2. Badr AM, Attia HA, Al-Rasheed N. 2020; Oleuropein reverses repeated corticosterone-induced depressive-like behavior in mice: evidence of modulating effect on biogenic amines. Sci Rep. 10:3336. DOI:
10.1038/s41598-020-60026-1. PMID:
32094406. PMCID:
PMC7040186.

3. Mohammad-Beigi H, Aliakbari F, Sahin C, Lomax C, Tawfike A, Schafer NP, et al. 2019; Oleuropein derivatives from olive fruit extracts reduce α-synuclein fibrillation and oligomer toxicity. J Biol Chem. 294:4215–32. DOI:
10.1074/jbc.RA118.005723. PMID:
30655291. PMCID:
PMC6422090.

4. Fki I, Sayadi S, Mahmoudi A, Daoued I, Marrekchi R, Ghorbel H. 2020; Comparative study on beneficial effects of hydroxytyrosol- and oleuropein-rich olive leaf extracts on high-fat diet-induced lipid metabolism disturbance and liver injury in rats. Biomed Res Int. 2020:1315202. DOI:
10.1155/2020/1315202. PMID:
31998777. PMCID:
PMC6970490.

5. Czerwińska ME, Gąsińska E, Leśniak A, Krawczyk P, Kiss AK, Naruszewicz M, et al. 2018; Inhibitory effect of Ligustrum vulgare leaf extract on the development of neuropathic pain in a streptozotocin-induced rat model of diabetes. Phytomedicine. 49:75–82. DOI:
10.1016/j.phymed.2018.06.006. PMID:
30217264.

6. Mao X, Xia B, Zheng M, Zhou Z. 2019; Assessment of the anti-inflammatory, analgesic and sedative effects of oleuropein from Olea europaea L. Cell Mol Biol (Noisy-le-grand). 65:52–5. DOI:
10.14715/cmb/2019.65.1.9. PMID:
30782294.

7. Zare L, Esmaeili-Mahani S, Abbasnejad M, Rasoulian B, Sheibani V, Sahraei H, et al. 2012; Oleuropein, chief constituent of olive leaf extract, prevents the development of morphine antinociceptive tolerance through inhibition of morphine-induced L-type calcium channel overexpression. Phytother Res. 26:1731–7. DOI:
10.1002/ptr.4634. PMID:
22422486.

9. Wu D, Hu Q, Zhu D. 2018; An update on hydrogen sulfide and nitric oxide interactions in the cardiovascular system. Oxid Med Cell Longev. 2018:4579140. DOI:
10.1155/2018/4579140. PMID:
30271527. PMCID:
PMC6151216.

10. Sheibani L, Lechuga TJ, Zhang H, Hameed A, Wing DA, Kumar S, et al. 2017; Augmented H
2S production via cystathionine-beta-synthase upregulation plays a role in pregnancy-associated uterine vasodilation. Biol Reprod. 96:664–72. DOI:
10.1095/biolreprod.116.143834. PMID:
28339573. PMCID:
PMC6366540.
11. Lin JQ, Luo HQ, Lin CZ, Chen JZ, Lin XZ. 2014; Sodium hydrosulfide relieves neuropathic pain in chronic constriction injured rats. Evid Based Complement Alternat Med. 2014:514898. DOI:
10.1155/2014/514898. PMID:
25506383. PMCID:
PMC4260443.

12. Chen H, Xie K, Chen Y, Wang Y, Wang Y, Lian N, et al. 2019; Nrf2/HO-1 signaling pathway participated in the protection of hydrogen sulfide on neuropathic pain in rats. Int Immunopharmacol. 75:105746. DOI:
10.1016/j.intimp.2019.105746. PMID:
31325725.

13. Lucarini E, Micheli L, Trallori E, Citi V, Martelli A, Testai L, et al. 2018; Effect of glucoraphanin and sulforaphane against chemotherapy-induced neuropathic pain: Kv7 potassium channels modulation by H2 S release in vivo. Phytother Res. 32:2226–34. DOI:
10.1002/ptr.6159. PMID:
30069944.
15. Suyama H, Kawamoto M, Shiraishi S, Gaus S, Kajiyama S, Yuge O. 2004; Analgesic effect of intrathecal administration of orexin on neuropathic pain in rats. In Vivo. 18:119–23. PMID:
15113038.
16. Toyama S, Shimoyama N, Shimoyama M. 2017; The analgesic effect of orexin-A in a murine model of chemotherapy-induced neuropathic pain. Neuropeptides. 61:95–100. DOI:
10.1016/j.npep.2016.12.007. PMID:
28041630.

