1. Hatch MN, Cushing TR, Carlson GD, Chang EY. Neuropathic pain and SCI: identification and treatment strategies in the 21st century. J Neurol Sci. 2018; 384:75–83. DOI:
10.1016/j.jns.2017.11.018. PMID:
29249383.

3. Cao Q, Benton RL, Whittemore SR. Stem cell repair of central nervous system injury. J Neurosci Res. 2002; 68:501–10. DOI:
10.1002/jnr.10240. PMID:
12111840.

5. Lindvall O, Kokaia Z. Stem cells for the treatment of neurological disorders. Nature. 2006; 441:1094–6. DOI:
10.1038/nature04960. PMID:
16810245.

6. Klass M, Gavrikov V, Drury D, Stewart B, Hunter S, Denson DD, et al. Intravenous mononuclear marrow cells reverse neuropathic pain from experimental mononeuropathy. Anesth Analg. 2007; 104:944–8. DOI:
10.1213/01.ane.0000258021.03211.d0. PMID:
17377111.

7. Meirelles Lda S, Nardi NB. Methodology, biology and clinical applications of mesenchymal stem cells. Front Biosci (Landmark Ed). 2009; 14:4281–98. DOI:
10.2741/3528. PMID:
19273350.
8. Amemori T, Jendelová P, Růzicková K, Arboleda D, Syková E. Co-transplantation of olfactory ensheathing glia and mesenchymal stromal cells does not have synergistic effects after spinal cord injury in the rat. Cytotherapy. 2010; 12:212–25. DOI:
10.3109/14653240903440103. PMID:
20196694.

9. Schäfer S, Berger JV, Deumens R, Goursaud S, Hanisch UK, Hermans E. Influence of intrathecal delivery of bone marrow-derived mesenchymal stem cells on spinal inflammation and pain hypersensitivity in a rat model of peripheral nerve injury. J Neuroinflammation. 2014; 11:157. DOI:
10.1186/s12974-014-0157-8. PMID:
25212534. PMCID:
4172959.

10. Siniscalco D, Giordano C, Galderisi U, Luongo L, de Novellis V, Rossi F, et al. Long-lasting effects of human mesenchymal stem cell systemic administration on pain-like behaviors, cellular, and biomolecular modifications in neuropathic mice. Front Integr Neurosci. 2011; 5:79. DOI:
10.3389/fnint.2011.00079. PMID:
22164136. PMCID:
3230031.

11. Zhang EJ, Song CH, Ko YK, Lee WH. Intrathecal administration of mesenchymal stem cells reduces the reactive oxygen species and pain behavior in neuropathic rats. Korean J Pain. 2014; 27:239–45. DOI:
10.3344/kjp.2014.27.3.239. PMID:
25031809. PMCID:
4099236.

12. Franchi S, Valsecchi AE, Borsani E, Procacci P, Ferrari D, Zalfa C, et al. Intravenous neural stem cells abolish nociceptive hypersensitivity and trigger nerve regeneration in experimental neuropathy. Pain. 2012; 152:850–61. DOI:
10.1016/j.pain.2012.01.008. PMID:
22321918.

13. Liu L, Hua Z, Shen J, Yin Y, Yang J, Cheng K, et al. Comparative efficacy of multiple variables of mesenchymal stem cell transplantation for the treatment of neuropathic pain in rats. Mil Med. 2017; 182:175–84. DOI:
10.7205/MILMED-D-16-00096. PMID:
28291470.

14. Siniscalco D, Giordano C, Galderisi U, Luongo L, Alessio N, Di Bernardo G, et al. Intra-brain microinjection of human mesenchymal stem cells decreases allodynia in neuropathic mice. Cell Mol Life Sci. 2010; 67:655–69. DOI:
10.1007/s00018-009-0202-4. PMID:
19937263.

15. Vaquero J, Zurita M, Oya S, Santos M. Cell therapy using bone marrow stromal cells in chronic paraplegic rats: systemic or local administration? Neurosci Lett. 2006; 398:129–34. DOI:
10.1016/j.neulet.2005.12.072. PMID:
16423458.

16. Chen G, Park CK, Xie RG, Ji RR. Intrathecal bone marrow stromal cells inhibit neuropathic pain via TGF-β secretion. J Clin Invest. 2015; 125:3226–40. DOI:
10.1172/JCI80883. PMID:
26168219. PMCID:
4563753.

17. Chen C, Chen F, Yao C, Shu S, Feng J, Hu X, et al. Intrathecal injection of human umbilical cord-derived mesenchymal stem cells ameliorates neuropathic pain in rats. Neurochem Res. 2016; 41:3250–60. DOI:
10.1007/s11064-016-2051-5. PMID:
27655256.

