This article has been
cited by other articles in ScienceCentral.
Abstract
Local anesthetic (LA) injection outside the sheath in epineural or paraneural connective tissue is considered safe practice among regional anesthesiologists. There is limited evidence as to whether neurological complications occur if LA is injected inside the sheath (subepineural - intraneural). We performed ultrasound guided injections at the level of undivided sciatic nerve in four amputated lower limbs. In two specimens, LA was injected in epineural connective tissue (paraneural tissue) and in another two specimens by penetrating the outer nerve sheath (hyperechoic epineurium). Ultrasonography demonstrated an increase in the size of nerve and macroscopic findings revealed fascicular tracings with sub-epineural injections. Limbs were sent for histological analysis in formalin containers. Pathologist performed the analysis which demonstrated an intact perineurium and a breach in the epineurium. We conclude that sub-epineural injections are unsafe and injection should be done in paraneural tissue to ensure safety and avoid unwanted neurological sequelae after the block.
Go to :

Keywords: Anesthetics, Local, Injections, Nerve Block, Neuralgia, Pathology, Peripheral Nerves, Sciatic Nerve, Ultrasonography, Interventional
It is universally agreed that the epineurium should not be breached during an isolated nerve block. Breach in the epineurium, leads to intraneural placement of needle tip, which is not desirable [
1,
2]. Improved imaging techniques with ultrasound (US) has increased the possibility of narrowing the needle tip-nerve distance, although the optimal needle tip-nerve distance remains elusive and undefined. The concept of intraneural placement of local anesthetic (LA) has been erroneously described as a safe practice [
3]. Furthermore, there is limited evidence of absence of neurological complications if LAs are injected inside the sheath (sub-epineural-intraneural). Therefore, this practice should be discontinued in clinical settings.
To understand the histological changes in a nerve specimen after a sub-epineural injection, we conducted a US-guided needle tip placement in two popliteal sciatic nerves 4-5 cm above the level of division, following an above-knee amputation. A pathologist independently evaluated the histological changes in the nerves of the amputated limbs.
Our aim was to understand the dynamics of spread in sciatic nerve specimens under US with extra-neural and sub-epineural injections in recently amputated limbs.
Go to :

