INTRODUCTION
Lumbar radicular syndrome is a disease that involves radiating pain in one or more dermatomes, and may be accompanied by chronic nerve irritation and dysfunction. Lumbar radicular syndrome is a highly prevalent disease, but a challenging clinical problem. Interventional procedures such as epidural steroid injection (ESI) may be considered if conservative treatments, such as physical therapy or medication are initially tried and are not effective.
Pulsed radiofrequency (PRF) is a treatment modality that alleviates radicular pain by intermittently applying high-frequency currents adjacent to the dorsal root ganglion (DRG) [
1,
2]. Although the mechanism of PRF is not fully understood, the electric field reversibly blocks transmission without destroying the small unmyelinated fibers, and the large fibers continue to be protected by the myelin sheath [
3].
The DRG plays an important role in low back pain and radiating pain [
4]. The DRG is very sensitive to mechanical compression, and is closely related to abnormal sensation and radiating pain [
5]. Radiologically, the location of the DRG is divided into 3 types—the intraspinal, foraminal, and extraforaminal regions (
Fig. 1)—and most DRG neurons are of the foraminal type [
6]. This position corresponds to the dorsal-cranial quadrant of the intervertebral foramen on the lateral view in fluoroscopy, and the middle of the pedicle column on the anteroposterior (AP) view. However, if the arthritic degenerative changes and foraminal stenosis are severe, positioning the needle to target the DRG on fluoroscopy may be difficult [
7]. Accordingly, needle tips can be placed laterally on the side of the corresponding pedicle in the AP view.
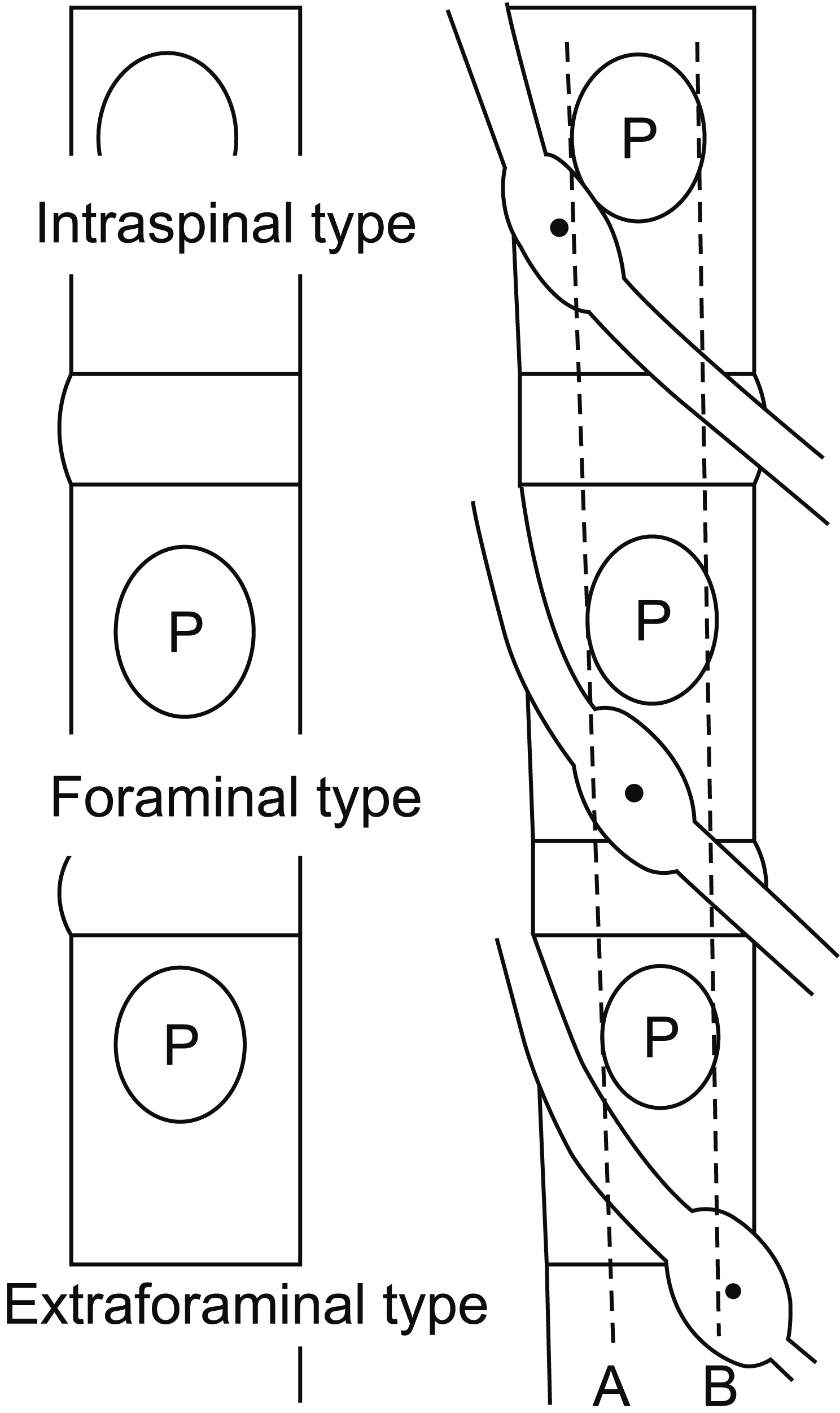 | Fig. 1Determination of the dorsal root ganglion (DRG) position. A and B are lines connecting the medial and lateral borders of the pedicles, respectively. If the midpoint of the DRG lies proximal to A, it is intraspinal type. If it is between A and B, it is foraminal type. If it is distal to B, it is extraforaminal type. 
