Abstract
Background
Low back pain secondary to discopathy is a common pain disorder. Multiple minimally invasive therapeutic modalities have been proposed; however, to date no study has compared percutaneous laser disc decompression (PLDD) with intradiscal injection of radiopaque gelified ethanol (DiscoGel®). We are introducing the first study on patient-reported outcomes of DiscoGel®
vs. PLDD for radiculopathy.
Methods
Seventy-two patients were randomly selected from either a previous strategy of PLDD or DiscoGel®, which had been performed in our center during 2016–2017. Participants were asked about their numeric rating scale (NRS) scores, Oswestry disability index (ODI) scores, and progression to secondary treatment.
Results
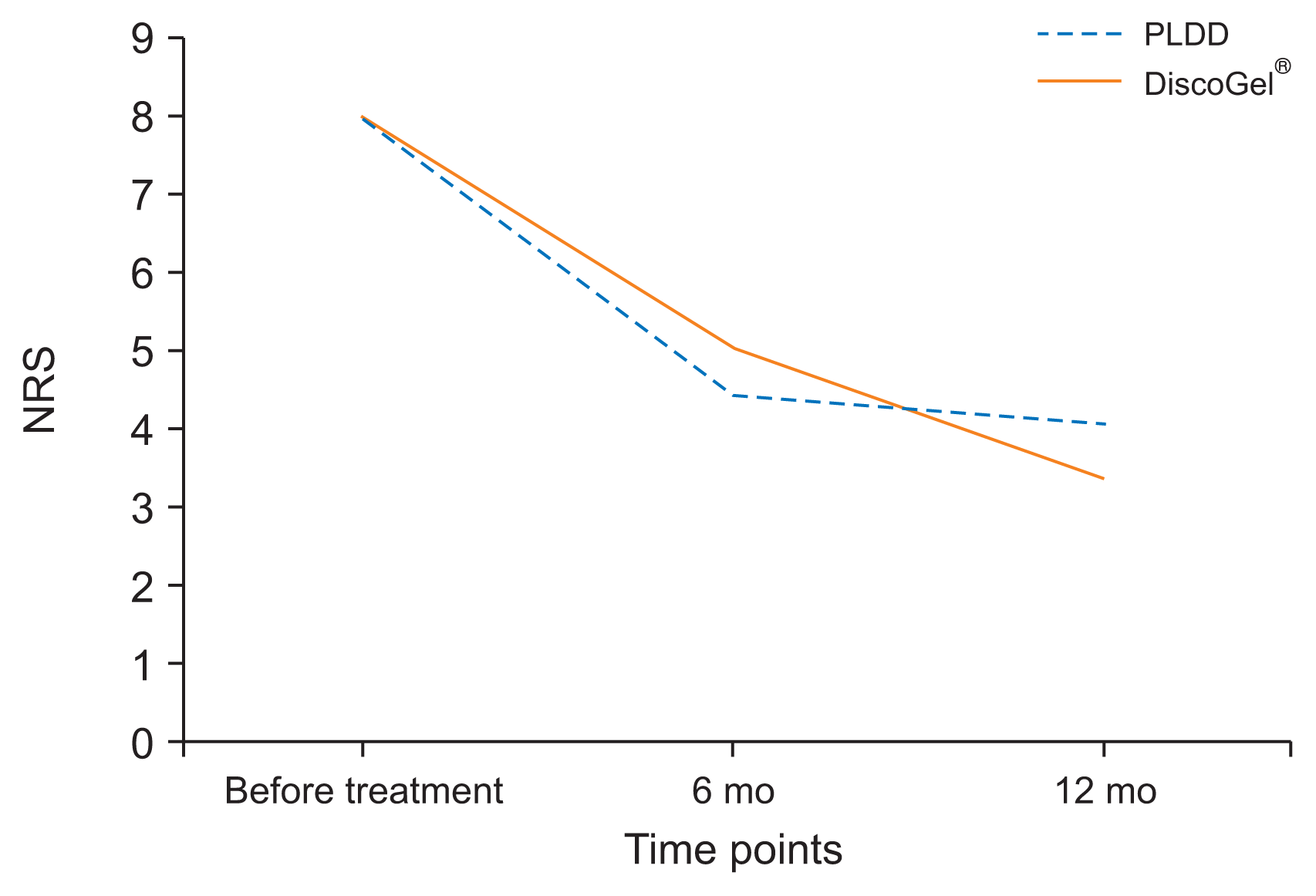
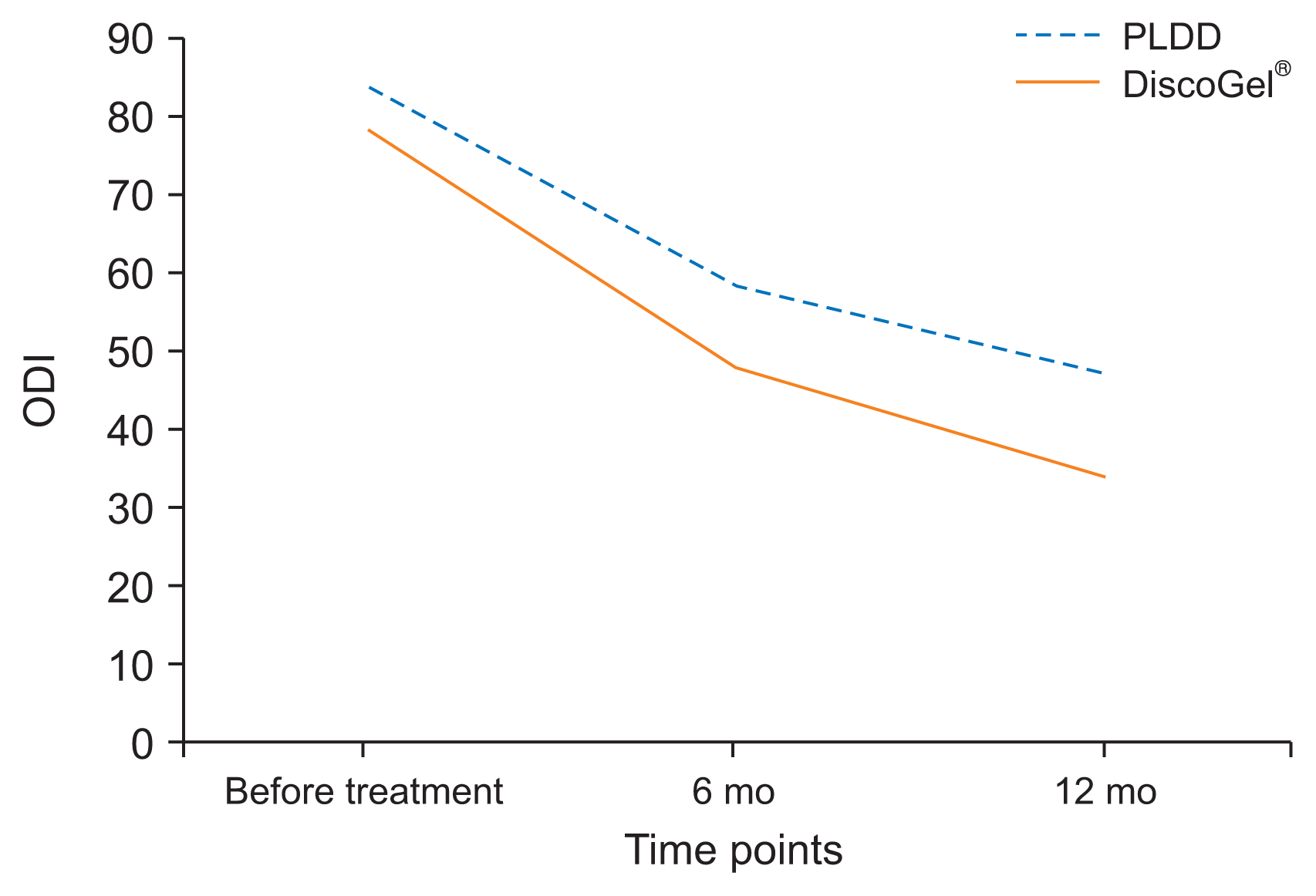
The mean NRS scores in the total cohort before intervention was 8.0, and was reduced to 4.3 in the DiscoGel® group and 4.2 in the PLDD group after 12 months, which was statistically significant. The mean ODI score before intervention was 81.25% which was reduced to 41.14% in the DiscoGel® group and 52.86% in the PLDD group after 12 months, which was statistically significant. Between-group comparison of NRS scores after two follow-ups were not statistically different (P = 0.62) but the ODI score in DiscoGel® was statistically lower (P = 0.001). Six cases (16.67%) from each group reported undergoing surgery after the follow-up period which was not statistically different.
Go to : 
Low back pain (LBP) and radiculopathy secondary to intervertebral disc pathology is one of the most common medical problems of all spinal, and even chronic pain disorders. Based on recent concepts, it is believed that the mechanisms of pain generation in radicular pain are simultaneous mechanical deformation of an intervertebral disc and irritation of the nerve root by chemical mediators originating from an injured disc, provoking inflammatory responses and increase the sensory neuron susceptibility, resulting in radicular pain [1–3].
Multiple therapeutic modalities, such as minimally invasive procedures and percutaneous techniques, have been recently proposed for the removal of herniated disc fragments (which have caused nerve root compression) and for developing the treatment of LBP and radiculopathy while minimizing procedure-related muscle and ligament injury [4–7].
Percutaneous laser disc decompression (PLDD) is another acceptable choice in the management of herniated lumbar disc disease and radiculopathy. PLDD is an indirect way of decompressing the compromised nerve root by using laser energy to vaporize a small volume of the nucleus pulposus and reducing the pressure in the intervertebral disc. Decreasing the intradiscal pressure would retract the herniation away from the compromised nerve root, thus reduce the amount of nerve root injury [8,9]. PLDD does not require hospitalization or anesthesia, and is therefore associated with lower healthcare costs than conventional surgery [10].
Another minimally invasive percutaneous treatment that has recently been made available by exploiting the chemical properties of pure ethanol is radiopaque gelified ethanol (DiscoGel®). DiscoGel® is a newly proposed sterile viscous intradiscal solution in the form of gelified ethanol associated with tungsten in suspension, which is more viscous than absolute alcohol and was introduced in 2007 for treatment of pain from lumbar discs that failed conservative treatment with an absence of neurological deficit. This class III intradiscal medical material is injected into the nucleus pulposus to decompress the intradiscal space. The presence of cellulose, which is a gelling agent, would decrease the possibility of epidural leakage from the administered agent that may occur with pure (not gelified) ethanol. The tungsten particles are used to control progression of the gel in the disc and through any annular fissures using fluoroscopic images [11–13].
Both treatments are performed in an outpatient setting with the patient in prone position, under local anesthesia and sterile conditions. In PLDD technique, an 18-G needle is placed centrally in the nucleus pulposus and parallel to the endplates in a posterolateral approach, guided by fluoroscopy. A glass fibre of 600 microns is advanced through the needle, and laser energy (Biolitec, Jena, Germany; 980 nm, 7 W, 0.6 sec pulses, interval 1 sec) is applied to a total energy delivery of 1,500 J (2,000 J for level L4-5) [8,9].
In the intradiscal injection of DiscoGel®, an 18-G needle is inserted into the median and posterior part of the disc and DiscoGel® is injected slowly (0.1 mL during a 30 sec period). The amount of DiscoGel® injected into the disc depends on the amplitude of the disc space and the relative capacity of the disc to accommodate the gel, usually between 0.5–0.8 mL. The needle is left in place for 2 minutes after the application to prevent late leakage [11,13].
Due to a paucity of evidence for prospective studies regarding the effectiveness of these techniques and patients’ long-term outcome in terms of radicular pain management and functional ability score improvement, this present study was designed to compare the effectiveness of intradiscal injection of DiscoGel® and PLDD in 72 patients with LBP and radiculopathy due to lumbar intervertebral disc herniation. Since the 1-year follow-up improvement would be the most important clinical landmark after these procedures, this report consists of patients’ reported results from a period of 12 months. Outcome measures included pain intensity and functional disability, assessed at baseline, 6 months, and 12 months following the treatment. Additional lumbar spine injections and/or progression to surgery were secondary outcome measures.
