1. Kamble SV, Motlekar SA, D’souza LL, Kudrigikar VN, Rao SE. Low doses of amitriptyline, pregabalin, and gabapentin are preferred for management of neuropathic pain in India: is there a need for revisiting dosing recommendations? Korean J Pain. 2017; 30:183–91. DOI:
10.3344/kjp.2017.30.3.183. PMID:
28757918. PMCID:
5532525.

2. Werhagen L, Budh CN, Hultling C, Molander C. Neuropathic pain after traumatic spinal cord injury--relations to gender, spinal level, completeness, and age at the time of injury. Spinal Cord. 2004; 42:665–73. DOI:
10.1038/sj.sc.3101641. PMID:
15289801.

3. Castro MM, Daltro C. Sleep patterns and symptoms of anxiety and depression in patients with chronic pain. Arq Neuropsiquiatr. 2009; 67:25–8. DOI:
10.1590/S0004-282X2009000100007. PMID:
19330205.

4. Gore M, Brandenburg NA, Dukes E, Hoffman DL, Tai KS, Stacey B. Pain severity in diabetic peripheral neuropathy is associated with patient functioning, symptom levels of anxiety and depression, and sleep. J Pain Symptom Manage. 2005; 30:374–85. DOI:
10.1016/j.jpainsymman.2005.04.009. PMID:
16256902.

5. Gormsen L, Rosenberg R, Bach FW, Jensen TS. Depression, anxiety, health-related quality of life and pain in patients with chronic fibromyalgia and neuropathic pain. Eur J Pain. 2010; 14:127.e1–8. DOI:
10.1016/j.ejpain.2009.03.010. PMID:
19473857.

6. McDermott AM, Toelle TR, Rowbotham DJ, Schaefer CP, Dukes EM. The burden of neuropathic pain: results from a cross-sectional survey. Eur J Pain. 2006; 10:127–35. DOI:
10.1016/j.ejpain.2005.01.014. PMID:
16310716.

7. Gustorff B, Dorner T, Likar R, Grisold W, Lawrence K, Schwarz F, et al. Prevalence of self-reported neuropathic pain and impact on quality of life: a prospective representative survey. Acta Anaesthesiol Scand. 2008; 52:132–6. DOI:
10.1111/j.1399-6576.2007.01486.x. PMID:
17976220.

8. Hassanijirdehi M, Khak M, Afshari-Mirak S, Holakouie-Naieni K, Saadat S, Taheri T, et al. Evaluation of pain and its effect on quality of life and functioning in men with spinal cord injury. Korean J Pain. 2015; 28:129–36. DOI:
10.3344/kjp.2015.28.2.129. PMID:
25852835. PMCID:
4387458.

9. Lude P, Kennedy P, Elfström ML, Ballert CS. Quality of life in and after spinal cord injury rehabilitation: a longitudinal multicenter study. Top Spinal Cord Inj Rehabil. 2014; 20:197–207. DOI:
10.1310/sci2003-197. PMID:
25484566. PMCID:
4257147.

10. Dermanovic Dobrota V, Hrabac P, Skegro D, Smiljanic R, Dobrota S, Prkacin I, et al. The impact of neuropathic pain and other comorbidities on the quality of life in patients with diabetes. Health Qual Life Outcomes. 2014; 12:171. DOI:
10.1186/s12955-014-0171-7. PMID:
25468384. PMCID:
4264315.

11. Geyh S, Ballert C, Sinnott A, Charlifue S, Catz A, D’Andrea Greve JM, et al. Quality of life after spinal cord injury: a comparison across six countries. Spinal Cord. 2013; 51:322–6. DOI:
10.1038/sc.2012.128. PMID:
23147129.

12. Leem YJ, Joh JW, Joeng KW, Suh JH, Shin JW, Leem JG. Central pain from excitotoxic spinal cord injury induced by intraspinal NMDA injection: a pilot study. Korean J Pain. 2010; 23:109–15. DOI:
10.3344/kjp.2010.23.2.109. PMID:
20556212. PMCID:
2886245.

13. Wollaars MM, Post MW, van Asbeck FW, Brand N. Spinal cord injury pain: the influence of psychologic factors and impact on quality of life. Clin J Pain. 2007; 23:383–91. DOI:
10.1097/AJP.0b013e31804463e5. PMID:
17515736.

14. Smith BH, Torrance N, Bennett MI, Lee AJ. Health and quality of life associated with chronic pain of predominantly neuropathic origin in the community. Clin J Pain. 2007; 23:143–9. DOI:
10.1097/01.ajp.0000210956.31997.89. PMID:
17237663.