|
To our knowledge, there has been no comparative study on how analgesic effects correspond to the position of the needle tip in PRF treatment. The purpose of this study was to evaluate the clinical effects of PRF according to the needle tip position.
Go to :

MATERIALS AND METHODS
1. Patient population
This study was approved by the Institutional Review Board (IRB) of Ewha Womans University Hospital (EUMC 2018-11-006). The patient’s information and records were anonymized, and then the IRB waived the written informed consent requirement. We collected clinical data through a hospital database and analyzed records for all patients with lumbar radiating pain who underwent PRF adjacent to the DRG between March 2016 and December 2017.
Inclusion criteria were as follows: (1) pain duration > 3 months; (2) unilateral radiating pain, suggesting involvement of the lumbar spinal nerves (L4 and L5) as well as affected nerve roots consistent with magnetic resonance imaging (MRI) findings; (3) being 20 years of age or older; (4) a score of 4 or more on an 11-point numerical rating scale (NRS) for pain scores after conservative treatment, including oral medication, physical therapy, or ESI; and (6) foraminal type on spine MRI (
Fig. 1) [
6].
Exclusion criteria were as follows: (1) absence of spinal MRI; (2) inadequate management of coexisting psychiatric diagnoses; (3) history of cancer, lumbar fracture, systemic disease, or connective tissue diseases; (4) no 3-month follow-up data; (5) pregnancy, coagulation disorders, systemic infection, fever, or local infection at the puncture site; (6) presence of a cardiac pacemaker or spinal cord stimulator; and (7) PRF treatment of the DRG in the last year; and (8) position of the needle tip in the caudal portion of the intervertebral foramen on the lateral view in fluoroscopy [
8,
9].
Patient characteristics assessed included age, sex, weight, height, comorbidities (such as hypertension and diabetes mellitus), and pain duration and location. The medication quantification scale III (MQS) was used to quantify changes in analgesics [
10]. We also analyzed clinical factors such as treatment level, spinal surgery history, MRI findings, the presence of disc herniation, target level foraminal stenosis, and central stenosis. We also analyzed the number of ESIs performed at the pain clinic for 3 months before PRF treatment.
2. PRF procedure
For PRF, the patient was placed in a prone position, and a pillow was placed under the lower abdomen. Disinfection was performed at the procedure site, and a sterile drape was put in place. Patients received at least 2 ESIs in the 3 months preceding PRF treatment, and a diagnostic root block was not performed immediately before PRF treatment. The entire course of the procedure was performed under fluoroscopic guidance. After local anesthesia with 1% lidocaine was applied to the skin, the RF needle (22 G, 10 cm, curved, with 10-mm active tip) was inserted into the target neuroforamen. The target point was the dorsal-cranial quadrant of the intervertebral foramen in the lateral view, and midway into the pedicle column in the AP view. When the RF needle was close to the target position, the stylet of the RF needle was removed, and the RF probe was inserted. The final position of the RF probe was determined with sensory stimulation (50 Hz), or when the patient felt a tingling sensation at a voltage below 0.5 V. If the threshold value exceeded 0.5 V, the needle was carefully advanced until the patient felt sensory stimulation. Pulsed current (20 msec, 2 Hz) was then applied 3 times for 120 seconds each time, with a 45-V output and short intervals between treatments. During this procedure, the temperature at the tip of the electrode was not supposed to exceed 42°C.