Go to : 
The present prospective cohort study was designed and conducted in the Anesthesiology Research Center (Tehran, Iran), during December 2018, aiming to compare the results from a one-year follow-up of two previously performed interventions of PLDD and intradiscal injection of DiscoGel® in patients with unilateral or bilateral radicular LBP due to lumbar intervertebral disc herniation.
The study protocol was approved by the Local Ethics Committee of the Shahid Beheshti University of Medical Sciences (IRB number: IR.SBMU.RETECH.REC.1397.1285) and the study was performed in accordance with the ethical standards of the 1964 Declaration of Helsinki. All data were kept confidential, patients’ anonymity was preserved, and the patients were not charged for the purposes of the study. The consent was previously taken from all patients, in this study we compared the results after one year.
The study population was made up of patients who had undergone either PLDD or intradiscal injection of DiscoGel® in the Anesthesiology Research Center during 2016–2017. Inclusion criteria were being aged 20–70 years, a history of unilateral or bilateral radicular pain to the lower extremities due to lumbar intervertebral disc herniation without neurologic deficits, whose diagnosis were proved by physical examination and medical imaging (radiologic imaging and magnetic resonance imaging), and were not responsive to conservative treatments during at least past 3 months. Exclusion criteria were a history of vertebral fracture, spinal canal stenosis, a history of any lumbar surgery or malignancy, peripheral nerve neuropathies, uncontrolled diabetes mellitus, and an inability to communicate in Persian language.
Sample size calculation was performed using the results from a previous pilot study on 20 cases (10 random cases from each procedure), assuming an α-error of 0.05, a power of 80%, an estimated difference between the two groups as 0.487, and standard error defined as 1.350. As a result, 60 cases were considered to be included. However, due to our prediction of cases with no follow-up, a 20% increase in sample size was certified and a total of 72 cases, who had undergone previous interventions and met the criteria, were included in the present study. Patients were randomly selected using the sort range randomly utility in Excel (Microsoft, Redmond, WA), according to whether they had received a previous PLDD or DiscoGel®.
Baseline and demographic data for all patients were recorded in the patients’ profiles and all of the participants were called by one independent researcher. If any patient was unreachable after 3 calls at different times of a day and different days of a week, the patient was excluded from the study.
During the phone call interview, the study aim and objective were described to each patient and participants were instructed to respond to the questions or rate each scale independently.
Pain intensity was evaluated based on a verbal numeric rating scale (NRS). The NRS is one of the most commonly used self-reporting scales for measuring pain, likely due to its ease of use (it requires no specialized equipment) and also because its 0 to 10 metric is preferred by health care professionals. The validity and reliability of this scale has been previously established [14]. Patients typically were asked, “How strong is your pain during the past 14 days, where 0 is no pain and 10 is the strongest or worst pain you can imagine?”.
Functional ability was evaluated based on the Oswestry disability index (ODI). The ODI is a self-administered questionnaire measuring “back-specific function” on a 10-item scale with six response categories each. Each item scores from 0 to 5, higher scores being worse, which is transformed into a 0% to 100% scale. The ten items include pain intensity, personal care, lifting, walking, sitting, standing, sleeping, work, social life, and traveling. Patients with scores between 0% to 20% have minimal disability, between 21% to 40% have moderate disability, between 41% to 60% have severe disability, 61% to 80% are crippled and 81% to 100% are bed-bound or exaggerating their symptoms. The validity and reliability of this scale has been previously established [15].
Additional lumbar spine injections and/or a progression to lumbar decompression surgery, diskectomy, or any other treatments after 1-year post-intervention were assessed and the answers were documented in their profile.
Due to the duration of follow-up and for reasons such as death, migration, or changes in the status of sample cases over time, the presence of cases with no follow-up (loss-to-follow-up) and therefore non-response (or response refusal) bias was predictable. To minimize this bias, besides considering a 20% increase in sample size (as mentioned before), inclusion and exclusion criteria were limited, and therefore, the samples were condition-specific and homogeneous. As a result, the sample population would represent the community studied.
In order to avoid recall bias, the primary outcome measured concentrated on the current condition of the patients (specifically the previous 2 wk). In order to avoid response bias, patients were provided with adequate details and necessary clarifications about the questions and the correct way of responding to the questionnaires.
Statistical analysis was performed using SPSS ver. 18 (IBM Corp., Armonk, NY).