18. Bonfield TL, Caplan AI. Adult mesenchymal stem cells: an innovative therapeutic for lung diseases. Discov Med. 2010; 9:337–45. PMID:
20423678.
19. Pan HC, Cheng FC, Chen CJ, Lai SZ, Lee CW, Yang DY, et al. Post-injury regeneration in rat sciatic nerve facilitated by neurotrophic factors secreted by amniotic fluid mesenchymal stem cells. J Clin Neurosci. 2007; 14:1089–98. DOI:
10.1016/j.jocn.2006.08.008. PMID:
17954375.

20. Kim HK, Park SK, Zhou JL, Taglialatela G, Chung K, Coggeshall RE, Chung JM. Reactive oxygen species (ROS) play an important role in a rat model of neuropathic pain. Pain. 2004; 111:116–24. DOI:
10.1016/j.pain.2004.06.008. PMID:
15327815.

21. Pisati F, Bossolasco P, Meregalli M, Cova L, Belicchi M, Gavina M, et al. Induction of neurotrophin expression via human adult mesenchymal stem cells: implication for cell therapy in neurodegenerative diseases. Cell Transplant. 2007; 16:41–55. DOI:
10.3727/000000007783464443. PMID:
17436854.

22. Siniscalco D, Rossi F, Maione S. Stem cell therapy for neuropathic pain treatment. J Stem Cells Regen Med. 2007; 3:2–11. PMID:
24693013. PMCID:
3908122.

23. Coderre TJ, Xanthos DN, Francis L, Bennett GJ. Chronic post-ischemia pain (CPIP): a novel animal model of complex regional pain syndrome-type I (CRPS-I; reflex sympathetic dystrophy) produced by prolonged hindpaw ischemia and reperfusion in the rat. Pain. 2004; 112:94–105. DOI:
10.1016/j.pain.2004.08.001. PMID:
15494189.

25. Kim H, Kim HY, Choi MR, Hwang S, Nam KH, Kim HC, et al. Dose-dependent efficacy of ALS-human mesenchymal stem cells transplantation into cisterna magna in SOD1-G93A ALS mice. Neurosci Lett. 2010; 468:190–4. DOI:
10.1016/j.neulet.2009.10.074. PMID:
19879334.

26. Bonin RP, Bories C, De Koninck Y. A simplified up-down method (SUDO) for measuring mechanical nociception in rodents using von Frey filaments. Mol Pain. 2014; 10:26. DOI:
10.1186/1744-8069-10-26. PMID:
24739328. PMCID:
4020614.

27. Giordano A, Galderisi U, Marino IR. From the laboratory bench to the patient’s bedside: an update on clinical trials with mesenchymal stem cells. J Cell Physiol. 2007; 211:27–35. DOI:
10.1002/jcp.20959. PMID:
17226788.

28. Lin CR, Wu PC, Shih HC, Cheng JT, Lu CY, Chou AK, et al. Intrathecal spinal progenitor cell transplantation for the treatment of neuropathic pain. Cell Transplant. 2002; 11:17–24. DOI:
10.3727/096020198389744. PMID:
12095216.

29. Eaton MJ, Plunkett JA, Martinez MA, Lopez T, Karmally S, Cejas P, et al. Transplants of neuronal cells bioengineered to synthesize GABA alleviate chronic neuropathic pain. Cell Transplant. 1999; 8:87–101. DOI:
10.1177/096368979900800102. PMID:
10338278.

30. Franchi S, Castelli M, Amodeo G, Niada S, Ferrari D, Vescovi A, et al. Adult stem cell as new advanced therapy for experimental neuropathic pain treatment. Biomed Res Int. 2014; 2014:470983. DOI:
10.1155/2014/470983. PMID:
25197647. PMCID:
4147203.

31. Siniscalco D, Giordano C, Galderisi U, Luongo L, Alessio N, Di Bernardo G, et al. Human mesenchymal stem cells as novel neuropathic pain tool. J Stem Cells Regen Med. 2010; 6:127. PMID:
24693137.
32. Zurita M, Vaquero J. Functional recovery in chronic paraplegia after bone marrow stromal cells transplantation. Neuroreport. 2004; 15:1105–8. DOI:
10.1097/00001756-200405190-00004. PMID:
15129154.

33. Soleymaninejadian E, Pramanik K, Samadian E. Immunomodulatory properties of mesenchymal stem cells: cytokines and factors. Am J Reprod Immunol. 2012; 67:1–8. DOI:
10.1111/j.1600-0897.2011.01069.x. PMID:
21951555.