3. Clinical data
The success of the procedure was defined as a decrease of 50% or more in the NRS score without increasing analgesic consumption [
11,
12]. All other responses were deemed negative responses. The patients were classified into 2 groups, group IP (group inside of pedicle) and group OP (group outside of pedicle), according to the position of the needle tip in the AP view. The position of the needle tip was analyzed by two physicians who were not involved in the procedure: one was an experienced pain physician, and the other was a radiologist. Using an AP view, the needle tip was advanced further inward than the lateral aspect of the corresponding pedicle in group IP. However, the tip of the needle did not move forward in group OP (
Fig. 2). We evaluated the success rate of treatment and NRS scores at 4, 8, and 12 weeks after PRF, and reviewed all complications within 3 months after the procedure.
 | Fig. 2Position of the needle tip. (A) The needle tip was advanced medially further than the lateral aspect of the corresponding pedicle. (B) The needle tip was in the lateral aspect of the corresponding pedicle. P: pedicle. 
|
4. Statistical analysis
Continuous variables are demonstrated as mean ± standard deviation or medians (interquartile ranges), and categorical variables are displayed as numbers (percentages). Demographic and clinical data were compared between the 2 groups by t-test, chi-square test, or Mann–Whitney U-testing. P values less than 0.05 were considered statistically significant. All statistical analyses were performed using PASW ver. 18.0 (IBM Corp., Armonk, NY).
Go to :

RESULTS
We reviewed the medical records of 86 consecutive patients who received PRF during the study period. Of these patients, 49 patients were included in our study, and 37 patients were excluded (
Fig. 3).
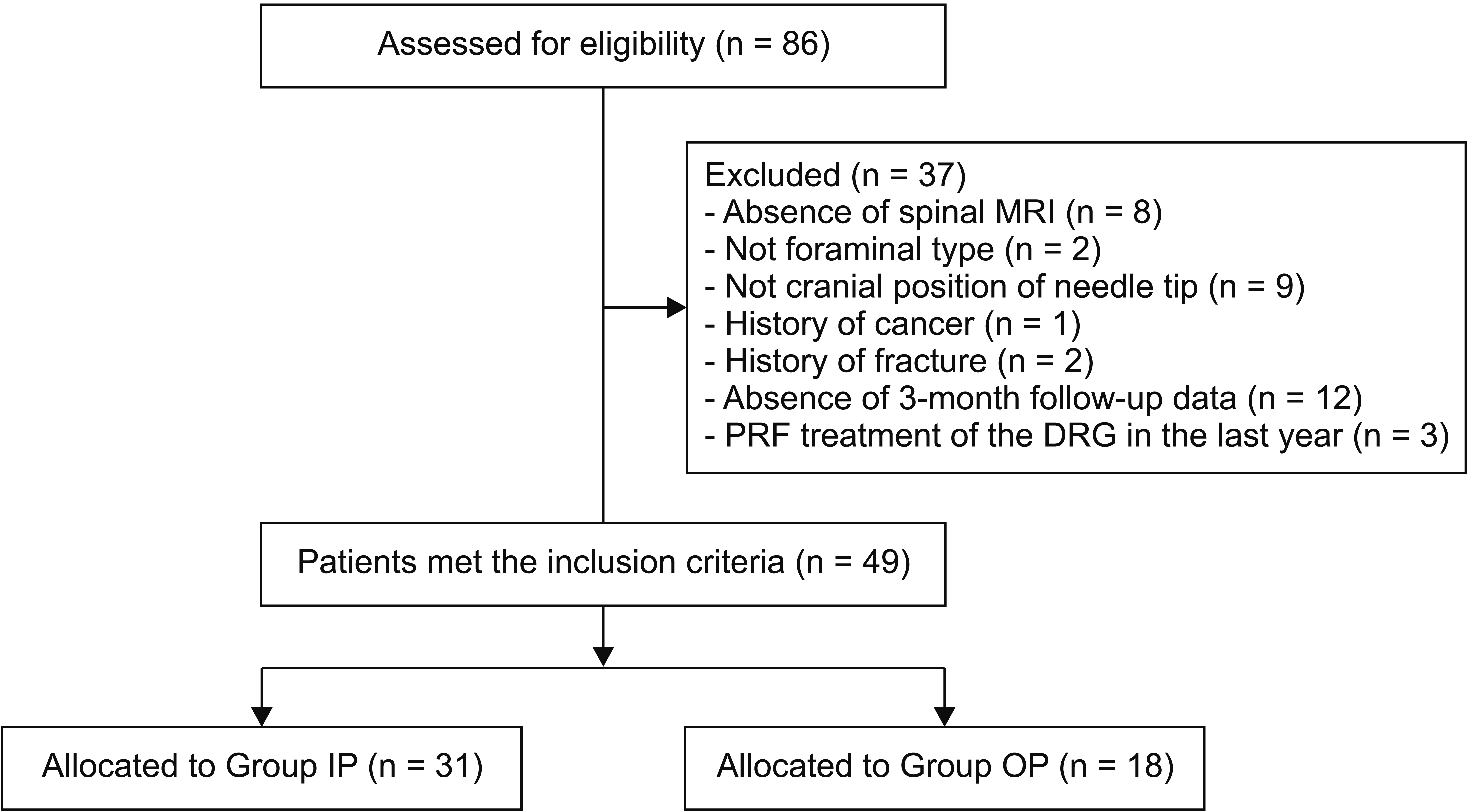 | Fig. 3Flow diagram of the patient inclusion process. MRI: magnetic resonance imaging, PRF: pulsed radiofrequency, DRG: dorsal root ganglion, Group IP: group inside of pedicle, Group OP: group outside of pedicle. 
|
Patient demographics and characteristics are shown in