Demographic factors were presented by frequencies and related percentages; continuous data were displayed by means and standard deviation. The statistical analysis is based on the assessment of differences in outcome measurements between both groups and comparison of averages at single time points. An independent-samples t-test, chi-square, or student t-test, as appropriate, and repeated measurements using analysis of variance were used to compare the variables at baseline, 6 months and 12 months after follow-up. P values < 0.05 were considered to be statistically significant.
Go to : 
During the recruitment period (2016–2017), a total of 72 subjects with unilateral or bilateral radicular pain, secondary to lumbar intervertebral disc herniation, had undergone either an intradiscal injection of DiscoGel® or PLDD in the Anesthesiology Research Center. Twelve months after the procedures, all 72 subjects (100%) were available for follow-up.
Baseline and demographic characteristics of the entire cohort were analyzed. The average age of the participants was 48.2 years old, ranged between 21 and 70 years old, with 43 subjects being male (59.72%). Twenty-six cases (36.11%) were smokers. The mean duration of pain and radiculopathy prior to the intervention was 12.19 months, ranged between 3 and 60 months. Mean pain intensity (NRS scores) was 8.0 (before the procedures). The mean functional disability index (ODI score) was 81.25% before the procedures. Twelve cases (16.67%) reported undergoing surgery at least 6 months after the 1-year follow-up.
Upon analyzing baseline data from PLDD group, the average age of participants was 44.6 years, ranged between 21 and 70 years, with 21 subjects being male (58.33%). Fourteen cases (38.89%) were smokers. Mean duration of pain and radiculopathy was 13.30 months, ranged between 3 and 60 months. Mean pain intensity at baseline was 8.00. Mean functional disability at baseline was 83.84%. Six cases (16.67%) reported undergoing surgery at least 8 months after the 1-year follow-up.
Upon analyzing baseline data from DiscoGel® group, the average age of participants was 47.3 years, ranged between 26 and 70 years, with 22 subjects being male (61.11%). Twelve cases (33.33%) were smokers. Mean duration of pain and radiculopathy was 10.88 months, ranged between 3 and 36 months. Mean pain intensity at baseline was 8.02. Mean functional disability at baseline was 78.35%. Six cases (16.67%) reported undergoing surgery at least 6 months after the 1-year follow-up. Data are demonstrated in Table 1.
Upon comparing and analyzing the outcome results between PLDD & DiscoGel® group, NRS scores in the total cohort before intervention was 8.0 (ranged from 5–10). After receiving the intervention and during the 12 months follow-up, the mean NRS score in the DiscoGel® group had decreased to 4.3. This difference in NRS scores before and after intervention was statistically significant (P = 0.005). After receiving the intervention and during the 12 months follow-up, the mean NRS score in the PLDD group had decreased to 4.2. This difference in NRS scores before and after intervention was statistically significant (P = 0.006). However, between-group comparison of NRS scores in the two groups after two follow-up visits showed no statistically significant difference between the two groups (P = 0.62). Data are demonstrated in Fig. 1. ODI score before intervention was 81.25% (ranged from 48%–100%).
After receiving the intervention and during the 12 months of follow-up, the mean ODI score in the DiscoGel® group had decreased to 41.14%. This difference in ODI scores before and after the intervention was statistically significant (P = 0.012).
After receiving the intervention and during the 12 months of follow-up, the mean ODI score in the PLDD group had decreased to 52.86%. This difference in ODI scores before and after the intervention was statistically significant (P = 0.019).
Between-group comparison of ODI score in the two groups after two follow-up visits showed a statistically significant difference between the two groups at P = 0.001 and the mean ODI score in the DiscoGel® group was statistically lower than the PLDD group. Data are demonstrated in Fig. 2.
Six cases from each group (16.66%, n = 36 per group) reported undergoing surgery after the follow-up period. This difference between the two groups was not statistically different.
Go to : 
A wide range of pain management modalities including minimally invasive percutaneous treatments have been suggested in recent years for discogenic pain secondary to lumbar disc herniation. In the present cohort study, we compared the effectiveness of two different modalities by evaluating the improvements in patients’ pain and functional scores.
PLDD is an attractive Food and Drug Administration-approved treatment modality for lumbar radiculopathy that reduces intradiscal pressure by vaporization of a small volume of water within the nucleus pulposus.
The result would be a decline in intradiscal pressure and also a more even distribution of weight across the disc, with a subsequent relief of discogenic pain; this is performed most commonly for lumbar disc pathologies [16,17]. Due to its minimally-invasive nature, the decrease in the risk of damage to the muscles, bone, ligaments, and nerves along with less back pain, a shorter hospital stay and shorter recovery period in comparison with conventional surgical methods are anticipated, and several cohort studies have previously showed the safety and potential benefits of PLDD [7,8,16,18–23]. On the other hand, multiple prospective randomized trials do exist that have revealed the lack of efficacy of PLDD alone or compared with conventional surgeries such as microdiscectomy [9,10,24].
