INTRODUCTION
The dorsal scapular nerve (DSN) arises from C5 within the posterior cervical triangle, procedes deep to the prevertebral fascia, and pierces the middle scalene muscle to innervate the rhomboid muscles. It passes under the levator scapulae muscle and then becomes more superficial between the rhomboid major and minor muscles, as it travels caudally along the medial border of the scapula [
1,
2]. The DSN, as well as other peripheral nerves, has the possibility of compression at any point along its course.
Entrapment neuropathy of the DSN is known to be one of common causes of pain in the interscapular region. Patients with entrapment neuropathy can experience sharp, stabbing, and burning pain [
3], or an itching sensation [
4] at the neck, shoulder, and arm, as well as in the interscapular region. Motor weakness in shoulder abduction and winged scapula have also been described as symptoms of this entrapment [
4,
5]. Because these symptoms may be often ambiguous, physicians can misdiagnose causes of pain in this region, which can lead to unnecessary or unsuccessful time-consuming management [
4].
Selective blockades using nerve stimulators [
6] and ultrasound (US) [
7,
8] have been conventionally used for diagnosing and treating DSN entrapment neuropathy, which has 2 approaches―anterior and posterior―depending on the access path of the needle used for blockade. The anterior approach is through the posterior triangle of the neck, while the posterior approach involves injection below the levator scapulae or rhomboid muscles in the back [
6,
8]. Contrary to myriad reports addressing the anterior approach, although it is regarded as a safer method for targeting the DSN only, there have been few reports describing topographical relationships between the DSN and the dorsal scapular artery (DSA) related to this posterior approach. Providing exact anatomical information related to the target structures is critical for more successful outcomes. As such, this study was performed to clarify the topographical relationships between the DSN and DSA in the interscapular region with the aim of facilitating the management of DSN entrapment by proposing new, safe, and convenient injection points related to DSN blockade using the posterior approach.
Go to :

MATERIALS AND METHODS
This study was approved by the Institutional Review Board of Wonkwang University (IRB ID No. WKIRB-201904-BM-027).
1. Cadaveric observation
Thirty shoulders of 15 embalmed Korean adult cadavers (9 males, 6 females; mean age 80.2 yr at death [range, 50–95 yr]) were dissected. All cadavers were stored at the Jesaeng-Euise Institute of Wonkwang University School of Medicine, and were qualified as materials for use in education and research according to domestic law.
None of the cadavers exhibited any evidence of gross pathology, previous surgical procedures, or traumatic lesions in the target region. After removing the skin and subcutaneous tissue, the trapezius was exposed. This muscle was cut at its origin and reflected laterally. The rhomboid major and minor were also cut at their origin sites and reflected laterally with careful confirmation of the pathway of the DSN and DSA without any changes in their locations. The DSN and DSA were then identified by sticking colored pin-heads at the levels of the defined points (
Fig. 1).
 | Fig. 1Marking method for delineating pathways of the dorsal scapular nerve (white pin heads) and dorsal scapular artery (blue pin heads). Ls: levator scapulae, Rmi: rhomboid minor, Rmaj: rhomboid major, Tz: trapezius, S: supraspinatus, SS: spine of scapula, I: infraspinatus. 
|
2. Measurement
The authors first defined a line and several points to analyze the pathways of the DSN and DSA in the anatomical position (
Fig. 2), which connected the superior and inferior angles of the scapula. The scapula was divided into 2 parts based on the scapular spine. The upper part was then divided into 3 equal parts over its entire length, whereas the lower part was divided into 3 equal parts until its midpoint line. Locations and distances of both the DSN and DSA were measured at each point of the medial border of the scapula, meeting perpendicular lines from the 7 defined points using a digital caliper (Mitutoyo, Tokyo, Japan).
 | Fig. 2Lines and points defined for analyzing the positional relationship between the dorsal scapular nerve and dorsal scapular artery and medial border of the scapula. Lines A and D indicate the superior angle and the spine of scapula, respectively. Line G indicates the midpoint between the spine of scapula (line D) and the inferior angle of scapula. Lines B, C, E, and F indicate each line divided into three equal parts between the lines A and D, and between the lines D and G, respectively. SA: superior angle of scapula, SS: spine of scapula, IA: inferior angle of scapula. 
|
3. US examination of live subjects
Fifty shoulders of 50 subjects (15 males, 35 females; mean age 59.2 yr [range, 20–83 yr]) were examined. Informed consent was obtained from the patients. None of the subjects had a history of trauma, surgery, or structural pathology in the target region. The transducer of the US device (Linear probe 12–5 MHz, Samsung HS 60; Samsung Medison, Seoul, Korea) was placed transversely at the levels of the defined lines A, B, and C.