Table 1. There were no significant differences between group IP and group OP in age, sex, height, weight, pain duration, or other past medical histories. There was no significant change in the MQS during the follow-up period, and there was no difference between the 2 groups. The presence of central stenosis, foraminal stenosis, or disc herniation on MRI did not differ, and the grade of central stenosis also did not exhibit a statistically significant difference between the 2 groups. In both groups, the median number of previous ESIs before PRF was 2, and there was no difference in injection target levels or injection sites.
Table 1
|
Variable |
Group IP (n = 31) |
Group OP (n = 18) |
P value |
|
Age (yr) |
71 ± 11 |
71 ± 12 |
0.93 |
|
Sex (male/female) |
19 (61.3)/12 (38.7) |
7 (38.9)/11 (61.1) |
0.13 |
|
Height (cm) |
162 ± 8 |
159 ± 10 |
0.13 |
|
Weight (kg) |
67 ± 10 |
64 ± 12 |
0.36 |
|
Hypertension |
18 (58.1) |
12 (66.6) |
0.55 |
|
Diabetes mellitus |
5 (16.1) |
2 (11.1) |
0.63 |
|
MQS |
|
Baseline |
9.3 (6.3) |
10.2 (4.6) |
0.98 |
|
4 wk after PRF |
5.6 (5.8) |
10.2 (5.3) |
0.95 |
|
8 wk after PRF |
7.9 (6.4) |
10.2 (5.3) |
0.99 |
|
12 wk after PRF |
7.9 (8.5) |
10.2 (5.3) |
0.86 |
|
Pain duration (mo) |
|
3–6/6–12/> 12 |
9 (29.0)/4 (12.9)/18 (58.1) |
2 (11.1)/5 (27.8)/11 (61.1) |
0.22 |
|
Location of pain (left/right) |
12 (38.7)/19 (61.3) |
11 (61.1)/7 (38.9) |
0.13 |
|
Levels treated (L4/L5) |
11 (35.5)/20 (64.5) |
9 (50.0)/9 (50.0) |
0.32 |
|
Failed back surgery syndrome |
4 (12.9) |
3 (16.7) |
0.72 |
|
Central stenosis |
23 (74.2) |
15 (83.3) |
0.46 |
|
Mild/Moderate/Severe |
13 (56.5)/8 (34.8)/2 (8.7) |
5 (33.3)/7 (46.7)/3 (20.0) |
0.33 |
|
Multiple central stenosis |
8 (25.8) |
5 (27.8) |
0.88 |
|
Foraminal stenosis |
10 (32.3) |
8 (44.4) |
0.39 |
|
Herniated intervertebral disc |
21 (67.7) |
13 (72.2) |
0.74 |
|
No. of previous ESI |
2 (2) |
2 (2) |
0.90 |

Table 2 shows the comparison of the success rates (based on predefined success criteria) for the 2 groups. Overall success rates were 57%, 53%, and 40% at 4 weeks, 8 weeks, and 12 weeks, respectively (not shown), but there was no significant difference in the percentage of successful responses between the 2 groups. There was no significant difference in baseline NRS scores. Both groups showed significant improvement in NRS scores at 4, 8, and 12 weeks after treatment, but no statistically significant differences were observed between the 2 groups (
Fig. 4).