After the introduction of a substance based on Disco-Gel® and its success in partial debulking of the nucleus pulposus with percutaneous intradiscal administration and consequent reduction of intradiscal pressure, this treatment has been tested in several controlled randomized studies. DiscoGel® is a newly proposed sterile viscous solution of DiscoGel® which is more viscous than absolute alcohol and has been recently used in minimally invasive procedures for treatment of discogenic lumbosciatia. After intradiscal injection of DiscoGel® no morpho-structural changes in the nuclear tissue or annulus were found [11], spurring hope that it could serve as an alternative for invasive conventional surgery or microdiscectomy in cases where there was no response to the other treatment modalities [25]. DiscoGel® induces its useful effects by some hypothetic mechanisms. Three significant hypotheses are:
- a diminishing of the intradiscal pressure as a result of induced dehydration of the nucleus
- a neurolytic effect on the growing neurons
- alcohol-induced necrosis of small piece of nucleus pulposus [12]
Initial and preliminary studies in 2007 and 2010 by Theron et al. [26,27] have defined satisfactory results along with the safety and efficacy of gelified ethanol in the percutaneous treatment of cervical and lumbar disc hernias. With no of adverse effects either during the procedure or after it, the authors showed promising results that suggest a feasible and safe alternative in the treatment of spinal disc hernias. Since 2014, many other investigations have shown that intradiscal DiscoGel® injection is a minimally invasive, low cost, safe, and effective intervention that may be a valuable choice in proper selected discopathies before making plans for surgery [12,13,28].
Compared with the findings obtained in the present study, using either PLDD or intradiscal injection of DiscoGel®, our results were satisfactory, as the overall therapeutic success rates were almost the same, although the decrease in functional disability was much greater in the DiscoGel® group. However, regarding the frequency of the need for secondary treatment options like conventional surgery after PLDD or intradiscal injection of DiscoGel®, a paucity of evidence exists.
In a newly designed retrospective, observational study by Klessinger [29] in 2018, the frequency of additional open surgery after PLDD in a long time period (10 yr) was examined retrospectively. The authors concluded that PLDD is not a replacement for open discectomy. Since it is broadly believed that conventional surgery is the gold standard treatment for patients with lumbar disc herniation and radiculopathies, PLDD and intradiscal injections of DiscoGel® need to be compared with conventional surgery, and the cost-effectiveness needs to be studied in multiple pain management centers along with larger sample sizes.
Along with documented benefits of DiscoGel®, an interesting finding was multiple reports concerning the role of disc microbial infection in disc degeneration. Pilot studies have shown infection rates when disc-only cultures are performed, and Propionibacterium acnes has been the predominant organism followed by Streptococcus sp. Therefore, the association of bacterial disc infection with the P. acnes strain in the induction of the same degenerative process as observed in patients with chronic LBP and Modic changes, have been recently proposed [30,31]. Many studies have claimed a strong connection between Modic changes and non-specific LBP and that is why so much attention is given to this pathologic change [32]. The prolonged antiseptic effect of gelified ethanol within a degenerative and potentially infected nucleus appears to be appropriate to fight infection, at least in theory, which can serve as an interesting issue for further studies on DiscoGel®.
The present analysis was performed in a Persian context, which limits the generalizability of findings since it may not be representative for other settings. Also, the lack of a comparison population for conservative therapies in the course of symptoms is another limitation for which future multi-central extensive studies with comparison groups are recommended to further document the safety, efficacy, and effectiveness of PLDD and intradiscal injection of DiscoGel® in discopathies. Although several cohort studies have been published, to date no study had been performed comparing PLDD with intradiscal injection of DiscoGel®.
In the present research, we introduced the first prospective cohort study on patient-reported outcomes of PLDD vs. intradiscal injection of DiscoGel® for radiculopathy in lumbar disc herniation from the points of pain and functional disability. Findings from our clinical investigations showed that both treatment modalities are equivalent in their clinical effects on pain, but DiscoGel® had a greater effect on decreasing the level of functional disability after 12 months of follow-up, although the rate of progression to secondary treatments and/or surgery were almost equal in the two groups.
Go to : 
ACKNOWLEDGMENTS
The authors would like to thank Atefe Alaee (aalaei@alum-nus.tums.ac.ir) for her critical reading of the manuscript and helpful comments.
Go to : 
Notes
Author contributions: Masoud Hashemi: Study conception; Payman Dadkhah: Methodology; Mehrdad Taheri: Investigation; Pegah Katibeh: Resources; Saman Asadi: Project administration.
Go to : 
REFERENCES
1. Govind J. Lumbar radicular pain. Aust Fam Physician. 2004; 33:409–12. PMID: 15253601.
2. Rhee JM, Schaufele M, Abdu WA. Radiculopathy and the herniated lumbar disc. Controversies regarding pathophysiology and management. J Bone Joint Surg Am. 2006; 88:2070–80. DOI: 10.2106/00004623-200609000-00023. PMID: 17036418.
3. Wheeler AH, Murrey DB. Chronic lumbar spine and radicular pain: pathophysiology and treatment. Curr Pain Headache Rep. 2002; 6:97–105. DOI: 10.1007/s11916-002-0005-x. PMID: 11872180.