34. Knaän-Shanzer S. Concise review: the immune status of mesenchymal stem cells and its relevance for therapeutic application. Stem Cells. 2014; 32:603–8. DOI:
10.1002/stem.1568. PMID:
24123756.

36. Goris RJ. Reflex sympathetic dystrophy: model of a severe regional inflammatory response syndrome. World J Surg. 1998; 22:197–202. DOI:
10.1007/s002689900369. PMID:
9451936.

37. Guo W, Wang H, Zou S, Gu M, Watanabe M, Wei F, et al. Bone marrow stromal cells produce long-term pain relief in rat models of persistent pain. Stem Cells. 2011; 29:1294–303. DOI:
10.1002/stem.667. PMID:
21630378. PMCID:
3277433.

38. Siniscalco D. Transplantation of human mesenchymal stem cells in the study of neuropathic pain. Methods Mol Biol. 2010; 617:337–45. DOI:
10.1007/978-1-60327-323-7_25. PMID:
20336433.

39. Musolino PL, Coronel MF, Hökfelt T, Villar MJ. Bone marrow stromal cells induce changes in pain behavior after sciatic nerve constriction. Neurosci Lett. 2007; 418:97–101. DOI:
10.1016/j.neulet.2007.03.001. PMID:
17379405.

40. Naruse K, Sato J, Funakubo M, Hata M, Nakamura N, Kobayashi Y, et al. Transplantation of bone marrow-derived mononuclear cells improves mechanical hyperalgesia, cold allodynia and nerve function in diabetic neuropathy. PLoS One. 2011; 6:e27458. DOI:
10.1371/journal.pone.0027458. PMID:
22125614. PMCID:
3220696.

41. Allen G, Galer BS, Schwartz L. Epidemiology of complex regional pain syndrome: a retrospective chart review of 134 patients. Pain. 1999; 80:539–44. DOI:
10.1016/S0304-3959(98)00246-2. PMID:
10342415.

42. Maleki J, LeBel AA, Bennett GJ, Schwartzman RJ. Patterns of spread in complex regional pain syndrome, type I (reflex sympathetic dystrophy). Pain. 2000; 88:259–66. DOI:
10.1016/S0304-3959(00)00332-8. PMID:
11068113.