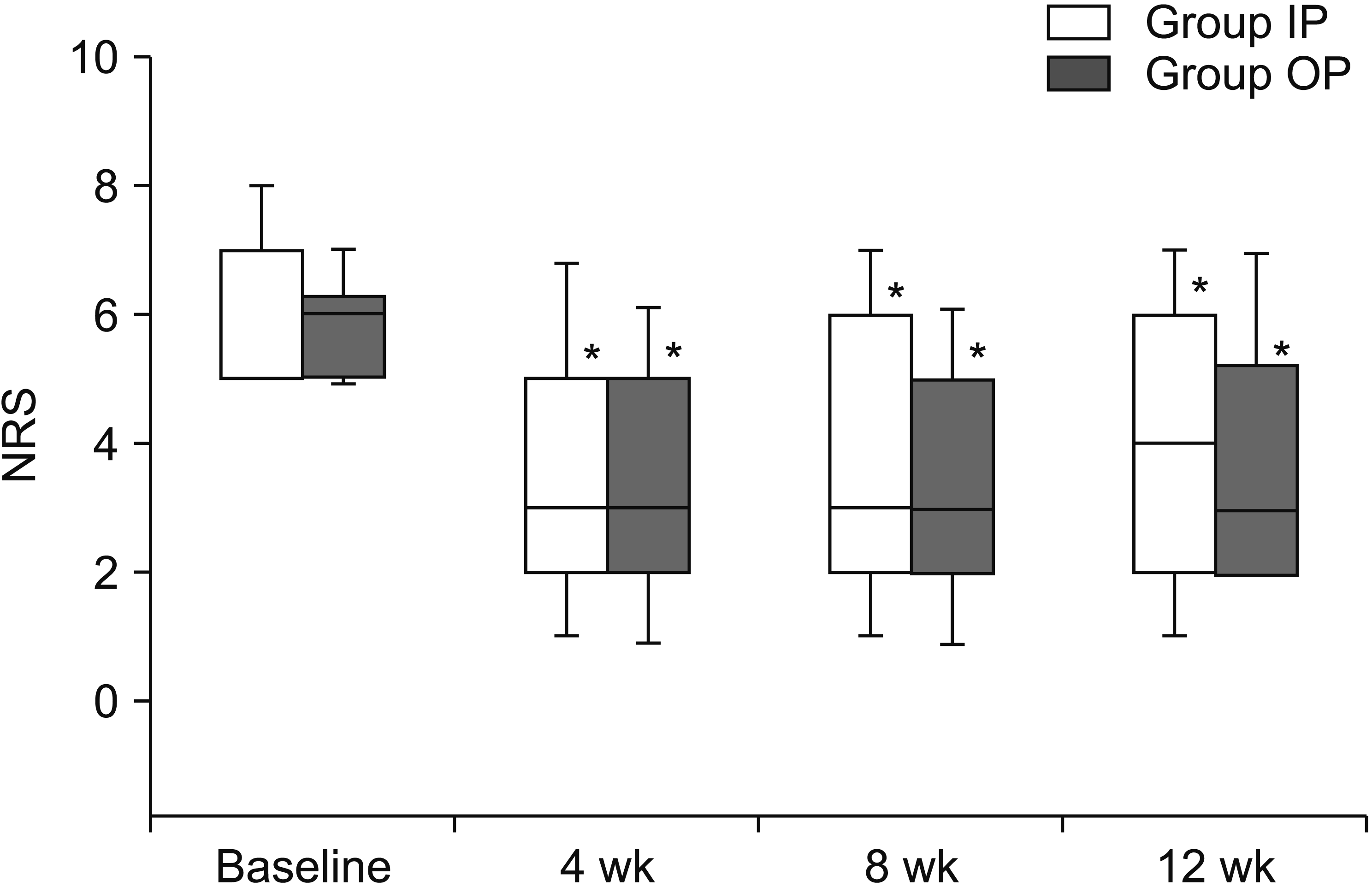 | Fig. 4Numerical rating scale (NRS). Group IP: group inside of pedicle, Group OP: group outside of pedicle. *P < 0.05 relative to baseline. 
|
Table 2
|
Variable |
Group IP (n = 31) |
Group OP (n = 18) |
P value |
|
Success at 4 wk after PRF |
17 (54.8) |
11 (61.1) |
0.67 |
|
Success at 8 wk after PRF |
15 (48.4) |
11 (61.1) |
0.39 |
|
Success at 12 wk after PRF |
12 (38.7) |
8 (44.4) |
0.69 |

Go to :

DISCUSSION
Our results demonstrated that there was no significant difference in the percentage of successful response rates and pain scores based on position of the needle tip.