4. Buenaventura RM, Datta S, Abdi S, Smith HS. Systematic review of therapeutic lumbar transforaminal epidural steroid injections. Pain Physician. 2009; 12:233–51. PMID: 19165306.
5. Hahne AJ, Ford JJ, McMeeken JM. Conservative management of lumbar disc herniation with associated radiculopathy: a systematic review. Spine (Phila Pa 1976). 2010; 35:E488–504. DOI: 10.1097/BRS.0b013e3181cc3f56. PMID: 20421859.
6. Menchetti PPM, Bini W. Percutaneous treatment in lumbar disc herniation. Minimally invasive surgery of the lumbar spine. Menchetti PPM, editor. London: Springer;2014. p. 83–105. DOI: 10.1007/978-1-4471-5280-4_4.

7. Skovrlj B, Gilligan J, Cutler HS, Qureshi SA. Minimally invasive procedures on the lumbar spine. World J Clin Cases. 2015; 3:1–9. DOI: 10.12998/wjcc.v3.i1.1. PMID: 25610845. PMCID: 4295214.

8. Ren L, Guo H, Zhang T, Han Z, Zhang L, Zeng Y. Efficacy evaluation of percutaneous laser disc decompression in the treatment of lumbar disc herniation. Photomed Laser Surg. 2013; 31:174–8. DOI: 10.1089/pho.2012.3402. PMID: 23565889.