CASE REPORT
This study was approved by the Institutional Ethics Committee, Sancheti Institute for Orthopedics and Rehabilitation, Pune (EC-SIOR/Agenda 060). Written informed consent was obtained from four patients and their next of kin. The lower limbs of these four patients suffered from severe superficial femoral arterial thrombosis, and the surgeon deemed amputation the best option. The sensations were attenuated below the mid-calf level and minimal motor movements in the form of plantarflexion and dorsiflexion of the foot and toe were possible. The time to surgery after the injury was more than 8 hours in each case. After above knee amputations the limbs were placed prone on a sterile sheet (
Fig. 1). We performed injection of methylene blue dye (MBD) with sterile water under US guidance (linear-array probe, 3-12 Hz, Sonosite M-Turbo; FUJIFILM Sonosite Inc., Bothell, WA) outside the epineurium in the epineural connective tissue (EPI-ct) in 2 specimens (labelled as L1 and L2) and below the epineurium after penetrating the epineurium – which was appreciated as a ‘pop’ – in 2 other specimens (L3 and L4). An in-plane technique was chosen to place a 50 mm needle (Stimuplex
®; B. Braun, Melsungen, Germany) from the lateral to medial aspect of the sciatic nerve (
Fig. 1). In L1 and L2, the needle tip was tangential to the nerve at a 6-7 o’clock position, and was positioned in the EPI-ct. Three mL sterile water with MBD was injected without any resistance. In L3 and L4, with the needle tip close to the nerve at a 9 o’clock position, the tip was advanced until a ‘pop’ was appreciated, and 3 mL sterile water with MBD was injected, below the epineurium in the ‘sub-epineural area’.
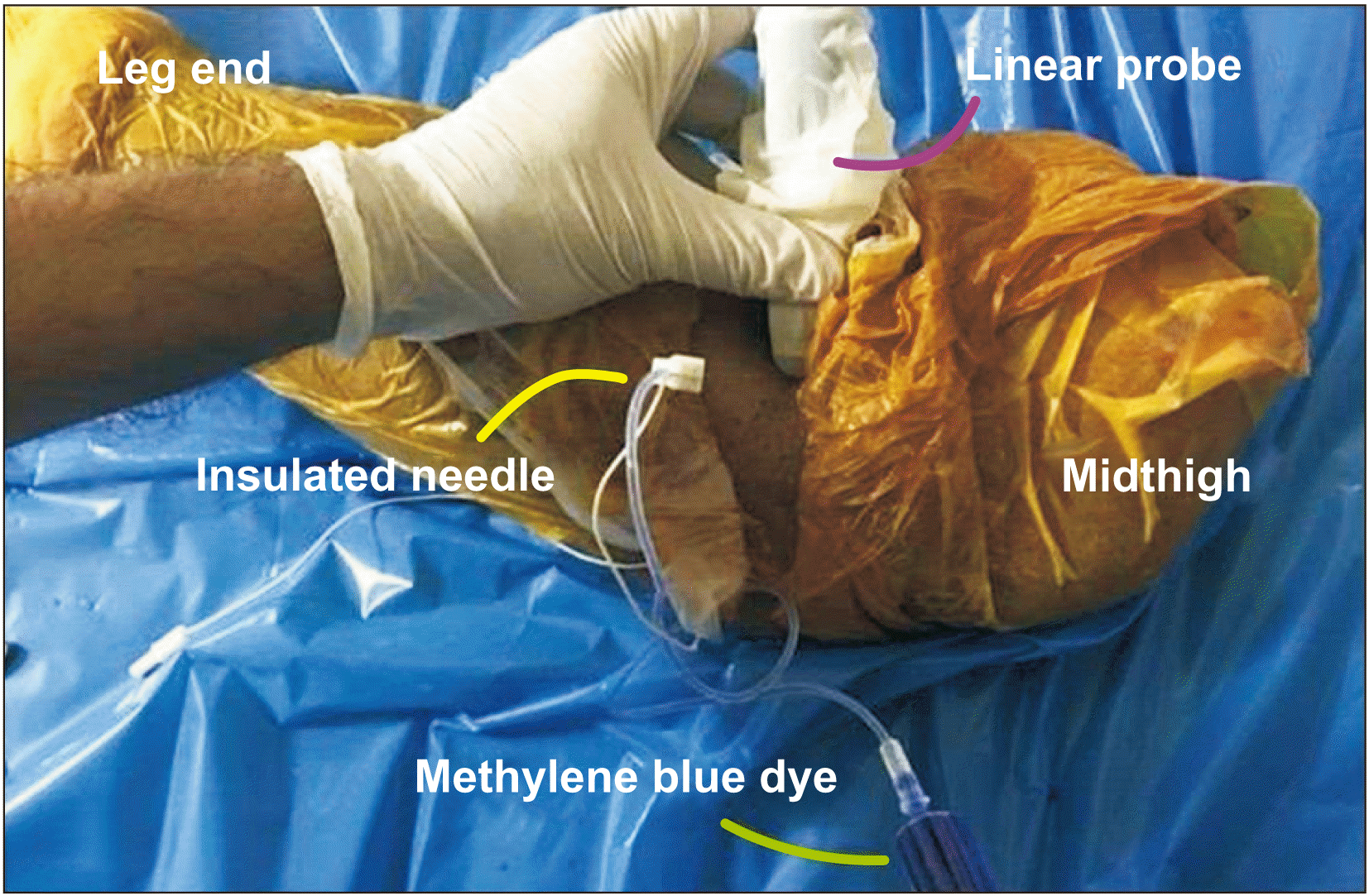 | Fig. 1L1 specimen in prone position, with ultrasound guided injection at the popliteal sciatic nerve through a sterile insulated needle under a linear probe. 