17. Kajiyama S, Kawamoto M, Shiraishi S, Gaus S, Matsunaga A, Suyama H, et al. 2005; Spinal orexin-1 receptors mediate anti-hyperalgesic effects of intrathecally-administered orexins in diabetic neuropathic pain model rats. Brain Res. 1044:76–86. DOI:
10.1016/j.brainres.2005.03.007. PMID:
15862792.

19. Yetik-Anacak G, Sevin G, Ozzayım O, Dereli MV, Ahmed A. 2016; Hydrogen sulfide: a novel mechanism for the vascular protection by resveratrol under oxidative stress in mouse aorta. Vascul Pharmacol. 87:76–82. DOI:
10.1016/j.vph.2016.08.003. PMID:
27538867.

20. Wang Z, Yan Y, Wang Y, Tong F. 2019; The interaction between CSE/H
2S and the iNOS/NO-mediated resveratrol/poly(ethylene glycol)-poly(phenylalanine) complex alleviates intestinal ischemia/reperfusion injuries in diabetic rats. Biomed Pharmacother. 112:108736. DOI:
10.1016/j.biopha.2019.108736. PMID:
30970526.
21. Vitalone A, Di Sotto A, Mammola CL, Heyn R, Miglietta S, Mariani P, et al. 2017; Phytochemical analysis and effects on ingestive behaviour of a Caralluma fimbriata extract. Food Chem Toxicol. 108(Pt A):63–73. DOI:
10.1016/j.fct.2017.07.027. PMID:
28713048.

22. Guo C, Bi J, Li X, Lyu J, Liu X, Wu X, et al. 2021; Immunomodulation effects of polyphenols from thinned peach treated by different drying methods on RAW264.7 cells through the NF-κB and Nrf2 pathways. Food Chem. 340:127931. DOI:
10.1016/j.foodchem.2020.127931. PMID:
32871358.

23. Castejon ML, Sánchez-Hidalgo M, Aparicio-Soto M, Montoya T, Martín-LaCave I, Fernández-Bolaños JG, et al. 2019; Dietary oleuropein and its new acyl-derivate attenuate murine lupus nephritis through HO-1/Nrf2 activation and suppressing JAK/STAT, NF-κB, MAPK and NLRP3 inflammasome signaling pathways. J Nutr Biochem. 74:108229. DOI:
10.1016/j.jnutbio.2019.108229. PMID:
31698204.

24. Sanchez-Alavez M, Benedict J, Wills DN, Ehlers CL. 2019; Effect of suvorexant on event-related oscillations and EEG sleep in rats exposed to chronic intermittent ethanol vapor and protracted withdrawal. Sleep. 42:zsz020. DOI:
10.1093/sleep/zsz020. PMID:
30715515. PMCID:
PMC6448295.

25. Janahmadi Z, Nekooeian AA, Moaref AR, Emamghoreishi M. 2017; Oleuropein attenuates the progression of heart failure in rats by antioxidant and antiinflammatory effects. Naunyn Schmiedebergs Arch Pharmacol. 390:245–52. DOI:
10.1007/s00210-016-1323-6. PMID:
27928616.

26. Bennett GJ, Xie YK. 1988; A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 33:87–107. DOI:
10.1016/0304-3959(88)90209-6. PMID:
2837713.

27. Ma L, Liu H, Chen G, Chen M, Wang L, Zhang X, et al. 2018; Sulfasalazine attenuates chronic constriction injury-induced neuroinflammation and mechanical hypersensitivity in rats. Neurosci Lett. 683:174–80. DOI:
10.1016/j.neulet.2018.07.042. PMID:
30075286.

28. Shibayama M, Kuniyoshi K, Suzuki T, Yamauchi K, Ohtori S, Takahashi K. 2014; The effects of locally injected triamcinolone on entrapment neuropathy in a rat chronic constriction injury model. J Hand Surg Am. 39:1714–21. DOI:
10.1016/j.jhsa.2014.05.026. PMID:
25017582.

