Saf 2019; 10: 2042098619836032. [45] | Therapeutic doses of mirogabalin (30 mg/d) demonstrated limited evidence of abuse potential, which was lower than for diazepam and pregabalin. | |
| Arnold et al. | Efficacy and safety of mirogabalin for the treatment of fibromyalgia: results from three 13-week randomized, double-blind, placebo- and active-controlled, parallel-group studies and a 52-week open-label extension study. | Curr Med Res Opin 2019; 35: 1825-35. [35] | Both mirogabalin 15 mg once daily and mirogabalin 15 mg twice daily did not improve in weekly average daily worst pain score at week 13 in patients with FM. | |
| Vinik et al. | Efficacy and safety of mirogabalin (DS-5565) for the treatment of diabetic peripheral neuropathic pain: a randomized, double-blind, placebo- and active comparator-controlled, adaptive proof-of-concept phase 2 study. | Diabetes Care 2014; 37: 3253-61. [30] | Mirogabalin 15, 20, and 30 mg/day had significant reductions the weekly change in average daily pain score in patients with DPNP. | |
| Brown et al. | Tolerability, pharmacokinetics, and pharmacodynamics of mirogabalin in healthy subjects: results from phase 1 studies. | Pharmacol Res Perspect 2018; 6: e00418. [44] | There was no difference of bioavailability between in fed and fasted states after single doses of mirogabalin 15 mg. Therefore, mirogabalin can be taken without food restrictions. | |
| Merante et al. | Efficacy of mirogabalin (DS-5565) on patient-reported pain and sleep interference in patients with diabetic neuropathic pain: secondary outcomes of a phase II proof-of-concept study. | Pain Med 2017; 18: 2198-207. [31] | Mirogabalin from 15 mg/d to 30 mg/d improved pain and sleep interference in DPNP. | |
| Kato et al. | Pharmacokinetics and safety of a single oral dose of mirogabalin in Japanese subjects with varying degrees of renal impairment. | J Clin Pharmacol 2018; 58: 57-63. [38] | A mirogabalin dose adjustment will be needed in Japanese subjects with moderate to severe renal impairment and end-stage renal disease. The most common ADRs were dizziness, somnolence, and vomiting in patients with end-stage renal disease. | |
| Tachibana et al. | Coadministration of probenecid and cimetidine with mirogabalin in healthy subjects: a phase 1, randomized, open-label, drug-drug interaction study. | Br J Clin Pharmacol 2018; 84: 2317-24. [42] | Coadministration of mirogabalin 15 mg and probenecid 500 mg inhibited both renal and metabolic clearance. Coadministration of mirogabalin 15 mg and cimetidine 400 mg inhibited renal clearance only. However, a priori dose adjustment is not recommended. | |
| Jansen et al. | Pharmacokinetics, pharmacodynamics, safety, and tolerability of mirogabalin when coadministered with lorazepam, zolpidem, tramadol, or ethanol: results from drug-drug interaction studies in healthy subjects. | Clin Pharmacol Drug Dev 2018; 7: 597-612. [43] | Peak mirogabalin plasma concentration decreased by 28% following tramadol coadministration, and increased by 20% following ethanol 20% 240 mL for men and 200 mL for women coadministration. Coadministration of mirogabalin 10 mg twice a day with either lorazepam 2 mg or ethanol increased the pharmacodynamic effects. Mirogabalin/lorazepam and mirogabalin 10 mg/zolpidem 10 mg increased incidence of somnolence. Mirogabalin 10 mg/tramadol 100 mg and mirogabalin/ethanol increased incidence of nausea and headache, respectively. | |
| Jansen et al. | A randomized, placebo-controlled, double-blind study of the safety, tolerability, pharmacokinetics, and pharmacodynamics of single and repeated doses of mirogabalin in healthy Asian volunteers. | Clin Pharmacol Drug Dev 2018; 7: 661-9. [24] | Mirogabalin was safe and tolerable in Asian and white subjects at doses up to 15 mg twice a day for 7 days. The most common ADRs were somnolence, headache, and dizziness. | |
| Duchin et al. | Open-label single-dose study to assess the effect of mild and moderate hepatic impairment on the pharmacokinetics of mirogabalin. | Clin Drug Investig 2018; 38: 1001-9. [40] | A single dose of mirogabalin 15 mg did not show effects on its pharmacokinetics in mild-to-moderate hepatic impairment. | |
| Yin et al. | Population pharmacokinetic modeling and simulation for assessing renal impairment effect on the pharmacokinetics of mirogabalin. | J Clin Pharmacol 2016; 56: 203-12. [39] | A dose reduction by 50% or 75% in patients was needed in moderate or severe renal impairment, respectively. However, no dose adjustment seemed necessary for patients with mild renal impairment. | |
| Baba et al. | Results of mirogabalin treatment for diabetic peripheral neuropathic pain in Asian subjects: a phase 2, double-blind, randomized, placebo-controlled, study. | Pain Ther 2020; 9: 261-78. [32] | In Asian patients with DPNP, mirogabalin (5, 10, and 15 mg twice a day) was well tolerated. There was a tendency toward improvement of pain with mirogabalin treatment. | |
| Baba et al. | Long-term safety and efficacy of mirogabalin in Asian patients with diabetic peripheral neuropathic pain. | J Diabetes Investig 2020; 11: 693-8. [33] | It was safe and effective for the Asian patients with DPNP to a long-term flexible dosing regimen of mirogabalin 10 or 15 mg twice a day. | |