|
Within 20 minutes after injection, with all specimens in prone position (L1, L2, L3, and L4), gentle dissection was performed by the surgeon and anesthesiologist by peeling layer by layer to identify the superficial dye spread. Tissue was handled carefully to avoid unwanted spread of the dye in different tissue planes. The findings were noted and the specimens were immersed in the chamber filled with 10% formalin and sent for histopathological analysis. The histological findings were reported independently by a pathologist. The tissues were fixed in formalin and subjected to increasing concentration of alcohol. The dehydrated tissues were dipped in molten wax. The tissue blocks were cut into thin ribbons through a microtome. After passing the ribbons through a decreasing concentration of alcohol, the tissue slides were stained with hematoxylin and eosin.
In L1 and L2, the spread was observed and images were downloaded. The spread was in the EPI-ct (outside the sheath), and no spread occurred in the sub-epineural area (inside the sheath). There was no swelling of the nerve. (
Fig. 2). In L3 and L4, upon sub-epineural injection, the nerve size briefly increased in all planes, evident from the dissipation of the solution in several directions (
Fig. 3). The nerve returned to near normal size within a few seconds. In L3 and L4, upon sub-epineural injection, the nerve size also briefly increased in all planes, evident from the dissipation of the solution in several directions (
Fig. 3). The nerve returned to near normal size within a few seconds. Macroscopic examination of L1 and L2 revealed a uniform, dense, circumferential spread of MBD in the specimens in which injection was performed EPI-ct (outside the sheath), and a distal cross-section of the nerve at the point of division revealed a spread in the EPI-ct between the 2 nerves (
