1. Woodworth GE, Ivie RMJ, Nelson SM, Walker CM, Maniker RB. Perioperative breast analgesia: a qualitative review of anatomy and regional techniques. Reg Anesth Pain Med. 2017; 42:609–31. DOI:
10.1097/AAP.0000000000000641. PMID:
28820803.
2. Wildsmith JA. Continuous thoracic epidural block for surgery: gold standard or debased currency? Br J Anaesth. 2012; 109:9–12. DOI:
10.1093/bja/aes177. PMID:
22696554.

3. Choe WJ, Kim JY, Yeo HJ, Kim JH, Lee SI, Kim KT, et al. Postpartum spinal subdural hematoma: irrelevant epidural blood patch: a case report. Korean J Anesthesiol. 2016; 69:189–92. DOI:
10.4097/kjae.2016.69.2.189. PMID:
27066211. PMCID:
4823418.

4. Yeung JH, Gates S, Naidu BV, Wilson MJ, Gao Smith F. Paravertebral block versus thoracic epidural for patients undergoing thoracotomy. Cochrane Database Syst Rev. 2016; 2:CD009121. PMID:
26897642.

6. Marhofer D, Marhofer P, Kettner SC, Fleischmann E, Prayer D, Schernthaner M, et al. Magnetic resonance imaging analysis of the spread of local anesthetic solution after ultrasound-guided lateral thoracic paravertebral blockade: a volunteer study. Anesthesiology. 2013; 118:1106–12. DOI:
10.1097/ALN.0b013e318289465f. PMID:
23442752.
8. Pace MM, Sharma B, Anderson-Dam J, Fleischmann K, Warren L, Stefanovich P. Ultrasound-guided thoracic paravertebral blockade: a retrospective study of the incidence of complications. Anesth Analg. 2016; 122:1186–91. DOI:
10.1213/ANE.0000000000001117. PMID:
26756911.
10. Bonvicini D, Giacomazzi A, Pizzirani E. Use of the ultrasound-guided erector spinae plane block in breast surgery. Minerva Anestesiol. 2017; 83:1111–2. PMID:
28492298.

11. Finneran JJ 4th, Gabriel RA, Khatibi B. Erector spinae plane blocks provide analgesia for breast and axillary surgery: a series of 3 cases. Reg Anesth Pain Med. 2018; 43:101–2. DOI:
10.1097/AAP.0000000000000695. PMID:
29261601.
12. Forero M, Adhikary SD, Lopez H, Tsui C, Chin KJ. The erector spinae plane block: a novel analgesic technique in thoracic neuropathic pain. Reg Anesth Pain Med. 2016; 41:621–7. DOI:
10.1097/AAP.0000000000000451. PMID:
27501016.
13. Scimia P, Basso Ricci E, Droghetti A, Fusco P. The ultrasound-guided continuous erector spinae plane block for postoperative analgesia in video-assisted thoracoscopic lobectomy. Reg Anesth Pain Med. 2017; 42:537. DOI:
10.1097/AAP.0000000000000616. PMID:
28632673.

14. Forero M, Rajarathinam M, Adhikary S, Chin KJ. Erector spinae plane (ESP) block in the management of post thoracotomy pain syndrome: a case series. Scand J Pain. 2017; 17:325–9. DOI:
10.1016/j.sjpain.2017.08.013. PMID:
28919152.

15. Chin KJ, Adhikary S, Sarwani N, Forero M. The analgesic efficacy of pre-operative bilateral erector spinae plane (ESP) blocks in patients having ventral hernia repair. Anaesthesia. 2017; 72:452–60. DOI:
10.1111/anae.13814. PMID:
28188621.

16. Kline J, Chin KJ. Modified dual-injection lumbar erector spine plane (ESP) block for opioid-free anesthesia in multilevel lumbar laminectomy. Korean J Anesthesiol. 2019; 72:188–90. DOI:
10.4097/kja.d.18.00289. PMID:
30392347. PMCID:
PMC6458518.

17. Kim E, Kwon W, Oh S, Bang S. The erector spinae plane block for postoperative analgesia after percutaneous nephrolithotomy. Chin Med J (Engl). 2018; 131:1877–8. DOI:
10.4103/0366-6999.237408. PMID:
30058589. PMCID:
PMC6071450.

19. Ramos J, Peng P, Forero M. Long-term continuous erector spinae plane block for palliative pain control in a patient with pleural mesothelioma. Can J Anaesth. 2018; 65:852–3. DOI:
10.1007/s12630-018-1097-z. PMID:
29488178.

20. Chin KJ, Malhas L, Perlas A. The erector spinae plane block provides visceral abdominal analgesia in bariatric surgery: a report of 3 cases. Reg Anesth Pain Med. 2017; 42:372–6. DOI:
10.1097/AAP.0000000000000581. PMID:
28272292.
21. Masaracchia MM, Herrick MD, Seiffert EA, Sites BD. Nerve blocks under general anesthesia: time to liberalize indications? Reg Anesth Pain Med. 2017; 42:299–301. DOI:
10.1097/AAP.0000000000000579. PMID:
28257389.
22. Ho D, Imai K, King G, Stuart EA. MatchIt: nonparametric preprocessing for parametric causal inference. J Stat Softw. 2011; 42:1–28. DOI:
10.18637/jss.v042.i08.