| Hutmacher et al. | Exposure-response modeling of average daily pain score, and dizziness and somnolence, for mirogabalin (DS-5565) in patients with diabetic peripheral neuropathic pain. | J Clin Pharmacol 2016; 56: 67-77. [34] | Mirogabalin was known to be 17-fold more potent to pregabalin. It provided an equianalgesic effect to pregabalin 300 mg in patients with DPNP. ADRs were lower in the group of twice-daily regimen compared to in the group of once-daily regimen. | |
| Ye et al. | Cost-effectiveness of mirogabalin for the treatment of post-herpetic neuralgia in Taiwan. | J Med Econ 2020; 23: 529-36. [28] | Mirogabalin 30 mg, a potent and selective α2δ ligand, is a cost-effective treatment option for PHN in Taiwan, with ICERs below the willingness-to-pay threshold. | |
| Tetsunaga et al. | Short-term outcomes of mirogabalin in patients with peripheral neuropathic pain: a retrospective study. | J Orthop Surg Res 2020; 15: 191. [25] | Mirogabalin 5-15 mg twice a day is safe and effective for control peripheral neuropathic pain. | |
| Case reports (2) | Takahashi et al. | A probable case of mirogabalin-induced neutropenia. | Cureus 2020; 12: e10182. [46] | They reported a case of development of neutropenia in a 77-year-old woman with squamous cell carcinoma of the lung, after taking mirogabalin at 10 mg/d for 6 weeks. |
| Matsuda et al. | A case of trigeminal trophic syndrome responding to mirogabalin. | Eur J Dermatol 2020. doi: 10.1684/ejd.2020.3746. [47] | They reported a case of successful treatment of trigeminal trophic syndrome secondary to herpes zoster in an 89-year-old female patient. | |
| Animal research (6) | Kitano et al. | Effects of mirogabalin, a novel ligand for the α₂δ subunit of voltage-gated calcium channels, on N-type calcium channel currents of rat dorsal root ganglion culture neurons. | Pharmazie 2019; 74: 147-9. [49] | Mirogabalin inhibits N-type calcium channel currents in rat DRG culture neurons. Mirogabalin and pregabalin inhibited the calcium channel currents of rat DRG neurons at 50 μM and 200 μM, respectively. |
| Domon et al. | Analgesic effects of the novel α₂δ ligand mirogabalin in a rat model of spinal cord injury. | Pharmazie 2018; 73: 659-61. [50] | Mirogabalin showed potent and long-lasting analgesic effects in a rat model of spinal cord injury, suggesting its effective pain relief for patients with central neuropathic pain. | |
| Murasawa et al. | Anxiolytic effects of the novel α₂δ ligand mirogabalin in a rat model of chronic constriction injury, an experimental model of neuropathic pain. | Psychopharmacology (Berl) 2020; 237: 189-97. [51] | Mirogabalin alleviated both anxiety-related behaviors and tactile allodynia in CCI model rats. It may provide both anxiety and pain relief in patients with neuropathic pain. | |
| Murasawa et al. | Anxiolytic-like effects of mirogabalin, a novel ligand for α₂δ ligand of voltage-gated calcium channels, in rats repeatedly injected with acidic saline intramuscularly, as an experimental model of fibromyalgia. | Pharmacol Rep 2020; 72: 571-9. [52] | Mirogabalin alleviated anxiety-related behaviors in an animal model, suggesting the potential to relieve anxiety in FM patients. | |
| Saeki et al. | Analgesic effects of mirogabalin, a novel ligand for α2δ subunit of voltage-gated calcium channels, in experimental animal models of fibromyalgia. | Naunyn Schmiedebergs Arch Pharmacol 2019; 392: 723-8. [53] | Mirogabalin showed analgesic effects in intermittent cold stress model mice and in Sluka model rats, suggesting the potential to alleviate pain in patients with FM. | |
| Iwai et al. | Mirogabalin prevents repeated restraint stress-induced dysfunction in mice. | Behav Brain Res 2020; 383: 112506. [54] | Mirogabalin prevents anxiety-like behavior and memory impairment induced by repeated restraint stress, related to hippocampal neurons. |
ALDAY: a very large, multicenter program made by three, randomized, double-blind, placebo and active-controlled (pregabalin), 13-week studies, evaluating mirogabalin for the treatment of pain associated with fibromyalgia, aged 18 years or older, DPNP: diabetic peripheral neuropathic pain, PHN: postherpetic neuralgia, US FDA: United States Food and Drug Administration, ADRs: adverse reactions, FM: fibromyalgia, ICER: incremental cost-effectiveness ratio, DRG: dorsal root ganglion, CCI: chronic constriction injury.
1) Review articles
2) Clinical trials
(1) Heathy volunteers
(2) Patients with PHN
(3) Patients with DPNP
(5) Patients with neuropathic pain from lumbar spine disease
(6) Patients with neuropathic pain in renal or hepatic dysfunction
(7) Coadministration with other drugs
(8) Bioavailability to food intake
(9) Abuse potential
3) Case reports
4) Animal research
4. Clinical application of mirogabalin
1) Pharmacokinetics
Table 3
The initial dose for adults without renal dysfunction is recommended starting from 5 mg, given orally twice a day. The dose is gradually increased by 5 mg, at an interval of at least a week, to 15 mg twice a day at the 3rd week [2,3,25-33].
Adapted from Kitano et al. [Pharmacological, pharmacodynamics, and clinical profile of mirogabalin besylate (Tarlige® tablets 2.5 mg ∙ 5 mg ∙ 10 mg ∙ 15 mg)]. Nihon Yakurigaku Zasshi 2019; 154: 352-61. Japanese [55].




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download