Fig. 4A, B), and partially around it. In the specimens of L3 and L4 with injections performed in sub-epineural area (below the sheath), the spread was not dense, and was non-uniform around the nerve (
Fig. 4C, D). Fascicular tracings of MBD were observed with sub-epineural injections.
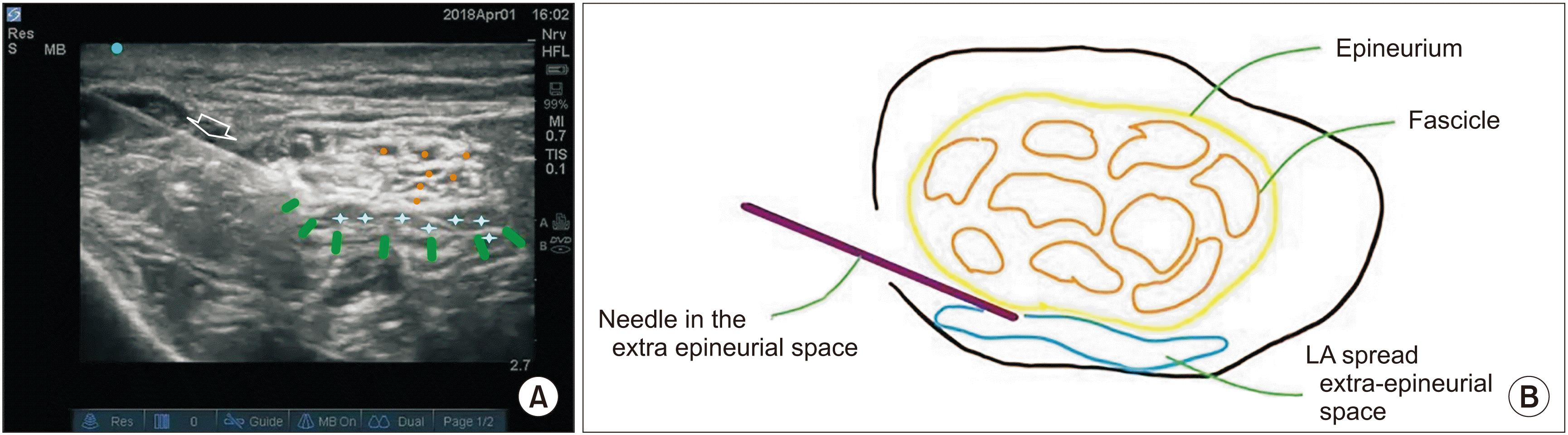 | Fig. 2L1 specimen in prone position. (A) The needle in-plane (white arrow) inserted from lateral to medial. The marker – dark blue – is on lateral side depicts the orientation marker. Green line denotes paraneural covering i.e., epineural connective tissue. The blue cross is the paraneural spread of the solution. Orange dots are fascicles. (B) Schematic diagram of Fig. 2A. LA: local anesthetic. 
|
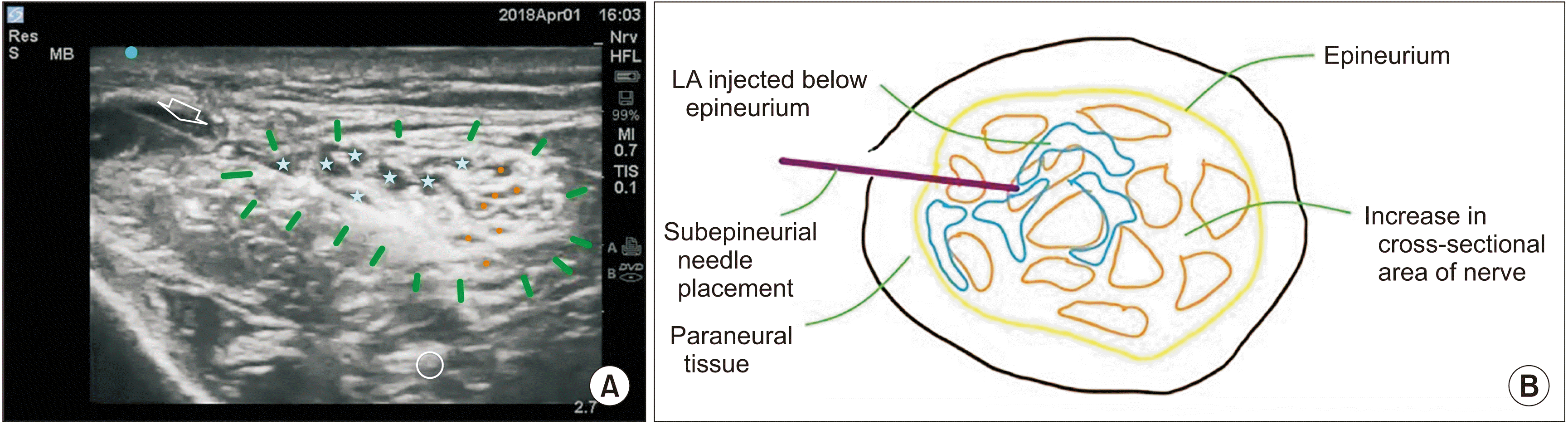 | Fig. 3L3 specimen in prone position. (A) The needle in-plane (white arrow) inserted from lateral to medial in L3. The marker – dark blue – is on lateral side depicts the orientation marker. Green line denotes paraneural covering i.e., epineural connective tissue. Blue asterisk is the intraneural solution spread. Orange dots are fascicles. (B) Schematic diagram of Fig. 3A. LA: local anesthetic. 
|
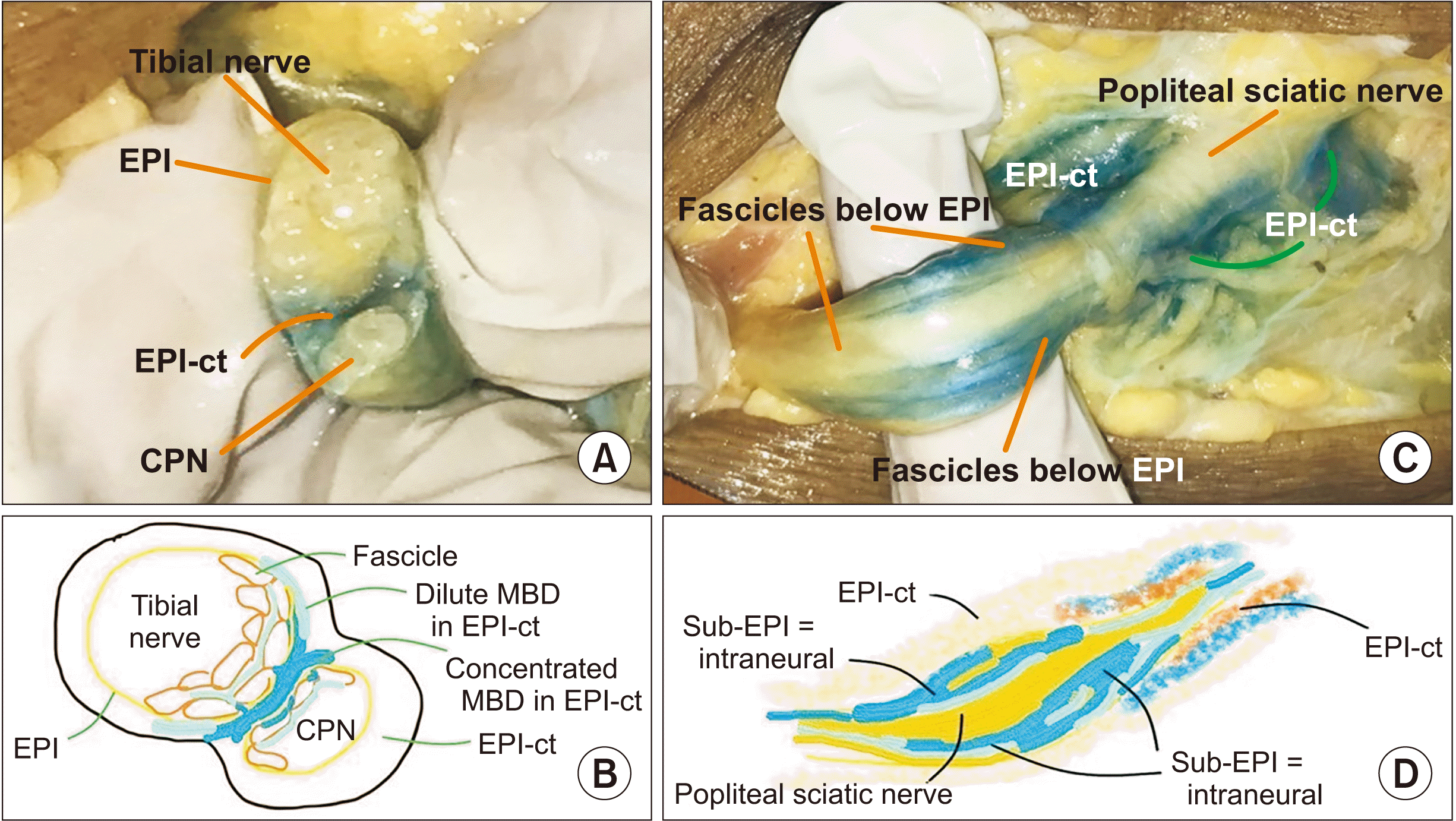 | Fig. 4L1 and L3 specimen in prone position. (A) Macroscopic findings of solution dispersal in L1. In the epineural connective tissue (EPI-ct) (paraneural) needle placement and injection of methylene blue dye (MBD) revealed an EPI-ct (paraneural) spread of MBD between the two nerves at the level of division. The EPI-ct (paraneural) and the epineural coverings of the nerves are well delineated. (B) Schematic diagram of Fig. 4A. (C) Macroscopic findings of solution dispersal in L3 specimen. (D) Schematic diagram of Fig. 4C. CPN: common peroneal nerve. 
|
A histological transverse section of a thin nerve (
