2. Villain N, Fouquet M, Baron JC, Mézenge F, Landeau B, de La Sayette V, et al. Sequential relationships between grey matter and white matter atrophy and brain metabolic abnormalities in early Alzheimer’s disease. Brain. 2010; 133:3301–14. DOI:
10.1093/brain/awq203. PMID:
20688814. PMCID:
PMC3291528.

3. Manning KY, Schranz A, Bartha R, Dekaban GA, Barreira C, Brown A, et al. Multiparametric MRI changes persist beyond recovery in concussed adolescent hockey players. Neurology. 2017; 89:2157–66. DOI:
10.1212/WNL.0000000000004669. PMID:
29070666. PMCID:
PMC5696642.

4. Arvanitakis Z, Fleischman DA, Arfanakis K, Leurgans SE, Barnes LL, Bennett DA. Association of white matter hyperintensities and gray matter volume with cognition in older individuals without cognitive impairment. Brain Struct Funct. 2016; 221:2135–46. DOI:
10.1007/s00429-015-1034-7. PMID:
25833685. PMCID:
PMC4592368.

6. Draganski B, Moser T, Lummel N, Gänssbauer S, Bogdahn U, Haas F, et al. Decrease of thalamic gray matter following limb amputation. Neuroimage. 2006; 31:951–7. DOI:
10.1016/j.neuroimage.2006.01.018. PMID:
16520065.

7. Flor H. Maladaptive plasticity, memory for pain and phantom limb pain: review and suggestions for new therapies. Expert Rev Neurother. 2008; 8:809–18. DOI:
10.1586/14737175.8.5.809. PMID:
18457537.

9. Makin TR, Scholz J, Henderson Slater D, Johansen-Berg H, Tracey I. Reassessing cortical reorganization in the primary sensorimotor cortex following arm amputation. Brain. 2015; 138(Pt 8):2140–6. DOI:
10.1093/brain/awv161. PMID:
26072517. PMCID:
PMC4511862.

10. Vogt BA. Pain and emotion interactions in subregions of the cingulate gyrus. Nat Rev Neurosci. 2005; 6:533–44. DOI:
10.1038/nrn1704. PMID:
15995724. PMCID:
PMC2659949.

11. Seo CH, Park CH, Jung MH, Jang S, Joo SY, Kang Y, et al. Preliminary investigation of pain-related changes in cerebral blood volume in patients with phantom limb pain. Arch Phys Med Rehabil. 2017; 98:2206–12. DOI:
10.1016/j.apmr.2017.03.010. PMID:
28392326.

12. Assaf Y, Pasternak O. Diffusion tensor imaging (DTI)-based white matter mapping in brain research: a review. J Mol Neurosci. 2008; 34:51–61. DOI:
10.1007/s12031-007-0029-0. PMID:
18157658.

13. Neil JJ. Diffusion imaging concepts for clinicians. J Magn Reson Imaging. 2008; 27:1–7. DOI:
10.1002/jmri.21087. PMID:
18050325.

14. Yi JS, Bae SO, Ahn YM, Park DB, Noh KS, Shin HK, et al. Validity and reliability of the Korean Version of the Hamilton Depression Rating Scale (K-HDRS). J Korean Neuropsychiatr Assoc. 2005; 44:456–65.
15. Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006; 31:1487–505. DOI:
10.1016/j.neuroimage.2006.02.024. PMID:
16624579.

16. Kieseppä T, Eerola M, Mäntylä R, Neuvonen T, Poutanen VP, Luoma K, et al. Major depressive disorder and white matter abnormalities: a diffusion tensor imaging study with tract-based spatial statistics. J Affect Disord. 2010; 120:240–4. DOI:
10.1016/j.jad.2009.04.023. PMID:
19467559.

17. Lindsay PG, Wyckoff M. The depression-pain syndrome and its response to antidepressants. Psychosomatics. 1981; 22:571–3. 576–7. DOI:
10.1016/S0033-3182(81)73478-9. PMID:
7267947.

19. Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009; 44:83–98. DOI:
10.1016/j.neuroimage.2008.03.061. PMID:
18501637.

20. Song SK, Sun SW, Ju WK, Lin SJ, Cross AH, Neufeld AH. Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage. 2003; 20:1714–22. DOI:
10.1016/j.neuroimage.2003.07.005. PMID:
14642481.