15. Gálvez R, Marsal C, Vidal J, Ruiz M, Rejas J. Cross-sectional evaluation of patient functioning and health-related quality of life in patients with neuropathic pain under standard care conditions. Eur J Pain. 2007; 11:244–55. DOI:
10.1016/j.ejpain.2006.02.002. PMID:
16563819.

16. Davies M, Brophy S, Williams R, Taylor A. The prevalence, severity, and impact of painful diabetic peripheral neuropathy in type 2 diabetes. Diabetes Care. 2006; 29:1518–22. DOI:
10.2337/dc05-2228. PMID:
16801572.

17. Teasell RW, Mehta S, Aubut JA, Foulon B, Wolfe DL, Hsieh JT, et al. Spinal Cord Injury Rehabilitation Evidence Research Team. A systematic review of pharmacologic treatments of pain after spinal cord injury. Arch Phys Med Rehabil. 2010; 91:816–31. DOI:
10.1016/j.apmr.2010.01.022. PMID:
20434623. PMCID:
3218077.

20. Rowbotham M, Harden N, Stacey B, Bernstein P, Magnus-Miller L. Gabapentin for the treatment of postherpetic neuralgia: a randomized controlled trial. JAMA. 1998; 280:1837–42. DOI:
10.1001/jama.280.21.1837. PMID:
9846778.

21. Rice AS, Maton S. Postherpetic Neuralgia Study Group. Gabapentin in postherpetic neuralgia: a randomised, double blind, placebo controlled study. Pain. 2001; 94:215–24. DOI:
10.1016/S0304-3959(01)00407-9. PMID:
11690735.

22. Sabatowski R, Gálvez R, Cherry DA, Jacquot F, Vincent E, Maisonobe P, et al. 1008-045 Study Group. Pregabalin reduces pain and improves sleep and mood disturbances in patients with post-herpetic neuralgia: results of a randomised, placebo-controlled clinical trial. Pain. 2004; 109:26–35. DOI:
10.1016/j.pain.2004.01.001. PMID:
15082123.

23. van Seventer R, Feister HA, Young JP Jr, Stoker M, Versavel M, Rigaudy L. Efficacy and tolerability of twice-daily pregabalin for treating pain and related sleep interference in postherpetic neuralgia: a 13-week, randomized trial. Curr Med Res Opin. 2006; 22:375–84. DOI:
10.1185/030079906X80404. PMID:
16466610.

24. Moon DE, Lee DI, Lee SC, Song SO, Yoon DM, Yoon MH, et al. Efficacy and tolerability of pregabalin using a flexible, optimized dose schedule in Korean patients with peripheral neuropathic pain: a 10-week, randomized, double-blind, placebo-controlled, multicenter study. Clin Ther. 2010; 32:2370–85. DOI:
10.1016/j.clinthera.2011.01.014. PMID:
21353106.

25. Xochilcal-Morales M, Castro EM, Guajardo-Rosas J, Obregón TN, Acevedo JC, Chucan JM, et al. A prospective, open-label, multicentre study of pregabalin in the treatment of neuropathic pain in Latin America. Int J Clin Pract. 2010; 64:1301–9. DOI:
10.1111/j.1742-1241.2010.02389.x. PMID:
20487048.

26. Guan Y, Ding X, Cheng Y, Fan D, Tan L, Wang Y, et al. Efficacy of pregabalin for peripheral neuropathic pain: results of an 8-week, flexible-dose, double-blind, placebo-controlled study conducted in China. Clin Ther. 2011; 33:159–66. DOI:
10.1016/j.clinthera.2011.02.007. PMID:
21444113.

27. Backonja M, Beydoun A, Edwards KR, Schwartz SL, Fonseca V, Hes M, et al. Gabapentin for the symptomatic treatment of painful neuropathy in patients with diabetes mellitus: a randomized controlled trial. JAMA. 1998; 280:1831–6. DOI:
10.1001/jama.280.21.1831. PMID:
9846777.

28. Sandercock D, Cramer M, Wu J, Chiang YK, Biton V, Heritier M. Gabapentin extended release for the treatment of painful diabetic peripheral neuropathy: efficacy and tolerability in a double-blind, randomized, controlled clinical trial. Diabetes Care. 2009; 32:e20. DOI:
10.2337/dc08-1450. PMID:
19171730. PMCID:
6905491.
29. Tölle T, Freynhagen R, Versavel M, Trostmann U, Young JP Jr. Pregabalin for relief of neuropathic pain associated with diabetic neuropathy: a randomized, double-blind study. Eur J Pain. 2008; 12:203–13. DOI:
10.1016/j.ejpain.2007.05.003. PMID:
17631400.