In an animal study, acute lumbar nerve root compression did not cause repetitive firing for more than a few seconds, but produced an instantaneous discharge in Aδ and C fibers. However, acute compression of the DRG caused a long-term repeated firing (5–25 min); however, this discharge occurred in both rapidly-conducting and slowly-conducting fibers (Aβ, Aδ, and C fibers) [
13]. PRF was found to block the signal only in unmyelinated C fibers, leaving myelinated Aδ fibers functioning and able to transmit pain signals [
14]. Das et al. [
12] reported that chronic radicular pain is a centrally mediated neuroimmune phenomenon, and the mechanism of action of DRG PRF is immunomodulatory: CD56
+, CD3
−, natural killer (NK) cell frequencies, and interferon-c (IFN-c) levels decreased, while CD8
+ T cell frequencies and interleukin-6 (IL-6) levels increased in treatment responders. There was an inverse correlation between IL-17 and pain severity scores after treatment.
Some physicians have suggested that PRF of the DRG is not a validated technique, and is instead a sham procedure for the treatment of lumbar radicular pain syndromes [
15]; other physicians have reported that PRF is a useful intervention. Chao et al. [
16] reported that at 3 months after lumbar PRF, 44.83% of patients experienced pain reduction of more than 50%. In another study, 41% of patients had pain relief of more than 50% at 6 months after PRF [
1]. In a study by Simopoulos et al. [
17], the pain intensity of 70% of the patients was successfully reduced at 2 months after PRF treatment. Therefore, whether PRF is efficacious in the treatment of lumbar radicular pain should be considered carefully.
Radiologically, DRG positions can be classified into 3 types: intraspinal, foraminal, and extraforaminal. These types depend on the relationship between the midpoint of the DRG and the borderline of the DRG. In the intraspinal type, the midpoint of the DRG is located inside the pedicle, and in the extraforaminal type, the midpoint of the DRG is located outside the pedicle. In the foraminal type, the midpoint of the DRG is located between the medial and lateral margins of the pedicle (
Fig. 1) [
6]. Most DRG neurons in the upper lumbar spine (L1–3) are of the foraminal or extraforaminal type, while the DRG neurons of the lower lumbar spine (L4–5) have little extraforaminal type DRGs [
6,
18].
Generally, a spinal nerve root block (SNRB) is performed for diagnostic purposes before DRG PRF. If there is pain reduction after SNRB, the corresponding DRG is thought to have a pathological cause. However, PRF can be performed even when there is no effect on conservative treatments, such as SNRB, in clinical practice. Furthermore, PRF may be performed to extend the effect when the SNRB has a short analgesic effect [
11].
This retrospective, observational study had some limitations. First, the widths and lengths of DRG neurons are variable [
6], and the asymmetry of the location of DRG neurons may be caused by disc herniation or degenerative hypertrophic facets in symptomatic patients [
19,
20]. Second, the study was conducted in a single clinical setting, and the sample size was small. Third, we did not collect information on disability and quality of life parameters other than pain relief. Fourth, we did not perform subgroup analysis on different diagnoses, such as multilevel central canal stenosis, herniated intervertebral discs, and failed back surgery syndrome; however, there was no significant difference in the diagnosis percentage between the 2 groups. Fifth, a retrospective observational study has inherent limitations. But we believe that a prospective study in which the needle tip is positioned in the lateral aspect of the pedicle intentionally would be an ethical issue, and thus, a retrospective study was best. Our results provide a basis for future prospective studies on the clinical effects of PRF according to the position of the needle tip.
In conclusion, the analgesic efficacy of PRF treatment was not statistically different based on the position of the needle tip. A larger, controlled, prospective study should be conducted to investigate the effect of needle tip position on the analgesic effects of PRF.
Go to :







 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download