22. Mac Donald CL, Dikranian K, Bayly P, Holtzman D, Brody D. Diffusion tensor imaging reliably detects experimental traumatic axonal injury and indicates approximate time of injury. J Neurosci. 2007; 27:11869–76. DOI:
10.1523/JNEUROSCI.3647-07.2007. PMID:
17978027. PMCID:
PMC2562788.

23. Budde MD, Janes L, Gold E, Turtzo LC, Frank JA. The contribution of gliosis to diffusion tensor anisotropy and tractography following traumatic brain injury: validation in the rat using Fourier analysis of stained tissue sections. Brain. 2011; 134(Pt 8):2248–60. DOI:
10.1093/brain/awr161. PMID:
21764818. PMCID:
PMC3155707.

24. Kumar R, Woo MA, Macey PM, Fonarow GC, Hamilton MA, Harper RM. Brain axonal and myelin evaluation in heart failure. J Neurol Sci. 2011; 307:106–13. DOI:
10.1016/j.jns.2011.04.028. PMID:
21612797. PMCID:
PMC3150745.

25. Harrison DM, Shiee N, Bazin PL, Newsome SD, Ratchford JN, Pham D, et al. Tract-specific quantitative MRI better correlates with disability than conventional MRI in multiple sclerosis. J Neurol. 2013; 260:397–406. DOI:
10.1007/s00415-012-6638-8. PMID:
22886062. PMCID:
PMC3753185.

26. Della Nave R, Ginestroni A, Diciotti S, Salvatore E, Soricelli A, Mascalchi M. Axial diffusivity is increased in the degenerating superior cerebellar peduncles of Friedreich’s ataxia. Neuroradiology. 2011; 53:367–72. DOI:
10.1007/s00234-010-0807-1. PMID:
21128070.

27. Weaver KE, Richards TL, Liang O, Laurino MY, Samii A, Aylward EH. Longitudinal diffusion tensor imaging in Huntington’s disease. Exp Neurol. 2009; 216:525–9. DOI:
10.1016/j.expneurol.2008.12.026. PMID:
19320010.

28. Cosottini M, Giannelli M, Siciliano G, Lazzarotti G, Michelassi MC, Del Corona A, et al. Diffusion-tensor MR imaging of corticospinal tract in amyotrophic lateral sclerosis and pro-gressive muscular atrophy. Radiology. 2005; 237:258–64. DOI:
10.1148/radiol.2371041506. PMID:
16183935.

29. Sullivan EV, Rohlfing T, Pfefferbaum A. Quantitative fiber tracking of lateral and interhemispheric white matter systems in normal aging: relations to timed performance. Neurobiol Aging. 2010; 31:464–81. DOI:
10.1016/j.neurobiolaging.2008.04.007. PMID:
18495300. PMCID:
PMC2815144.

30. Della Nave R, Ginestroni A, Tessa C, Giannelli M, Piacentini S, Filippi M, et al. Regional distribution and clinical correlates of white matter structural damage in Huntington disease: a tract-based spatial statistics study. AJNR Am J Neuroradiol. 2010; 31:1675–81. DOI:
10.3174/ajnr.A2128. PMID:
20488902.

31. Rosas HD, Lee SY, Bender AC, Zaleta AK, Vangel M, Yu P, et al. Altered white matter microstructure in the corpus callosum in Huntington’s disease: implications for cortical “disconnection”. Neuroimage. 2010; 49:2995–3004. DOI:
10.1016/j.neuroimage.2009.10.015. PMID:
19850138. PMCID:
PMC3725957.

32. Acosta-Cabronero J, Williams GB, Pengas G, Nestor PJ. Absolute diffusivities define the landscape of white matter degeneration in Alzheimer’s disease. Brain. 2010; 133(Pt 2):529–39. DOI:
10.1093/brain/awp257. PMID:
19914928.

34. Zhu T, Zhong J, Hu R, Tivarus M, Ekholm S, Harezlak J, et al. Patterns of white matter injury in HIV infection after partial immune reconstitution: a DTI tract-based spatial statistics study. J Neurovirol. 2013; 19:10–23. DOI:
10.1007/s13365-012-0135-9. PMID:
23179680. PMCID:
PMC3568248.