30. Guay DR. Pregabalin in neuropathic pain: a more “pharmaceutically elegant” gabapentin? Am J Geriatr Pharmacother. 2005; 3:274–87. DOI:
10.1016/j.amjopharm.2005.12.008.

31. Turner JA, Cardenas DD, Warms CA, McClellan CB. Chronic pain associated with spinal cord injuries: a community survey. Arch Phys Med Rehabil. 2001; 82:501–9. DOI:
10.1053/apmr.2001.21855. PMID:
11295011.

32. Tzellos TG, Papazisis G, Amaniti E, Kouvelas D. Efficacy of pregabalin and gabapentin for neuropathic pain in spinal-cord injury: an evidence-based evaluation of the literature. Eur J Clin Pharmacol. 2008; 64:851–8. DOI:
10.1007/s00228-008-0523-5. PMID:
18607580.

33. Attal N, Mazaltarine G, Perrouin-Verbe B, Albert T. SOFMER French Society for Physical Medicine and Rehabilitation. Chronic neuropathic pain management in spinal cord injury patients. What is the efficacy of pharmacological treatments with a general mode of administration? (oral, transdermal, intravenous). Ann Phys Rehabil Med. 2009; 52:124–41. DOI:
10.1016/j.rehab.2008.12.011. PMID:
19909703.

34. Snedecor SJ, Sudharshan L, Cappelleri JC, Sadosky A, Desai P, Jalundhwala YJ, et al. Systematic review and comparison of pharmacologic therapies for neuropathic pain associated with spinal cord injury. J Pain Res. 2013; 6:539–47. DOI:
10.2147/JPR.S45966. PMID:
23874121. PMCID:
3712802.

35. Guy S, Mehta S, Leff L, Teasell R, Loh E. Anticonvulsant medication use for the management of pain following spinal cord injury: systematic review and effectiveness analysis. Spinal Cord. 2014; 52:89–96. DOI:
10.1038/sc.2013.146. PMID:
24296804.

36. Mehta S, McIntyre A, Dijkers M, Loh E, Teasell RW. Gabapentinoids are effective in decreasing neuropathic pain and other secondary outcomes after spinal cord injury: a meta-analysis. Arch Phys Med Rehabil. 2014; 95:2180–6. DOI:
10.1016/j.apmr.2014.06.010. PMID:
24992021.

37. Song F, Altman DG, Glenny AM, Deeks JJ. Validity of indirect comparison for estimating efficacy of competing interventions: empirical evidence from published meta-analyses. BMJ. 2003; 326:472. DOI:
10.1136/bmj.326.7387.472. PMID:
12609941. PMCID:
150178.

38. Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009; 151:264–9. DOI:
10.7326/0003-4819-151-4-200908180-00135. PMID:
19622511.

39. Tai Q, Kirshblum S, Chen B, Millis S, Johnston M, DeLisa JA. Gabapentin in the treatment of neuropathic pain after spinal cord injury: a prospective, randomized, double-blind, crossover trial. J Spinal Cord Med. 2002; 25:100–5. DOI:
10.1080/10790268.2002.11753609. PMID:
12137213.

40. Levendoglu F, Ogün CO, Ozerbil O, Ogün TC, Ugurlu H. Gabapentin is a first line drug for the treatment of neuropathic pain in spinal cord injury. Spine (Phila Pa 1976). 2004; 29:743–51. DOI:
10.1097/01.BRS.0000112068.16108.3A. PMID:
15087796.