Go to :

RESULTS
In cadavers, the running patterns of the DSA and DSN in the interscapular region were classified into 3 types based on their locational relationships (
Fig. 3): type I were nerves that were medial to the artery and parallel without changing location throughout their length (80.0% of specimens); type II (13.3%) were nerves or arteries that traversed one another only one time throughout their length; and type III (6.7%) were nerves or arteries that traversed one another > 2 times, resembling a twist pattern.
 | Fig. 3Running patterns of the dorsal scapular nerve (DSN; yellow line) and dorsal scapular artery (DSA; red line) throughout their course of the cadavers. (A) The DSN and DSA run parallel throughout their course. The structures traverse one another only one time (B), or more than 2 times (C). MB: medial border of scapula. 
|
Locational relationships and distance between the DSA and DSN at the level of each defined line were analyzed (
Table 1). At each level, number of the specimens in which both structures could be simultaneously observed was 22, 29, 27, 12, 4, 1, and 0, respectively. At the levels of lines A, B, and C, the nerve was always medial to the artery (100% of specimens). At the level of line D (at the scapular spine), the nerve was medial and lateral to the artery in 75% and 25% of subjects, respectively. Below line D, the number of arteries that could be identified using the naked eye clearly decreased.
Table 1
Measurement of Distance between the Dorsal Scapular Nerve and DSA at Each Line of the Cadavers
|
Line |
Medial to the DSA |
Lateral to the DSA |
|
|
|
Mean ± SD (range), mm |
Specimens (n) |
Mean ± SD (range), mm |
Specimens (n) |
|
|
|
A |
7.4 ± 4.3 (0.9–15.2) |
22 |
|
0 |
|
B |
10.7 ± 5.0 (2.6–18.7) |
29 |
|
0 |
|
C |
10.3 ± 5.9 (1.5–21.7) |
27 |
|
0 |
|
D |
9.2 ± 5.3 (1.1–17.0) |
12 |
1.9 ± 1.9 (0.2–4.3) |
4 |
|
E |
9.1 ± 5.3 (2.9–15.6) |
4 |
2.5 |
1 |
|
F |
18 |
1 |
6.0 |
1 |
|
G |
|
0 |
3.4 |
1 |

The relationships between the DSA and medial border of the scapula as well as the DSN and medial border of the scapula were analyzed (
Fig. 4). At the level of each line, except for line A, most of the arteries were lateral to the medial border of the scapula, with frequencies of 86.2% (line B), 89.7% (line C), and 86.4% (line D). The arteries were located within approximately 8.4 mm medial and 13.0 mm lateral to the medial border of the scapula. In the cases of nerves, the frequency of the nerve being medial or lateral to the medial border of scapula was similar except for the level at line A. The nerves were located within approximately 10.8 mm medial and 13.4 mm lateral to the medial border of the scapula.