35. Rayhan RU, Stevens BW, Timbol CR, Adewuyi O, Walitt B, VanMeter JW, et al. Increased brain white matter axial diffusivity associated with fatigue, pain and hyperalgesia in Gulf War illness. PLoS One. 2013; 8:e58493. DOI:
10.1371/journal.pone.0058493. PMID:
23526988. PMCID:
PMC3603990.

36. Li Z, Li C, Fan L, Jiang G, Wu J, Jiang T, et al. Altered microstructure rather than morphology in the corpus callosum after lower limb amputation. Sci Rep. 2017; 7:44780. DOI:
10.1038/srep44780. PMID:
28303959. PMCID:
PMC5355997.

37. Simões EL, Bramati I, Rodrigues E, Franzoi A, Moll J, Lent R, et al. Functional expansion of sensorimotor representation and structural reorganization of callosal connections in lower limb amputees. J Neurosci. 2012; 32:3211–20. DOI:
10.1523/JNEUROSCI.4592-11.2012. PMID:
22378892. PMCID:
PMC6622024.

38. Jiang G, Yin X, Li C, Li L, Zhao L, Evans AC, et al. The plasticity of brain gray matter and white matter following lower limb amputation. Neural Plast. 2015; 2015:823185. DOI:
10.1155/2015/823185. PMID:
26587289. PMCID:
PMC4637496.

40. Love S, Hilton DA, Coakham HB. Central demyelination of the Vth nerve root in trigeminal neuralgia associated with vascular compression. Brain Pathol. 1998; 8:1–11. DOI:
10.1111/j.1750-3639.1998.tb00126.x. PMID:
9458161.

41. Ellingson BM, Mayer E, Harris RJ, Ashe-McNally C, Naliboff BD, Labus JS, et al. Diffusion tensor imaging detects microstructural reorganization in the brain associated with chronic irritable bowel syndrome. Pain. 2013; 154:1528–41. DOI:
10.1016/j.pain.2013.04.010. PMID:
23721972. PMCID:
PMC3758125.

42. Lutz J, Jäger L, de Quervain D, Krauseneck T, Padberg F, Wichnalek M, et al. White and gray matter abnormalities in the brain of patients with fibromyalgia: a diffusion-tensor and volumetric imaging study. Arthritis Rheum. 2008; 58:3960–9. DOI:
10.1002/art.24070. PMID:
19035484.

43. Sundgren PC, Petrou M, Harris RE, Fan X, Foerster B, Mehrotra N, et al. Diffusion-weighted and diffusion tensor imaging in fibromyalgia patients: a prospective study of whole brain diffusivity, apparent diffusion coefficient, and fraction anisotropy in different regions of the brain and correlation with symptom severity. Acad Radiol. 2007; 14:839–46. DOI:
10.1016/j.acra.2007.03.015. PMID:
17574134.

44. Gallagher R. Opioid-induced neurotoxicity. Can Fam Physician. 2007; 53:426–7. PMID:
17872676. PMCID:
PMC1949075.
45. Ohn SH, Chang WH, Park CH, Kim ST, Lee JI, Pascual-Leone A, et al. Neural correlates of the antinociceptive effects of repetitive transcranial magnetic stimulation on central pain after stroke. Neurorehabil Neural Repair. 2012; 26:344–52. DOI:
10.1177/1545968311423110. PMID:
21980153. PMCID:
PMC3541021.