9. Singh V, Manchikanti L, Calodney AK, Staats PS, Falco FJ, Caraway DL, et al. Percutaneous lumbar laser disc decompression: an update of current evidence. Pain Physician. 2013; 16(2 Suppl):SE229–60. PMID: 23615885.
10. Sobieraj A, Maksymowicz W, Barczewska M, Konopielko M, Mazur D. Early results of percutaneous laser disc decompression (PLDD) as a treatment of discopathic lumbar pain. Ortop Traumatol Rehabil. 2004; 6:264–9.
11. Guarnieri G, De Dominicis G, Muto M. Intradiscal and intramuscular injection of Discogel®—radiopaque gelified ethanol: pathological evaluation. Neuroradiol J. 2010; 23:249–52. DOI: 10.1177/197140091002300216. PMID: 24148546.

12. Léglise A, Lombard J, Moufid A. DiscoGel® in patients with discal lumbosciatica. Retrospective results in 25 consecutive patients. Orthop Traumatol Surg Res. 2015; 101:623–6. DOI: 10.1016/j.otsr.2015.05.007. PMID: 26215088.
13. Volpentesta G, De Rose M, Bosco D, Stroscio C, Guzzi G, Bombardieri C, et al. Lumbar percutaneous intradiscal injection of radiopaque gelified ethanol (“Discogel”) in patients with low back and radicular pain. J Pain Relief. 2014; 3:145. DOI: 10.4172/2167-0846.1000145.