Fig. 5A) showed the epineurium with MBD in the EPI-ct. The structures displaced included collagen fibers, elastin fibers, and fibroblasts. The transverse section of the thin nerve (
Fig. 5B) shows the injection site with a probable puncture of the epineurium, at the ragged end of the epineurium (
Fig. 5B). MBD is imbedded in the epineurium. The perineurium appeared to be intact, but the individual fascicles close to the ragged epineurium were darker stained (
Fig. 5B) than the rest of individual fascicles in the same nerve bundle. The nerve appeared to be edematous. The other individual nerve bundles were intact. The US and histological findings are summarized in
Table 1.
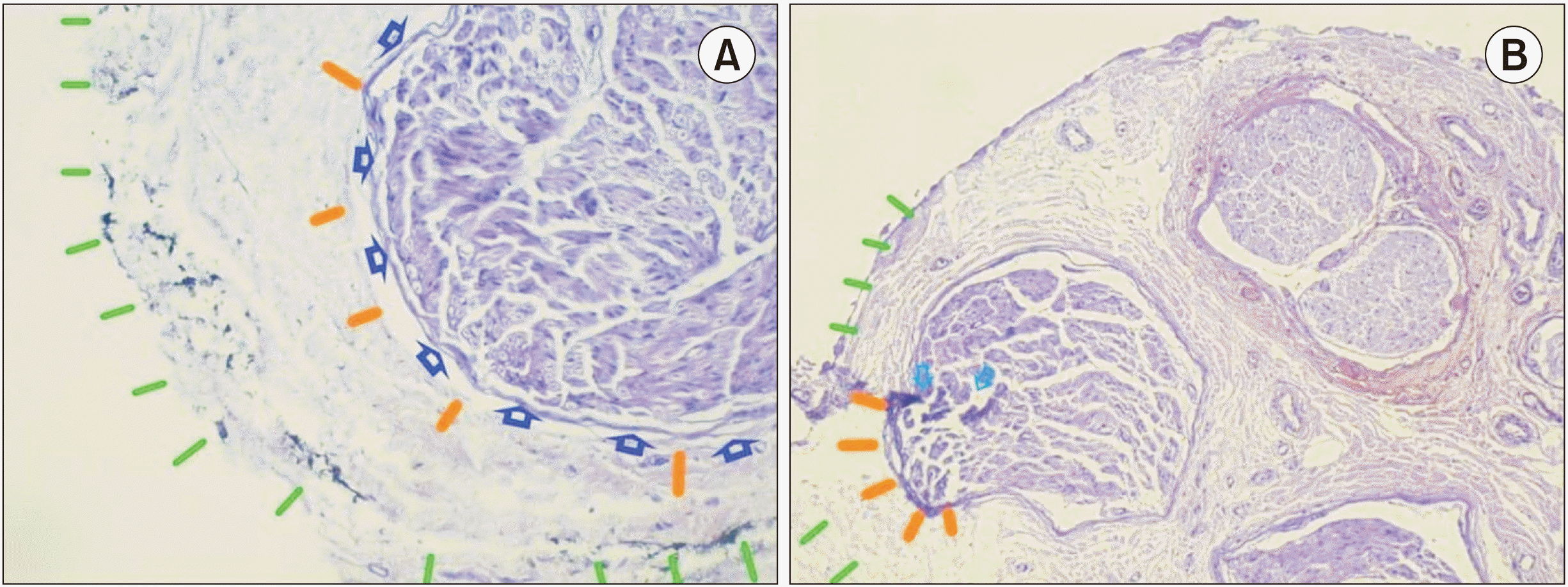 | Fig. 5Histological information about L1 and L3 specimen (hematoxylin and eosin stain, low power [×10]). (A) Histological findings of specimen L1. Epineural connective tissue (paraneural tissue-green lines) injection in L1, revealed localization of methylene blue dye in the epineural connective tissue (paraneural). The intact inner epineurium (gold) and the perineurium (dark blue arrows) and internal architecture of the nerve are well preserved. (B) Histological findings of specimen L3. Sub-epineural needle placement (inside the sheath) revealed the breach of inner epineurium (gold). 
|
Table 1
Ultrasound and macroscopic findings of 4 specimens
|
Specimen no. |
Ultrasound |
|
Macroscopic findings |
|
|
|
EPI-ct |
Sub-EPI |
EPI-ct |
Sub-EPI |
|
L1 |
Epineurium connective tissue spread |
NA |
|
Uniform and dense circumferential spread – MBD between TN and CPN |
NA |
|
L2 |
Epineurium connective tissue spread |
NA |
|
Uniform and dense circumferential spread |
NA |
|
L3 |
NA |
Increased diameter of nerve in all planes |
|
NA |
Fascicular tracings observed below sheath |
|
L4 |
NA |
Increased diameter of nerve in all planes |
|
NA |
Fascicular tracings observed below sheath |