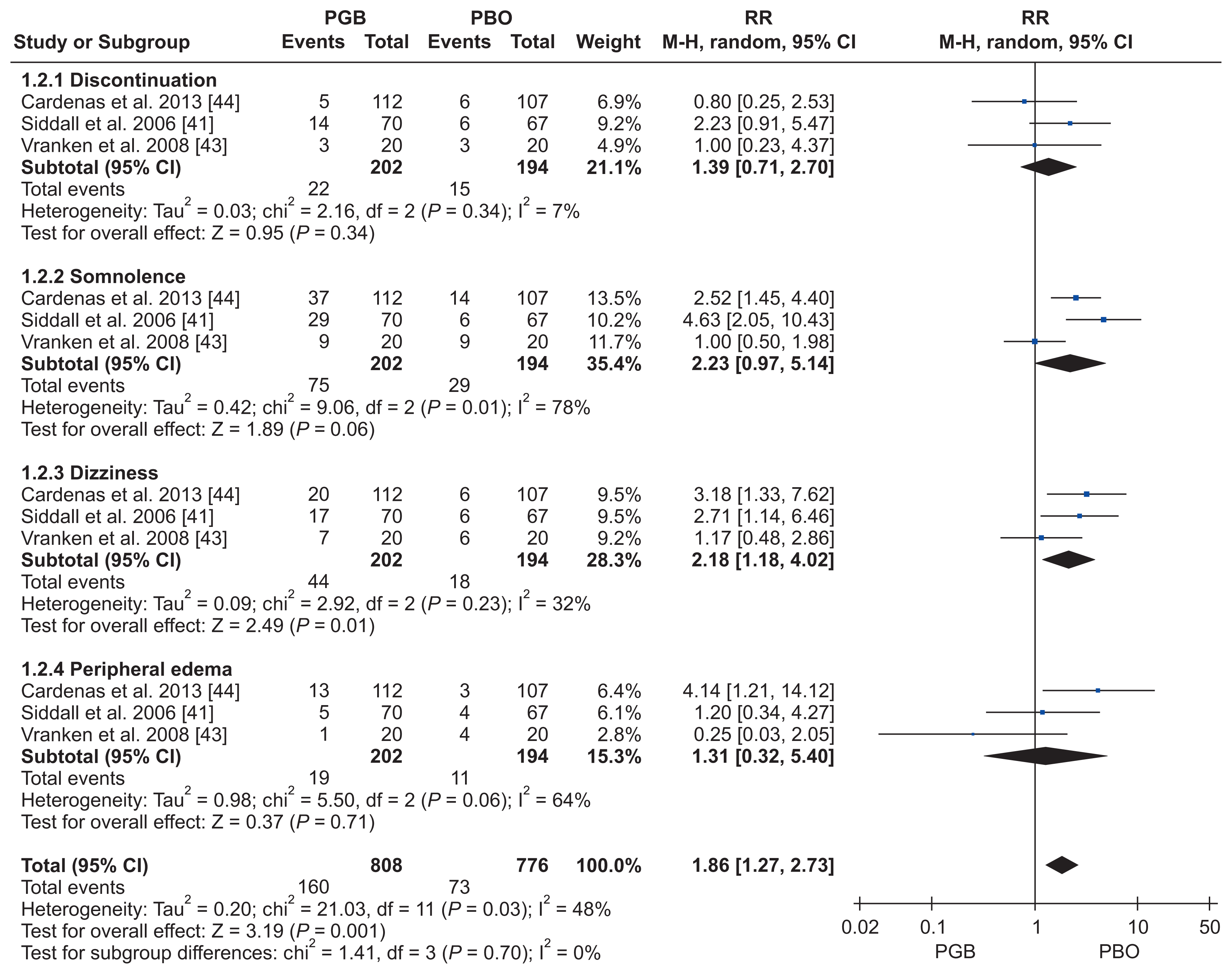
41. Siddall PJ, Cousins MJ, Otte A, Griesing T, Chambers R, Murphy TK. Pregabalin in central neuropathic pain associated with spinal cord injury: a placebo-controlled trial. Neurology. 2006; 67:1792–800. DOI:
10.1212/01.wnl.0000244422.45278.ff. PMID:
17130411.

42. Rintala DH, Holmes SA, Courtade D, Fiess RN, Tastard LV, Loubser PG. Comparison of the effectiveness of amitriptyline and gabapentin on chronic neuropathic pain in persons with spinal cord injury. Arch Phys Med Rehabil. 2007; 88:1547–60. DOI:
10.1016/j.apmr.2007.07.038. PMID:
18047869.

43. Vranken JH, Dijkgraaf MG, Kruis MR, van der Vegt MH, Hollmann MW, Heesen M. Pregabalin in patients with central neuropathic pain: a randomized, double-blind, placebo-controlled trial of a flexible-dose regimen. Pain. 2008; 136:150–7. DOI:
10.1016/j.pain.2007.06.033. PMID:
17703885.

44. Cardenas DD, Nieshoff EC, Suda K, Goto S, Sanin L, Kaneko T, et al. A randomized trial of pregabalin in patients with neuropathic pain due to spinal cord injury. Neurology. 2013; 80:533–9. DOI:
10.1212/WNL.0b013e318281546b. PMID:
23345639. PMCID:
3589291.

