INTRODUCTION
The acromion is a clinically important anatomical feature of the coracoacromical arch. Anatomic changes to the acromion and the acromioclavicular joint can reduce the volume of the supraspinatous opening and increase mechanical degeneration and wear of underlying soft tissue [
1]. Shoulder impingement syndrome (SIS) occurs between the rotator cuff and the acromion and involves the acromioclavicular joint and the coracoacromial ligament [
1–
4]. SIS typically leads to weakness and/or pain around the shoulder region, loss of movement in the affected shoulder, and difficulty in sleeping [
5,
6]. Osteophytes, hypertrophic changes, and bony spurs in the acromion have been considered major causes of SIS. Subacromial or acromioclavicular spurs were reported to be observed in about 50% of SIS cases [
6]. Most anatomic studies have focused on morphologic classifications of the acromion, such as being of a flat, curved, hooked, or convex type [
7–
9]. However, morphologic classification does not reflect hypertrophic changes in the acromion, such as osteophytes and bony spurs. Moreover, the shape of the acromion is not always regular, and the directions of the axis of the acromion surface cannot be determined [
10]. Thus, for evaluating hypertrophic changes in the whole acromion, a new morphological parameter called the acromion process cross-sectional area (APA) was devised by measuring the maximum cross-sectional area of the acromion process. Unlike conventional morphological classifications of the acromion, APA reflects hypertrophic changes. We hypothesized that the APA would be a key morphologic parameter in the diagnosis of SIS. There are no previous reports of an association between SIS and the whole cross-sectional acromion area as a morphologic parameter on magnetic resonance imaging (MRI). Thus, we compared the APA between SIS patients and healthy controls using MRI. The aims of this retrospective study were to investigate the optimal cut-off value of APA in patients with SIS and to evaluate the usefulness of the APA as an objective diagnostic tool in determining SIS.
Go to :

MATERIALS AND METHODS
1. Patients
We registered this study at the Catholic Kwandong University College of Medicine, Republic of Korea (IS17RISI0042), and the University’s Institutional Review Board reviewed and approved the research protocol. We retrospectively reviewed patients who had visited Catholic Kwandong University International St. Mary’s Hospital from March 2014 to June 2017 and who had been diagnosed with SIS. All patients were enrolled after the diagnosis of SIS was confirmed by two experienced, board-certified, musculoskeletal radiologists. The SIS group comprised 95 patients (41 males and 54 females).
The inclusion criteria for the SIS group were: 1) positive shoulder impingement signs (Hawkins–Kennedy test or Neer’s test); 2) shoulder pain during active arm elevation; 3) a shoulder MRI taken within 12 months of the first diagnosis and available for review; 4) a painful arc; and 5) weakness or pain with resisting scapular plane abduction on internal humeral rotation or resisting isometric external rotation. Exclusion criteria were: 1) a history of upper arm fracture; 2) shoulder pain with passive or active cervical spine motion; 3) a history of shoulder surgery; 4) evidence of frozen shoulder; and 5) a full-thickness rotator cuff tear.
To compare the APAs between patients with and without SIS, we also enrolled a control group of individuals who had undergone shoulder MRI as a part of routine medical examinations. We only enrolled patients in the control group who had no complaints of upper arm or shoulder pain, without matching them to the SIS group. The control group comprised 126 individuals (70 males and 56 females) with mean ages of 53.2 ± 8.7 years (range, 33–73 yr) in the male group and 53.3 ± 7.0 years (range, 39–71 yr) in the female group (
Table 1). We excluded patients from the control group if they reported any known shoulder pathology or met any of the above mentioned exclusion criteria.
Table 1
Comparison of the Characteristics of Control, and SIS Group
|
Parameter |
Male |
Female |
|
|
|
Control group (n = 70) |
SIS group (n = 41) |
Control group (n = 56) |
SIS group (n = 54) |
|
Age (yr) |
53.2 ± 8.7 |
57.6 ± 6.9 |
53.3 ± 7.0 |
58.1 ± 7.4 |
|
APA (mm2) |
136.5 ± 21.8 |
202.9 ± 31.8*
|
105.4 ± 19.1 |
147.6 ± 22.9*
|