Go to :

DISCUSSION
Our study demonstrated that in 2 specimens, at L3 and L4, a US-guided injection below the epineurium, which is a sub-perineural injection of the undivided sciatic nerve, disturbed the epineurium and increased the size of the nerve with 3 mL of solution. Close to the ragged appearance of the epineurium (
Fig. 3A) the fascicles were stained with MBD, suggesting a possible breach in the perineurium. In the other 2 specimens, at L1 and L2, the injection in the EPI-ct did not cause damage to any structures. In clinical practice at least 20 mL of LA is injected for an adequate block, so one can imagine the nerve damage this would produce, with a sub-epineural injection.
Tran’s study group [
4] concluded that in an US-guided popliteal sciatic nerve, a sub-epineural injection provides a higher success rate with a shorter performance time. Well-defined experimental animal models have concluded that a sub-epineural injection (intraneural) produces significant axonal damage and disruption of the blood-nerve barrier [
5-
7].
In clinical practice, a ‘pop’ is appreciated as the needle penetrates what we visualize as a sheath around the undivided sciatic nerve. This sheath is the epineurium, which should not be violated. This sub-neural injection is an intraneural injection [
8]. This is an unsafe practice and should be avoided in view of fascicular injury. Histologically, there was no spread of MBD below the epineurium after injecting into the EPI-ct. This EPI-ct is mentioned in the literature as the paraneural tissue [
9]. The paraneural injections are not accompanied by any damage of the axons. Clinically, in peripheral nerves, neither the pop, nor the current US resolution, differentiate between the epineurium and perineurium.
Through this study we demonstrate that a pop or a click in an US-guided popliteal sciatic nerve block could be detrimental regarding the nerve integrity, and recommend injections in the EPI-ct, better termed as paraneural tissue. Furthermore, the needle placement should be tangential (
Fig. 2A) and not perpendicular to the nerve [
10].
Some unanswered questions were the disturbed (ragged) epineurium, which appeared at the 6-7 o’clock position, the probable site of needle tip puncture (
Fig. 5B), while the rest of the neural architecture demonstrated a normal pattern (
Fig. 3A). Perhaps a larger sample size would have been more suited to answering the above-mentioned questions. A comparative study between high and low volumes in the paraneural and sub-epineural structure, as well as subsequent histological analysis would answer several queries. Recent practice patterns concerning isolated peripheral nerve injections suggest that paraneural injections are considered safe [
9,
11]. Our histological study demonstrates that an injection in the EPI-ct (paraneural tissue) is a safe practice. A large volume sub-epineural injection would be detrimental, with consequent nerve damage, considering the smaller volumes in our study producing histological changes suggestive of intraneural injection.
Though limited damage was evident on macroscopic and histological analysis of subepineural injections in our study, we strongly recommend paraneural injections in the isolated sciatic nerve.
Go to :