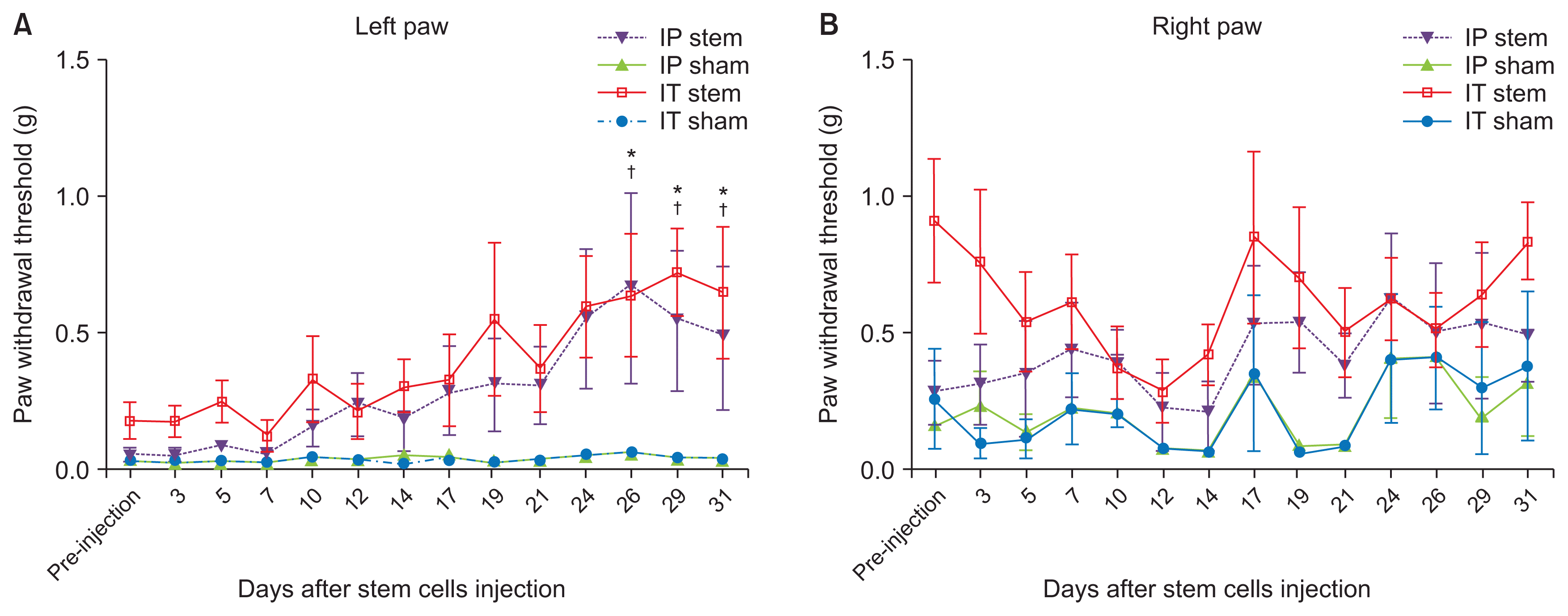
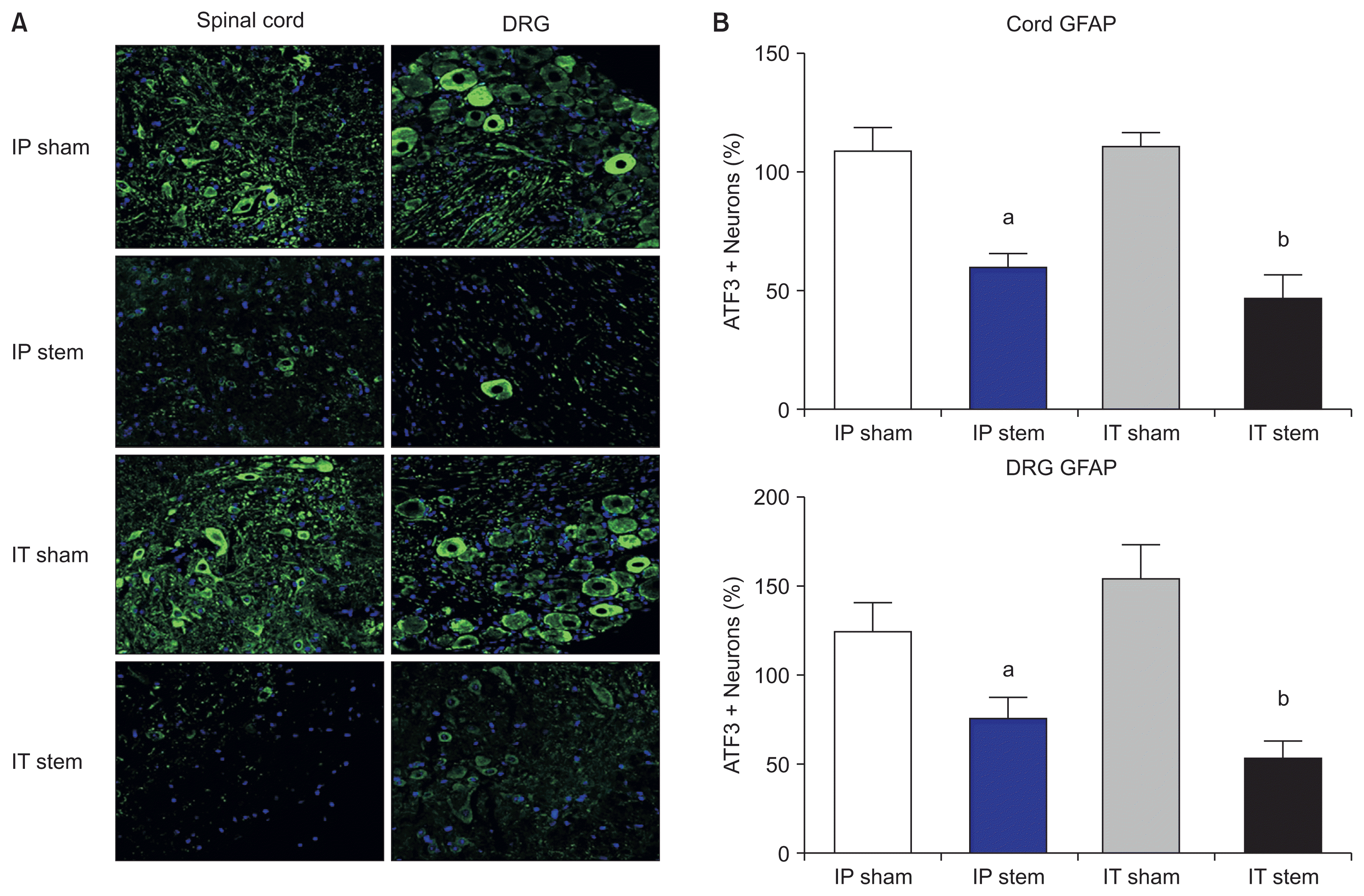




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download