2. MRI scanning protocol
All MRI scans were taken at the Department of Orthopedics and Shoulder Center, Catholic Kwandong University International St. Mary’s Hospital. In both groups, the MRIs were taken with the same technique and position using proton density fast spin-echo (FSE) imaging. The MRI scans were taken on 3T scanners (Magnetom Skyra, Sonata, Biograph, Avanto [Siemens Healthcare GmbH, Erlangen, Germany]; Philips Ingenia [R4] [Philips Medical Systems, Best, The Netherlands]) with a dedicated shoulder coil (Med Rad Multipurpose Array, Indianola, PA). Pulse sequence parameters included oblique coronal FSE fat suppression (repetition time [TR]: 3,000 msec, echo time [TE]: 90 msec, echo train length [ETL]: 15, matrix: 448 × 270, slice thickness: 4 mm, field of view: 16 cm). The following other parameters were used: a 0.4 mm intersection gap, 2,700 msec/95 msec TR/TE, 300 cm × 300 cm field of view, 358 × 512 matrix, and 15 ETL.
3. Image analysis
We measured the APA in the oblique coronal proton density FSE view using a picture archiving and communications system (INFINITT Healthcare, Seoul, Korea) by outlining the acromion process (
Fig. 1). Among several slices on the oblique coronal view, the one showing the maximal APA was selected and used for analysis.
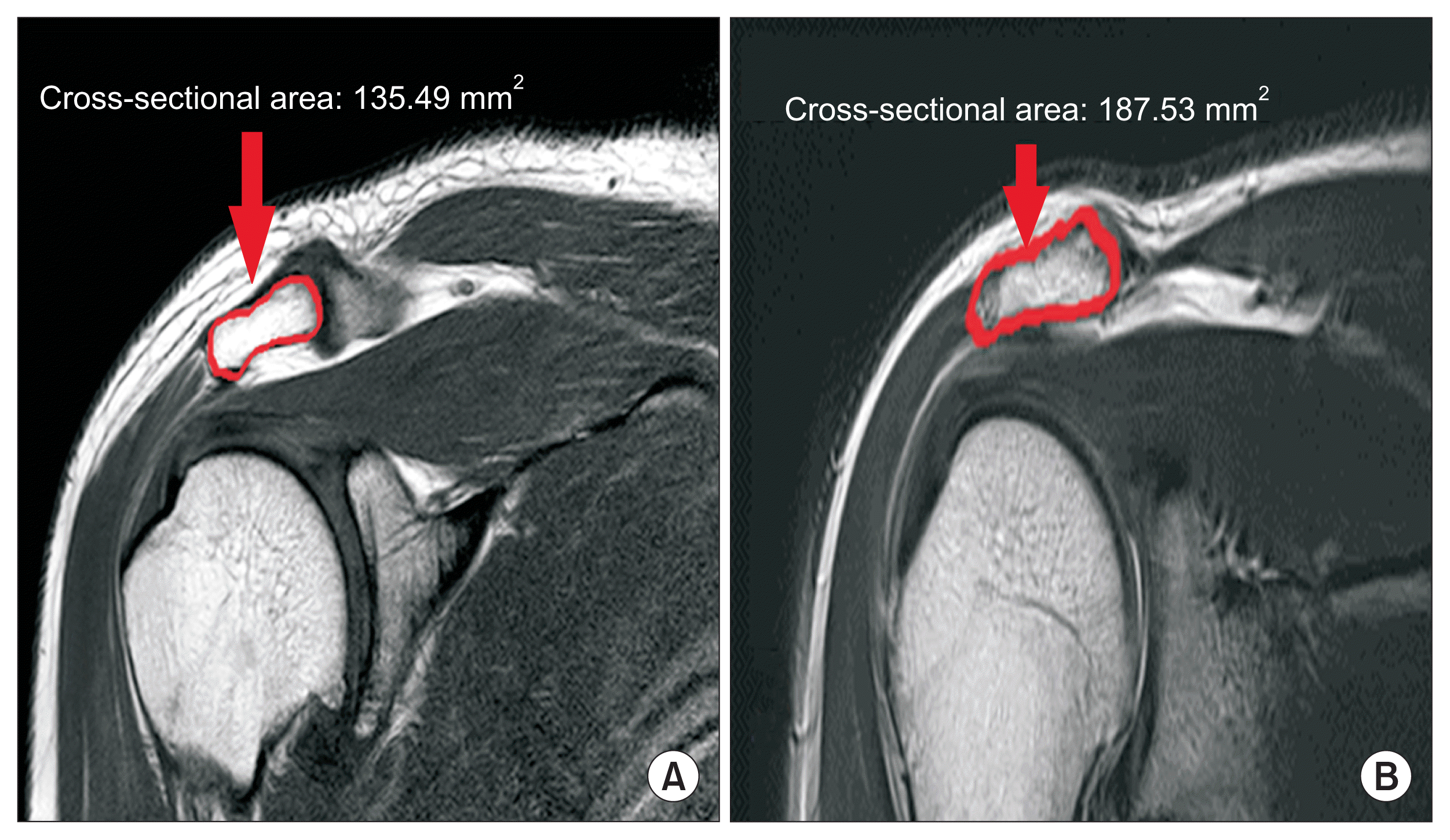 | Fig. 1Oblique coronal proton density fast spin-echo magnetic resonance imaging in acromion sections: (A) normal control male; (B) shoulder impingement syndrome. 
|
4. Statistical analyses
According to the previous study [
11], the effect size was 0.57, and it was calculated that 18 patients per group were required to obtain a power of 0.95, considering an alpha error of 0.05. When the dropout rate was set at approximately 20%, at least 95 medical records were reviewed for each group. Data are expressed as mean ± standard deviation. We compared the APAs between the control and SIS groups by demographic characteristics using unpaired
t-tests. We estimated the validity of the APA for diagnosis of SIS with receiver operator characteristic (ROC) curves, an optimal cut-off score, area under the curve (AUC), sensitivity, and specificity with 95% confidence intervals (CIs). We used IBM SPSS ver. 21 (IBM Corp., Armonk, NY) for the statistical analyses, and we considered
P < 0.05 to be statistically significant.
Go to :