ACKNOWLEDGEMENTS
We acknowledge Dr. Asawari Manjarekar (MD), Pathologist from CareFirst Diagnostics Pvt Ltd Pune, Maharashtra State, India for histopathological analysis of the slides involved in this manuscript.
Go to :

Notes
Go to :

REFERENCES
2. Al-Nasser B. 2007; Intraneural injection of local anesthetics during ultrasound-guided peripheral nerve block may lead to nerve injury. Anesthesiology. 106:1245–6. DOI:
10.1097/01.anes.0000265460.54744.6f. PMID:
17525607.

3. Bigeleisen PE. 2006; Nerve puncture and apparent intraneural injection during ultrasound-guided axillary block does not invariably result in neurologic injury. Anesthesiology. 105:779–83. DOI:
10.1097/00000542-200610000-00024. PMID:
17006077.

4. Tran DQ, Dugani S, Pham K, Al-Shaafi A, Finlayson RJ. 2011; A randomized comparison between subepineural and conventional ultrasound-guided popliteal sciatic nerve block. Reg Anesth Pain Med. 36:548–52. DOI:
10.1097/AAP.0b013e318235f566. PMID:
22005661.

5. Gentili F, Hudson AR, Hunter D, Kline DG. 1980; Nerve injection injury with local anesthetic agents: a light and electron microscopic, fluorescent microscopic, and horseradish peroxidase study. Neurosurgery. 6:263–72. DOI:
10.1227/00006123-198003000-00007. PMID:
7383289.

6. Westerlund T, Vuorinen V, Kirvelä O, Röyttä M. 1999; The endoneurial response to neurolytic agents is highly dependent on the mode of application. Reg Anesth Pain Med. 24:294–302. DOI:
10.1097/00115550-199924040-00004. PMID:
10445767.

7. Lupu CM, Kiehl TR, Chan VW, El-Beheiry H, Madden M, Brull R. 2010; Nerve expansion seen on ultrasound predicts histologic but not functional nerve injury after intraneural injection in pigs. Reg Anesth Pain Med. 35:132–9. DOI:
10.1097/AAP.0b013e3181d25cfe. PMID:
20216032.

9. Andersen HL, Andersen SL, Tranum-Jensen J. 2012; Injection inside the paraneural sheath of the sciatic nerve: direct comparison among ultrasound imaging, macroscopic anatomy, and histologic analysis. Reg Anesth Pain Med. 37:410–4. DOI:
10.1097/AAP.0b013e31825145f3. PMID:
22609646.
10. Sermeus LA, Sala-Blanch X, McDonnell JG, Lobo CA, Nicholls BJ, van Geffen GJ, et al. 2017; Ultrasound-guided approach to nerves (direct vs. tangential) and the incidence of intraneural injection: a cadaveric study. Anaesthesia. 72:461–9. DOI:
10.1111/anae.13787. PMID:
28185262.

11. Franco CD, Sala-Blanch X. 2019; Functional anatomy of the nerve and optimal placement of the needle for successful (and) safe nerve blocks. Curr Opin Anaesthesiol. 32:638–42. DOI:
10.1097/ACO.0000000000000776. PMID:
31415044.

Go to :









 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download