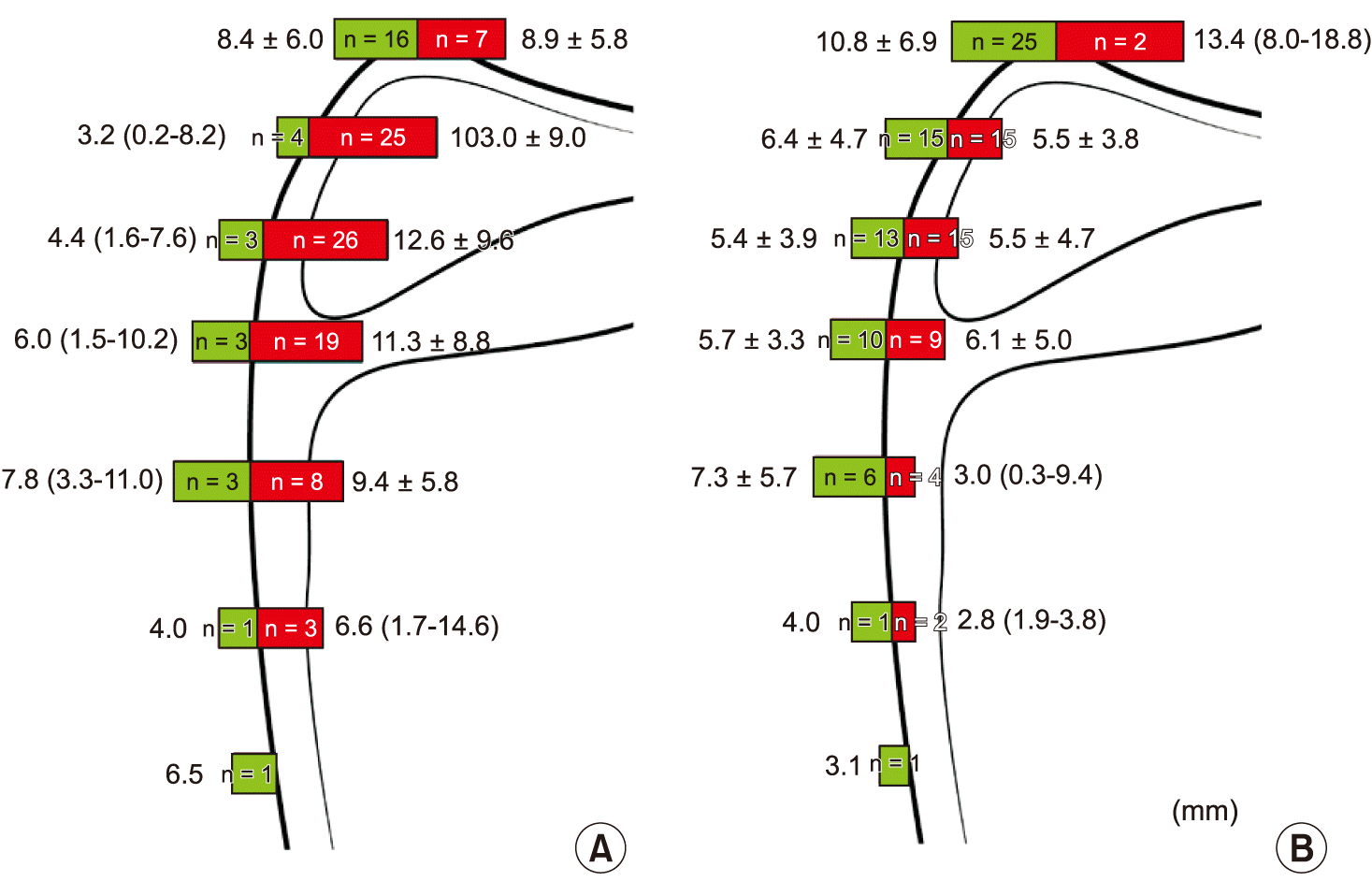 | Fig. 4Frequency and distance (mm) of the dorsal scapular artery (A) and dorsal scapular nerve (B) from the medial border of scapula at the level of each line of the cadavers. Green and red bars indicate the structures passing toward the medial and lateral sides of the medial border of scapula, respectively. 
|
In live subjects, the linear probe of the US was placed transversely at each levels of the defined lines A, B, and C, which were used in the cadaveric study. The DSA could be identified using US Doppler imaging, which depicted its pulsation. In the analysis of US images from 50 shoulders, the artery was observed on lines A (94.0%, n = 47), B (86.0%, n = 43), and C (78.0%, n = 39) (
Fig. 5). In 3 cases (6.0%), no DSA could be observed in any of the lines.
 | Fig. 5Ultrasound (US) images and frequency of the detected dorsal scapular artery (yellow arrow) in live subjects. Panels A, B, and C depict US images of the same patient at lines A, B, and C, respectively. Tz: trapezius muscle, Rb: rhomboid muscles, SA: superior angle of scapula, R: rib, MB: medial border of scapula. 
|
Go to :

DISCUSSION
Pain in the interscapular region can have various etiologies including discogenic or facet joint disorders, myofascial pain syndrome, back strain, and entrapment neuropathy [
9,
10]. Among these causes, DSN entrapment is associated with pain at the interscapular, shoulder and arm regions, weakness in arm abduction, and/or winged scapula [
3]. Neuropathic dysfunction related to chronic DSN entrapment may cause atrophies of the rhomboid or levator scapulae muscles [
11]. In these cases, the onset of pain can appear abruptly or develop slowly over time. DSN entrapment is typically caused by whiplash injury and overhead work, especially lifting overhead. In addition, an imbalance in the rotator cuff muscles and instability of the acromioclavicular joint can also lead to DSN entrapment [
5,
12]. For management of this neuropathy, various therapeutic interventions, including myofascial release, trigger point injections, postural re-education, nerve blockade, neurolysis, neurectomy, and radiofrequency denervation have been used [
11,
13]. Among these, precise DSN blockade has been known to be a valuable diagnostic and therapeutic tool [
6]. For precise and effective selective nerve blockade, however, an exact understanding of the topographical relationships between the DSN and DSA is essential.