RESULTS
The mean age of the male control and SIS group was 53.2 ± 8.7 and 57.6 ± 6.9 years, respectively. The mean age of the female control subjects was 53.3 ± 7.0 years and that of the female SIS patients was 58.1 ± 7.4 years. Sex and age were not significantly different between the groups. The average APA was 136.50 ± 21.75 mm
2 in the male control group and 202.91 ± 31.78 mm
2 in the male SIS group; the SIS patients had significantly greater APAs (
P < 0.001) than the male controls. The average APA in the female control and SIS group was 105.38 ± 19.07 and 147.62 ± 22.90 mm
2, respectively; these SIS patients also had significantly greater APAs (
P < 0.001) than the female controls (
Table 1). Regarding the validity of the APA as a predictor of SIS, ROC curve analysis showed that the optimal cut-off value of the APA in males was 165.14 mm
2 with 90.2% sensitivity, 91.4% specificity (
Table 2), and an AUC of 0.968 (95% CI: 0.941–0.995), and in females, it was 122.50 mm
2 with 85.2% sensitivity, 84.9% specificity (
Table 3), and an AUC of 0.928 (95% CI: 0.882–0.974).
Table 2
Sensitivity and Specificity of Each Cut-off Value of the APA in Male
|
APA (mm2) |
Sensitivity (%) |
Specificity (%) |
|
124.95 |
100.0 |
28.6 |
|
150.44 |
97.6 |
77.1 |
|
156.62 |
95.1 |
81.4 |
|
165.14a
|
90.2 |
91.4 |
|
172.53 |
80.5 |
92.9 |
|
191.67 |
61.0 |
100.0 |

Table 3
Sensitivity and Specificity of Each Cut-off Value of the APA in Female
|
APA (mm2) |
Sensitivity (%) |
Specificity (%) |
|
104.85 |
100.0 |
50 |
|
120.04 |
90.7 |
76.8 |
|
121.81 |
87.0 |
82.1 |
|
122.50a
|
85.2 |
84.9 |
|
130.73 |
74.1 |
89.3 |
|
156.51 |
31.5 |
100.0 |

Go to :