29. Khangura RK, Bali A, Kaur G, Singh N, Jaggi AS. 2017; Neuropathic pain attenuating effects of perampanel in an experimental model of chronic constriction injury in rats. Biomed Pharmacother. 94:557–63. DOI:
10.1016/j.biopha.2017.07.137. PMID:
28780471.

33. Vashistha B, Sharma A, Jain V. 2017; Ameliorative potential of ferulic acid in vincristine-induced painful neuropathy in rats: an evidence of behavioral and biochemical examination. Nutr Neurosci. 20:60–70. DOI:
10.1179/1476830514Y.0000000165. PMID:
25494651.

34. Choi Y, Yoon YW, Na HS, Kim SH, Chung JM. 1994; Behavioral signs of ongoing pain and cold allodynia in a rat model of neuropathic pain. Pain. 59:369–76. DOI:
10.1016/0304-3959(94)90023-X. PMID:
7708411.

35. Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. 1994; Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 53:55–63. DOI:
10.1016/0165-0270(94)90144-9. PMID:
7990513.

36. Wang G, Yang Y, Wang C, Huang J, Wang X, Liu Y, et al. 2020; Exploring the role and mechanisms of diallyl trisulfide and diallyl disulfide in chronic constriction-induced neuropathic pain in rats. Korean J Pain. 33:216–25. DOI:
10.3344/kjp.2020.33.3.216. PMID:
32606266. PMCID:
PMC7336342.

37. Xie T, Zhang J, Kang Z, Liu F, Lin Z. 2020; miR-101 down-regulates mTOR expression and attenuates neuropathic pain in chronic constriction injury rat models. Neurosci Res. 158:30–6. DOI:
10.1016/j.neures.2019.09.002. PMID:
31526851.

38. Yamamoto S, Suzuki Y, Ono H, Kume K, Ohsawa M. 2016; N- and L-type calcium channels blocker cilnidipine ameliorates neuropathic pain. Eur J Pharmacol. 793:66–75. DOI:
10.1016/j.ejphar.2016.11.001. PMID:
27823932.

39. Shen X, Chakraborty S, Dugas TR, Kevil CG. 2014; Hydrogen sulfide measurement using sulfide dibimane: critical evaluation with electrospray ion trap mass spectrometry. Nitric Oxide. 41:97–104. DOI:
10.1016/j.niox.2014.06.002. PMID:
24932544. PMCID:
PMC4413942.

40. Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. 1951; Protein measurement with the Folin phenol reagent. J Biol Chem. 193:265–75. PMID:
14907713.

41. Kim YH, Choi YJ, Kang MK, Lee EJ, Kim DY, Oh H, et al. 2018; Oleuropein curtails pulmonary inflammation and tissue destruction in models of experimental asthma and emphysema. J Agric Food Chem. 66:7643–54. DOI:
10.1021/acs.jafc.8b01808. PMID:
29945446.

42. Lechuga TJ, Qi QR, Kim T, Magness RR, Chen DB. 2019; E2β stimulates ovine uterine artery endothelial cell H
2S production in vitro by estrogen receptor-dependent upregulation of cystathionine β-synthase and cystathionine γ-lyase expression†. Biol Reprod. 100:514–22. DOI:
10.1093/biolre/ioy207. PMID:
30277497. PMCID:
PMC6378861.
43. Di Cesare Mannelli L, Lucarini E, Micheli L, Mosca I, Ambrosino P, Soldovieri MV, et al. 2017; Effects of natural and synthetic isothiocyanate-based H
2S-releasers against chemotherapy-induced neuropathic pain: role of Kv7 potassium channels. Neuropharmacology. 121:49–59. DOI:
10.1016/j.neuropharm.2017.04.029. PMID:
28431970.

44. Wu G, Ringkamp M, Hartke TV, Murinson BB, Campbell JN, Griffin JW, et al. 2001; Early onset of spontaneous activity in uninjured C-fiber nociceptors after injury to neighboring nerve fibers. J Neurosci. 21:RC140. DOI:
10.1523/JNEUROSCI.21-08-j0002.2001. PMCID:
PMC6762537. PMID:
11306646.