14. Haefeli M, Elfering A. Pain assessment. Eur Spine J. 2006; 15(Suppl 1):S17–24. DOI: 10.1007/s00586-005-1044-x. PMID: 16320034. PMCID: 3454549.

15. Fairbank JC, Pynsent PB. The Oswestry disability index. Spine (Phila Pa 1976). 2000; 25:2940–52. DOI: 10.1097/00007632-200011150-00017. PMID: 11074683.

16. Zhao XL, Fu ZJ, Xu YG, Zhao XJ, Song WG, Zheng H. Treatment of lumbar intervertebral disc herniation using C-arm fluoroscopy guided target percutaneous laser disc decompression. Photomed Laser Surg. 2012; 30:92–5. DOI: 10.1089/pho.2011.3050. PMID: 22150064. PMCID: 3270046.

17. Knezevic NN, Mandalia S, Raasch J, Knezevic I, Candido KD. Treatment of chronic low back pain - new approaches on the horizon. J Pain Res. 2017; 10:1111–23. DOI: 10.2147/JPR.S132769. PMID: 28546769. PMCID: 5436786.
18. Epstein NE. Should anyone perform percutaneous endoscopic laser diskectomy and percutaneous lumbar disc decompressions? Surg Neurol Int. 2016; 7(Suppl 42):S1080–4. DOI: 10.4103/2152-7806.196764. PMID: 28144489. PMCID: 5234304.