DISCUSSION
There are no objective morphologic parameters to indicate acromion process hypertrophic changes. We believed that the cross-sectional area of the acromion process could be an objective, precise, clear measurement parameter for evaluating hypertrophic changes in the acromion process. In our present research, we measured the APA from oblique coronal proton density FSE images. To our best knowledge, an association between SIS and APA as a morphologic parameter on MRI has not been reported previously.
SIS is a common disorder in elderly and middle-aged populations that usually involves shoulder pain [
10,
12]. Any abnormality that disturbs the relationship of the coracoacromial arch may lead to SIS [
9]. The acromion process projects forward at right angles from the lateral end of the scapular spine. It is an important component of the subacromial space, which forms the superior boundary of the coracoacromial arch [
9]. The coracoacromial ligament spans between the coracoid process and the tip of the acromion to form the coracoacromial arch [
13,
14]. The space below the arch gives passage to the rotator cuff tendons of muscles [
9]. Morphological changes and variations in the acromion process and its relationship with the coracoid process and glenoid cavity are the most relevant determinants of the coracoacromial arch space [
15]. Thus, previous studies focused on the cause-and-effect relationship between acromial morphology and SIS. Saha and Vasudeva [
9] reported three types of acromial morphology, flat, curved, and hooked, and Gagey et al. [
16] described a fourth type, convex. Kitay et al. [
17] described the acromial tilt. Banas et al. [
18] observed the frontal plane slope of the acromion process on MRI and found smaller lateral acromial angles in patients with SIS. Nyffeler et al. [
19] demonstrated that the acromion process in patients with SIS appeared to have more lateral extension than that in normal controls, and they described the acromion index. Despite these numerous previous studies, they did not reflect degenerative hypertrophic changes to the acromion process, such as enthesophytes, bony spurs, and osteophytes [
8].
Few studies have investigated the anatomical basis of the acromion process. Hughes et al. [
5] asserted that the lack of anatomical validity to support the use of clinical evaluations may explain their poor diagnostic accuracy, and Mayerhoefer et al. [
20] contended that acromial morphology is not a good descriptor of coracoaromial arch narrowing. Aydin et al. [
2] found that acromion type was not an important predictor of SIS. Our data demonstrate an association between APA and SIS; SIS patients had significantly greater APAs than those in the control subjects.
Our interpretation of this association is that hypertrophy of the acromion process might be related to continuous stress, which might increase the APA. The process of acromion hypertrophy begins with mechanical stress during shoulder movements, which leads to increased force on the acromion process and extensive abrasion. This etiology could alter the morphologic features of the acromion process. Takase and Yamamoto [
21] concluded that the advanced degenerative hypertrophic changes on the undersurface of the acromion process result in full-thickness cuff tears. ROC analysis showed that the APA had high diagnostic accuracy for detecting SIS (AUC: 0.968 in males and 0.928 in females). We suggest that the APA is a precise, objective, and clear morphological predictor of SIS.
The present study had several limitations. First, there might have been errors associated with measuring the APA on MRI. We attempted to measure the maximal APA at the level of the acromioclavicular joint. However, the method of analyzing a single MRI slice to measure the maximum cross-sectional area among several slices may be inconsistent due to technical problems, rater reliability, or individual anatomic variations. To overcome these shortcomings, it is necessary to measure the three dimensional (3D) reconstructed volume with less effect on cutting angle or anatomic variations. But we didn’t. Since the volume in 3D can reflect hypertrophy more accurately than the maximal APA, a study using 3D reconstructed volume may be needed in the future study. Also, it is required in further studies to establish reproducibility by measuring reliability to prevent intra-rater or inter-rater error. Second, SIS represents a combination of complex pathogenic causes, including the coracoacromial ligament, subacromial space [
22], and supraspinatous tendon thickness [
23], but we focused only on the acromion process. Future studies should assess the association between extrinsic direct mechanical compression and intrinsic changes in the supraspinatus tendon. Third, there are different stages of SIS, but in this study, we did not compare patients with intact rotator cuffs and patients with rotator cuff tears. Forth, this study is designed to evaluate only the relevance of APA and SIS. Further prospective study about the severity of disease or pain intensity related to APA is thought to be of great help to clinicians. In spite of these limitations, this is the first research to document that the APA is associated with SIS.
In conclusion, APA is a new, sensitive morphological parameter for evaluating SIS. The optimal APA cut-off points were 165.14 mm2 for males (90.2% sensitivity, 91.4% specificity, and an AUC of 0.968) and 122.50 mm2 for females (85.2% sensitivity, 84.9% specificity, and an AUC of 0.928). We hope that this new measurement technique will be helpful for assessing SIS.
Go to :





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download