45. Djouhri L, Koutsikou S, Fang X, McMullan S, Lawson SN. 2006; Spontaneous pain, both neuropathic and inflammatory, is related to frequency of spontaneous firing in intact C-fiber nociceptors. J Neurosci. 26:1281–92. DOI:
10.1523/JNEUROSCI.3388-05.2006. PMID:
16436616. PMCID:
PMC6674571.

46. Meacham K, Shepherd A, Mohapatra DP, Haroutounian S. 2017; Neuropathic pain: central vs. peripheral mechanisms. Curr Pain Headache Rep. 21:28. DOI:
10.1007/s11916-017-0629-5. PMID:
28432601.

48. Sorge RE, Mapplebeck JC, Rosen S, Beggs S, Taves S, Alexander JK, et al. 2015; Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat Neurosci. 18:1081–3. DOI:
10.1038/nn.4053. PMID:
26120961. PMCID:
PMC4772157.

49. Castejón ML, Rosillo MÁ, Montoya T, González-Benjumea A, Fernández-Bolaños JG, Alarcón-de-la-Lastra C. 2017; Oleuropein down-regulated IL-1β-induced inflammation and oxidative stress in human synovial fibroblast cell line SW982. Food Funct. 8:1890–8. DOI:
10.1039/C7FO00210F. PMID:
28426090.

50. Dubey AK, Handu SS, Mediratta PK. 2015; Suvorexant: the first orexin receptor antagonist to treat insomnia. J Pharmacol Pharmacother. 6:118–21. DOI:
10.4103/0976-500X.155496. PMID:
25969666. PMCID:
PMC4419247.