19. Ishiwata Y, Takada H, Gondo G, Osano S, Hashimoto T, Yamamoto I. Magnetic resonance-guided percutaneous laser disk decompression for lumbar disk herniation--relationship between clinical results and location of needle tip. Surg Neurol. 2007; 68:159–63. DOI: 10.1016/j.surneu.2006.10.058. PMID: 17662349.

20. Haufe SM, Mork AR, Pyne M, Baker RA. Percutaneous laser disc decompression for thoracic disc disease: report of 10 cases. Int J Med Sci. 2010; 7:155–9. DOI: 10.7150/ijms.7.155. PMID: 20567616. PMCID: 2880844.

21. Lee DY, Lee SH. Carbon dioxide (CO2) laser-assisted microdiscectomy for extraforaminal lumbar disc herniation at the L5-S1 level. Photomed Laser Surg. 2011; 29:531–5. DOI: 10.1089/pho.2010.2954. PMID: 21309702.

22. Ren L, Guo B, Zhang T, Bai Q, Wang XH, Zhang L, et al. Medium-term follow-up findings in imaging manifestation after percutaneous laser disc decompression. Photomed Laser Surg. 2013; 31:247–51. DOI: 10.1089/pho.2012.3433. PMID: 23741993.

23. Hashemi SM, Dadkhah P, Taheri M, Abootorabi SMHS. Effects of percutaneous laser disc decompression (PLDD) on clinical outcome of teared vs intact protruded intervertebral discs: a prospective cohort study. 2018; 2:279–85.
24. Brouwer PA, Brand R, van den Akker-van Marle ME, Jacobs WC, Schenk B, van den Berg-Huijsmans AA, et al. Percutaneous laser disc decompression versus conventional microdiscectomy in sciatica: a randomized controlled trial. Spine J. 2015; 15:857–65. DOI: 10.1016/j.spinee.2015.01.020. PMID: 25614151.

25. Asgharzadeh A, Khoshnood N. Evaluating the safety and efficacy of discogel in the treatment of herniated lumbar disc: a systematic review. Health Tech Asmnt Act. 2017; 1:e62329. DOI: 10.5812/htaa.62329.

26. Theron J, Cuellar H, Sola T, Guimaraens L, Casasco A, Courtheoux P. Percutaneous treatment of cervical disk hernias using gelified ethanol. AJNR Am J Neuroradiol. 2010; 31:1454–6. DOI: 10.3174/ajnr.A1923. PMID: 20053805.

27. Theron J, Guimaraens L, Casasco A, Sola T, Cuellar H, Courtheoux P. Percutaneous treatment of lumbar intervertebral disk hernias with radiopaque gelified ethanol: a preliminary study. J Spinal Disord Tech. 2007; 20:526–32. DOI: 10.1097/BSD.0b013e318033e860. PMID: 17912130.
28. Bellini M, Romano DG, Leonini S, Grazzini I, Tabano C, Ferrara M, et al. Percutaneous injection of radiopaque gelified ethanol for the treatment of lumbar and cervical intervertebral disk herniations: experience and clinical outcome in 80 patients. AJNR Am J Neuroradiol. 2015; 36:600–5. DOI: 10.3174/ajnr.A4166. PMID: 25395657.

29. Klessinger S. The frequency of resurgery after percutaneous lumbar surgery using dekompressor in a ten-year period. Minim Invasive Surg. 2018; 2018:5286760. DOI: 10.1155/2018/5286760. PMID: 30402284. PMCID: 6198552.

30. Zamora T, Palma J, Andia M, Garcia P, Wozniak A, Solar A, et al. Effect of propionibacterium acnes (PA) injection on intervertebral disc degeneration in a rat model: does it mimic modic changes? Orthop Traumatol Surg Res. 2017; 103:795–9. DOI: 10.1016/j.otsr.2017.04.005. PMID: 28552835.

31. Rao PJ, Phan K, Reddy R, Scherman DB, Taylor P, Mobbs RJ. DISC (degenerate-disc infection study with contaminant control): pilot study of Australian cohort of patients without the contaminant control. Spine (Phila Pa 1976). 2016; 41:935–9. DOI: 10.1097/BRS.0000000000001404. PMID: 26679882.
32. Chen Z, Zheng Y, Yuan Y, Jiao Y, Xiao J, Zhou Z, et al. Modic changes and disc degeneration caused by inoculation of propionibacterium acnes inside intervertebral discs of rabbits: a pilot study. Biomed Res Int. 2016; 2016:9612437. DOI: 10.1155/2016/9612437. PMID: 26925420. PMCID: 4746301.
Go to : 




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download