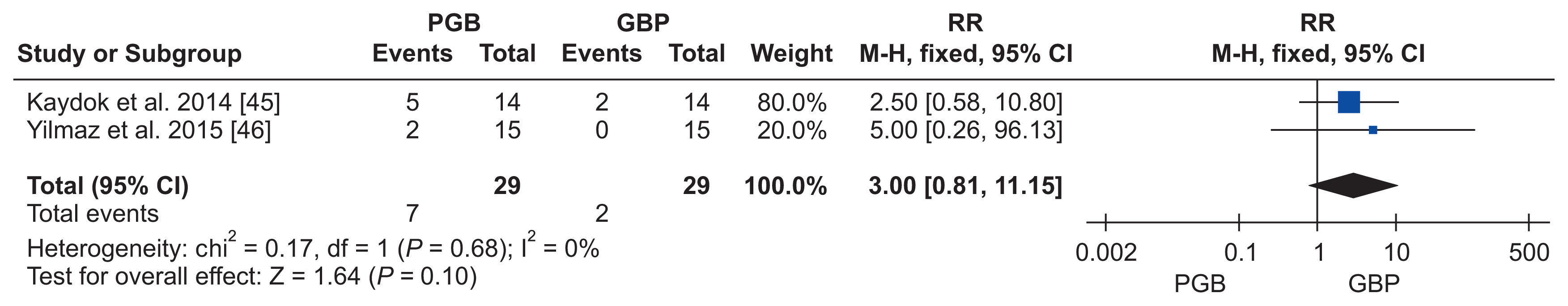
45. Kaydok E, Levendoglu F, Ozerbil MO, Karahan AY. Comparison of the efficacy of gabapentin and pregabalin for neuropathic pain in patients with spinal cord injury: a crossover study. Acta Med Mediterranea. 2014; 30:1343.
46. Yilmaz B, Yaşar E, Köroğlu Omaç Ö, Göktepe AS, Tan AK. Gabapentin vs. pregabalin for the treatment of neuropathic pain in patients with spinal cord injury: a crossover study. Turk J Phys Med Rehab. 2014; 61:1–5. DOI:
10.5152/tftrd.2015.79069.

47. Ghosh AK, Ghosh A, Kundu A, Das AK, Bhattacharya KB. Comparative study of efficacy and safety of pregabalin and gabapentin in neuropathic pain. Asian J Pharm Life Sci. 2012; 2:64–71.
48. Donegan S, Williamson P, Gamble C, Tudur-Smith C. Indirect comparisons: a review of reporting and methodological quality. PLoS One. 2010; 5:e11054. DOI:
10.1371/journal.pone.0011054. PMID:
21085712. PMCID:
2978085.

49. Dworkin RH, O’Connor AB, Backonja M, Farrar JT, Finnerup NB, Jensen TS, et al. Pharmacologic management of neuropathic pain: evidence-based recommendations. Pain. 2007; 132:237–51. DOI:
10.1016/j.pain.2007.08.033. PMID:
17920770.

50. Putzke JD, Richards JS, Kezar L, Hicken BL, Ness TJ. Long-term use of gabapentin for treatment of pain after traumatic spinal cord injury. Clin J Pain. 2002; 18:116–21. DOI:
10.1097/00002508-200203000-00007. PMID:
11882775.

51. To TP, Lim TC, Hill ST, Frauman AG, Cooper N, Kirsa SW, et al. Gabapentin for neuropathic pain following spinal cord injury. Spinal Cord. 2002; 40:282–5. DOI:
10.1038/sj.sc.3101300. PMID:
12037709.

52. Ahn SH, Park HW, Lee BS, Moon HW, Jang SH, Sakong J, et al. Gabapentin effect on neuropathic pain compared among patients with spinal cord injury and different durations of symptoms. Spine (Phila Pa 1976). 2003; 28:341–6. DOI:
10.1097/01.BRS.0000048464.57011.00. PMID:
12590206.

53. Song F, Loke YK, Walsh T, Glenny AM, Eastwood AJ, Altman DG. Methodological problems in the use of indirect comparisons for evaluating healthcare interventions: survey of published systematic reviews. BMJ. 2009; 338:b1147. DOI:
10.1136/bmj.b1147. PMID:
19346285. PMCID:
2665205.

54. Onouchi K, Koga H, Yokoyama K, Yoshiyama T. An open-label, long-term study examining the safety and tolerability of pregabalin in Japanese patients with central neuropathic pain. J Pain Res. 2014; 7:439–47. DOI:
10.2147/JPR.S63028. PMID:
25114584. PMCID:
4122555.





 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download