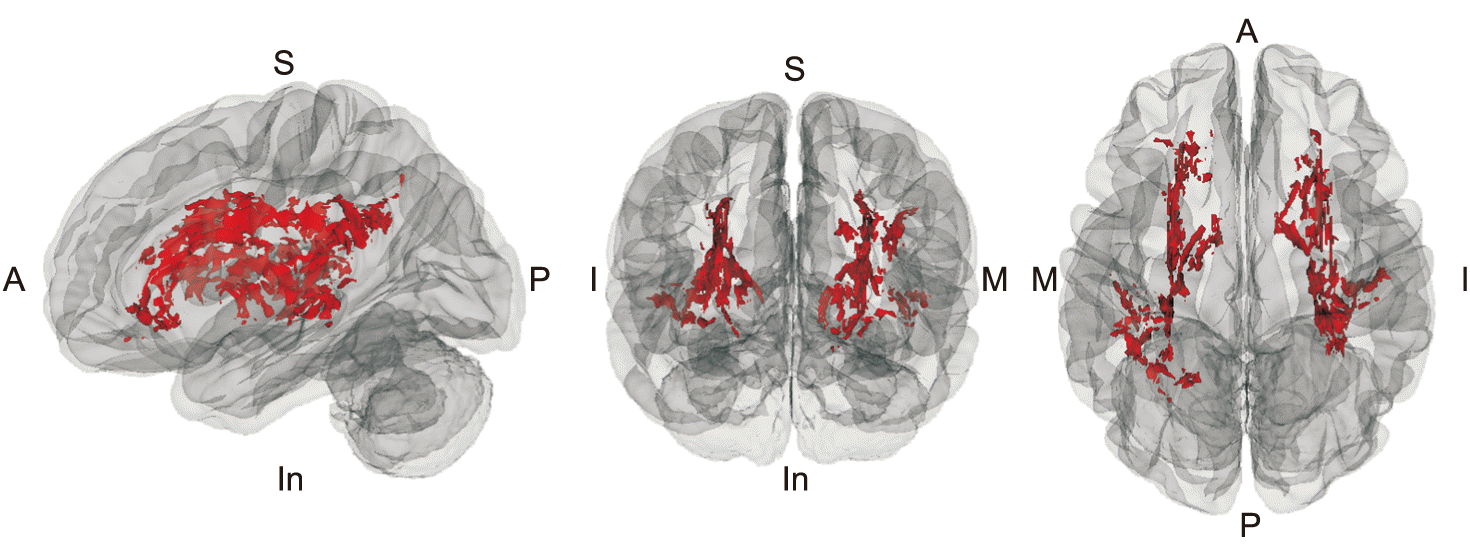
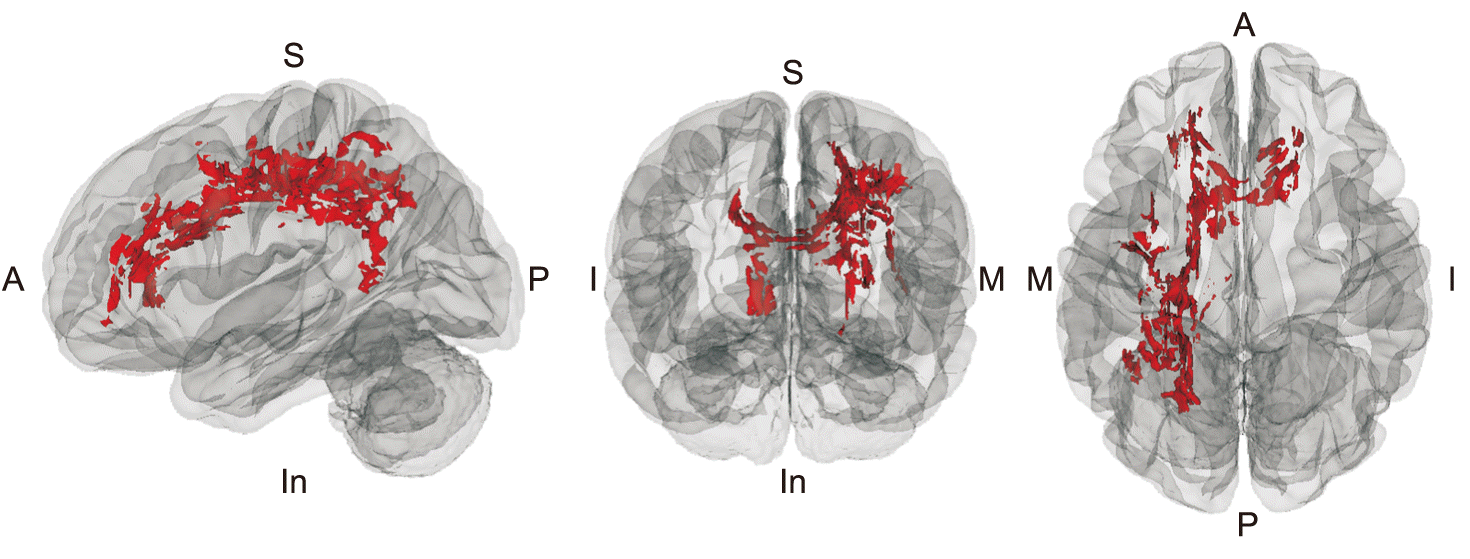




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download