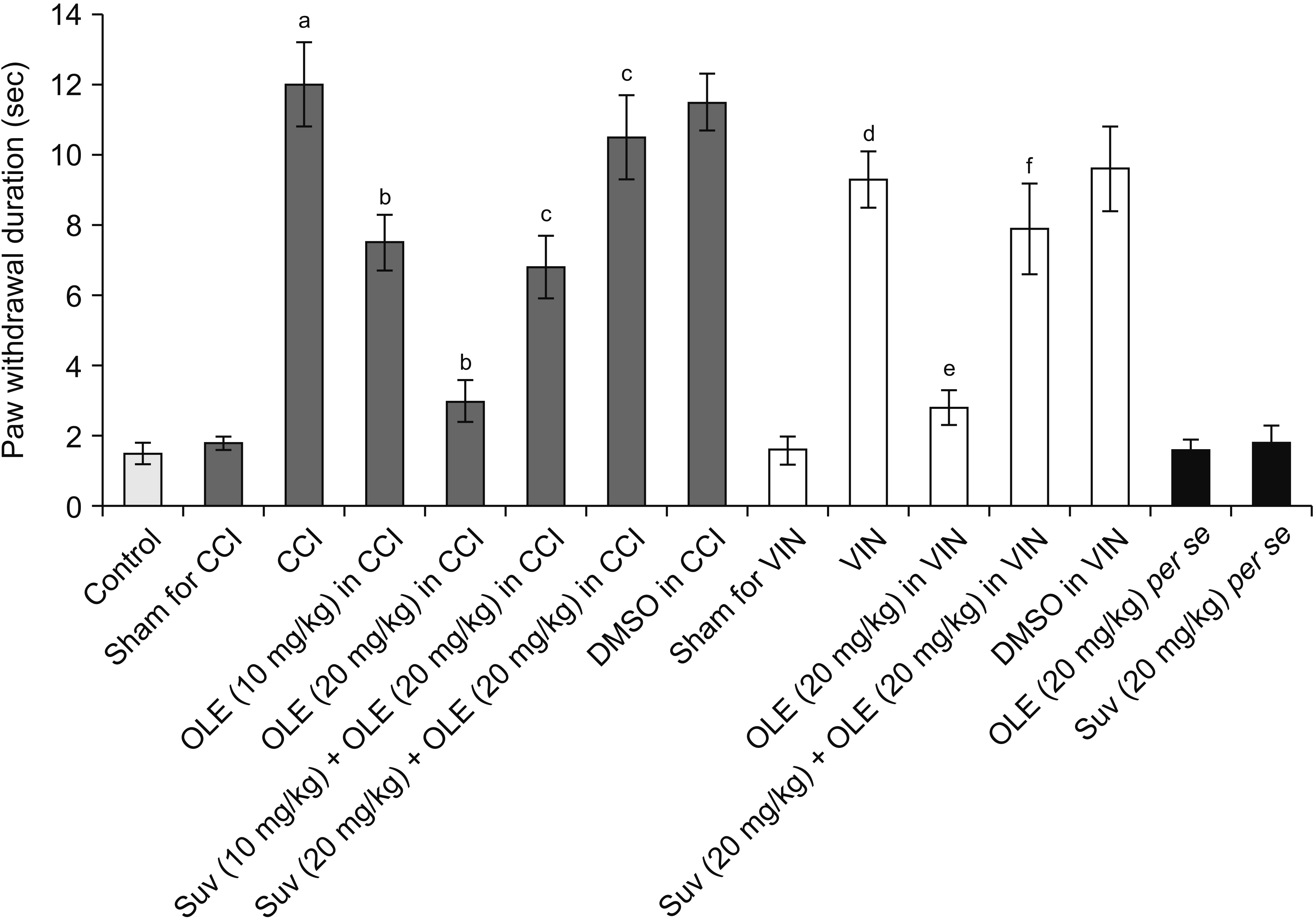
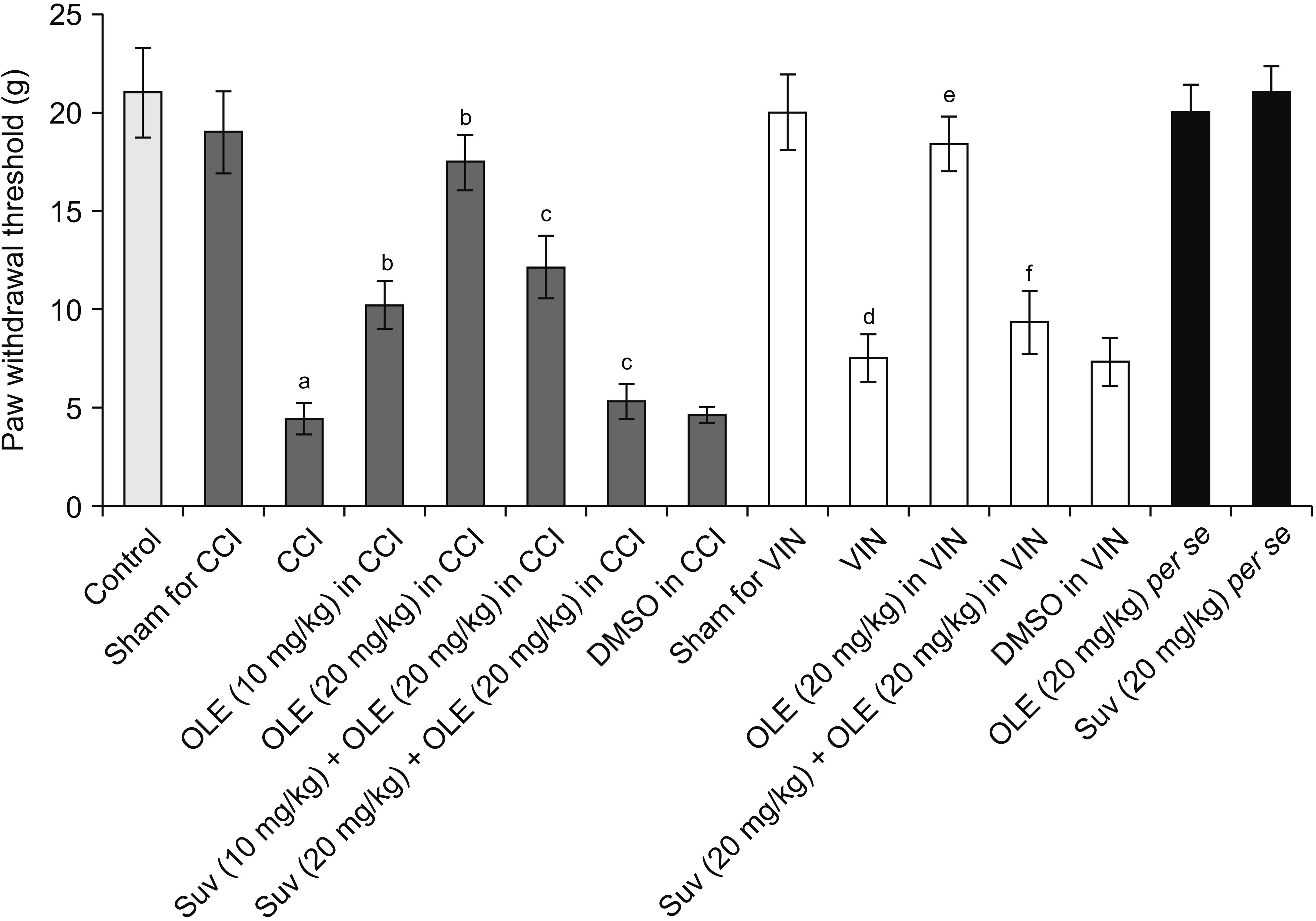