The DSN can be compressed at the scalene muscles, the posterior triangle of the neck, the junction between the levator scapulae and rhomboid minor muscles, and the inter-scapular region throughout its course [
1,
3,
14]. There are 2 approaches for DSN blockade related to managing DSN entrapment. One is the anterolateral approach within the middle scalene muscle through the posterior triangle of the neck, which is the most common method [
7]. However, there is high anatomical variability and, in some patients, the DSN may not visible even using US [
15]. In this case, blocking the entire brachial plexus may be an option for providing nonspecific analgesia to the scapula; however, there is concern about unintentional blockade or injury of the other nerves [
7]. Using a relatively high volume of anesthetics for a brachial plexus block may also cause unexpected side effects, such as paralysis of the phrenic nerve or paralysis or the nerves from the cervical plexus [
16,
17]. The other is the posterior approach through the interscapular region, which has been regarded to be a relatively safer method for DSN blockade because of the simplicity of the structures close to the levator scapulae and rhomboid muscles. However, to date, there have been only 2 reports describing the posterior approach for DSN blockade [
6,
8]. Based on our experience, however, the nerve cannot be easily identified, even if definite landmarks, such as arteries and bony structures, are used during US-guided blockade.
In the present study, we propose 3 important points for facilitating selective DSN block based on anatomical conformation related to the pathways of the DSN and DSA. First, the DSN was always medial to the DSA at the levels of lines A, B, and C (
Table 1); the mean distances between both structures at each line were 7.4 mm, 10.7 mm, and 10.3 mm, respectively. Thus, if the DSA is detected at the levels of lines A, B, and C under US, an injection with local anesthetic must be performed about 10 mm medial to the DSA. Second, the superior angle of the scapula can be easily identified using US. At this angle, approximately 70% of the DSA was observed to be medial to the medial border of the scapula (
Fig. 4A), whereas at the other lines, most of the DSA tended to be lateral to the medial border of the scapula. In the latter cases, the DSA was not likely to be identified in US imaging due to the shadow from the scapula. Third, > 90% of the DSN was always found around the points of the medial border of the scapula meeting lines A, B, and C (
Fig. 4B). For consideration in clinical practice, our results related to US observation also support the above recommendations. Because most of the DSNs were observed above line C in the cadaveric dissections, we performed US to find the DSA in the levels of the lines A, B, and C (
Fig. 5). The region where the DSA was mostly observed was line A (94.0% of patients). The artery could be also detected on lines B and C with high frequency, which was consistent with the results from the cadaveric dissection. Based on the above 3 points, physicians who want to effectively block the DSN using the posterior approach must try to identify the DSA using US at line A. When physicians succeed in finding the DSA, injection with local anesthetic must be performed about 10 mm medial to the DSA. At lines B and C, patients sometimes require a change of position with protraction of the scapula for exposing the DSA on US imaging. The DSA could not be detected on any lines using US in 6% of patients, which may have been due to physiological conditions, such as weak blood vessels, thickened muscle mass, and obesity. In these cases, DSN blockade is also possible using a simple injection below the levator scapulae or rhomboid muscles at the levels of lines A, B, and C. The above mentioned position (a position with protraction of the scapula) is also recommended in other lines when the DSA is difficult to find even in clinical practice.
In conclusion, the results of this study provide useful information regarding the posterior approach using US or blind technique in the clinical field. However, one limitation of this study is that it did not provide accurate success rates or suggestions as to the optimal quantity of local anesthetic for DSN blockade in the various regions due to its observational nature, the limited number of cadaveric dissections, and the comparative study of subjects using US. This could be resolved in future cadaveric studies using dye injection, or in clinical trials involving live subjects, with data from the present study used as reference points.
Go to :









 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download