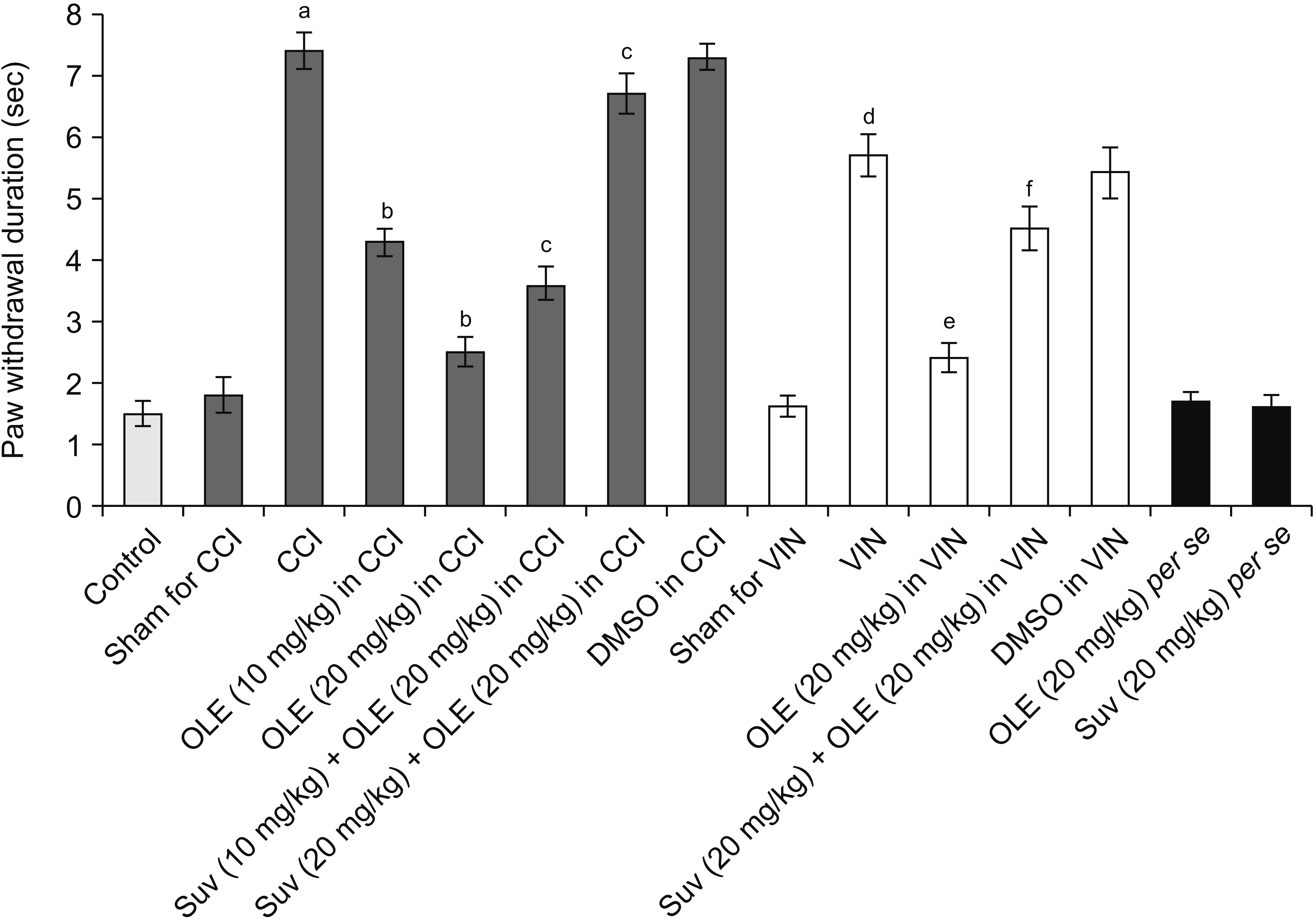
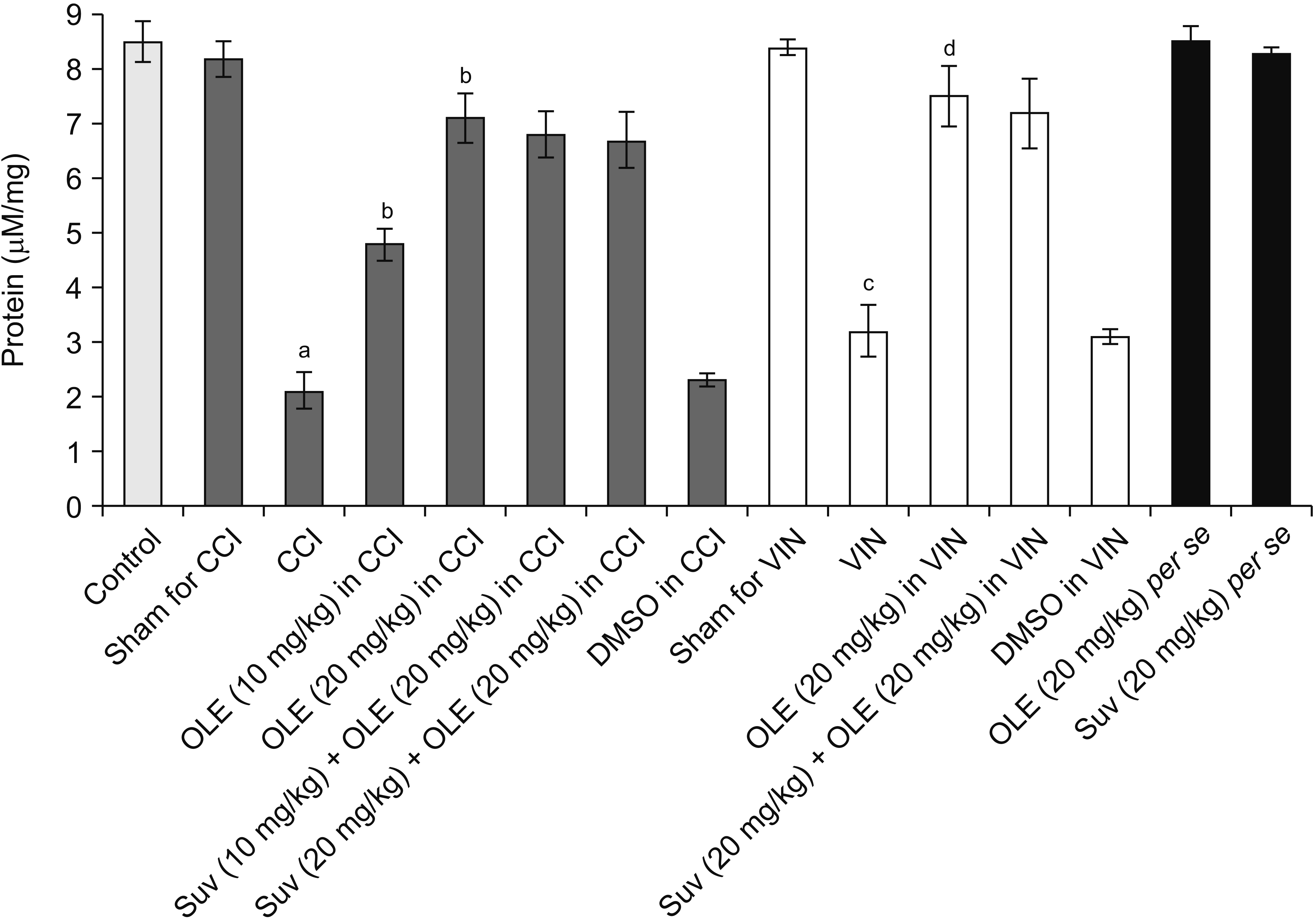




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download