24. Kumar A, Hulsey A, Martinez-Wilson H, Kim J, Gadsden J. The use of liposomal bupivacaine in erector spinae plane block to minimize opioid consumption for breast surgery: a case report. A A Pract. 2018; 10:239–41. DOI:
10.1213/XAA.0000000000000674. PMID:
29708919.

25. Tanaka N, Ueshima H, Otake H. Erector spinae plane block for combined lovectomy and radical mastectomys. J Clin Anesth. 2018; 45:27–8. DOI:
10.1016/j.jclinane.2017.12.012. PMID:
29274543.
26. Gürkan Y, Aksu C, Kuş A, Yörükoğlu UH, Kılıç CT. Ultrasound guided erector spinae plane block reduces postoperative opioid consumption following breast surgery: a randomized controlled study. J Clin Anesth. 2018; 50:65–8. DOI:
10.1016/j.jclinane.2018.06.033. PMID:
29980005.

27. Yoshizaki M, Murata H, Ogami-Takamura K, Hara T. Bilateral erector spinae plane block using a programmed intermittent bolus technique for pain management after Nuss procedure. J Clin Anesth. 2019; DOI:
10.1016/j.jclinane.2019.03.014. PMID:
30852328.

28. Tiburzi C, Cerotto V, Gargaglia E, Carli L, Gori F. Pectoral nerve block II with programmed intermittent bolus of local anesthetic and postoperative pain relief in breast surgery. Minerva Anestesiol. 2019; 85:201–2. DOI:
10.23736/S0375-9393.18.13027-6. PMID:
30207137.

29. Öbrink E, Jildenstål P, Oddby E, Jakobsson JG. Postoperative nausea and vomiting: update on predicting the probability and ways to minimize its occurrence, with focus on ambulatory surgery. Int J Surg. 2015; 15:100–6. DOI:
10.1016/j.ijsu.2015.01.024. PMID:
25638733.

30. Porreca F, Ossipov MH. Nausea and vomiting side effects with opioid analgesics during treatment of chronic pain: mechanisms, implications, and management options. Pain Med. 2009; 10:654–62. DOI:
10.1111/j.1526-4637.2009.00583.x. PMID:
19302436.

31. Ohgoshi Y, Ikeda T, Kurahashi K. Continuous erector spinae plane block provides effective perioperative analgesia for breast reconstruction using tissue expanders: a report of two cases. J Clin Anesth. 2018; 44:1–2. DOI:
10.1016/j.jclinane.2017.10.007. PMID:
29065334.

32. Kwon WJ, Bang SU, Sun WY. Erector spinae plane block for effective analgesia after total mastectomy with sentinel or axillary lymph node dissection: a report of three cases. J Korean Med Sci. 2018; 33:e291. DOI:
10.3346/jkms.2018.33.e291. PMID:
31044575. PMCID:
PMC6209766.

33. Kim D, Bang S, Sun WY. Erector spinae plane block with sedation for surgical anesthesia in breast conserving surgery. J Clin Anesth. 2019; DOI:
10.1016/j.jclinane.2019.03.003. PMID:
30852327.

34. Adhikary SD, Bernard S, Lopez H, Chin KJ. Erector spinae plane block versus retrolaminar block: a magnetic resonance imaging and anatomical study. Reg Anesth Pain Med. 2018; 43:756–62. PMID:
29794943.
35. Costache I, de Neumann L, Ramnanan CJ, Goodwin SL, Pawa A, Abdallah FW, et al. The mid-point transverse process to pleura (MTP) block: a new end-point for thoracic paravertebral block. Anaesthesia. 2017; 72:1230–6. DOI:
10.1111/anae.14004. PMID:
28762464.

36. Luyet C, Eichenberger U, Greif R, Vogt A, Szücs Farkas Z, Moriggl B. Ultrasound-guided paravertebral puncture and placement of catheters in human cadavers: an imaging study. Br J Anaesth. 2009; 102:534–9. DOI:
10.1093/bja/aep015. PMID:
19244265.

37. Damjanovska M, Stopar Pintaric T, Cvetko E, Vlassakov K. The ultrasound-guided retrolaminar block: volume-dependent injectate distribution. J Pain Res. 2018; 11:293–9. DOI:
10.2147/JPR.S153660. PMID:
29445296. PMCID:
5808708.

38. El-Boghdadly K, Pawa A, Chin KJ. Local anesthetic systemic toxicity: current perspectives. Local Reg Anesth. 2018; 11:35–44. DOI:
10.2147/LRA.S154512. PMID:
30122981. PMCID:
6087022.

39. De Cassai A, Bonvicini D, Correale C, Sandei L, Tulgar S, Tonetti T. Erector spinae plane block: a systematic qualitative review. Minerva Anestesiol. 2019; 85:308–19. DOI:
10.23736/S0375-9393.18.13341-4. PMID:
30621377.

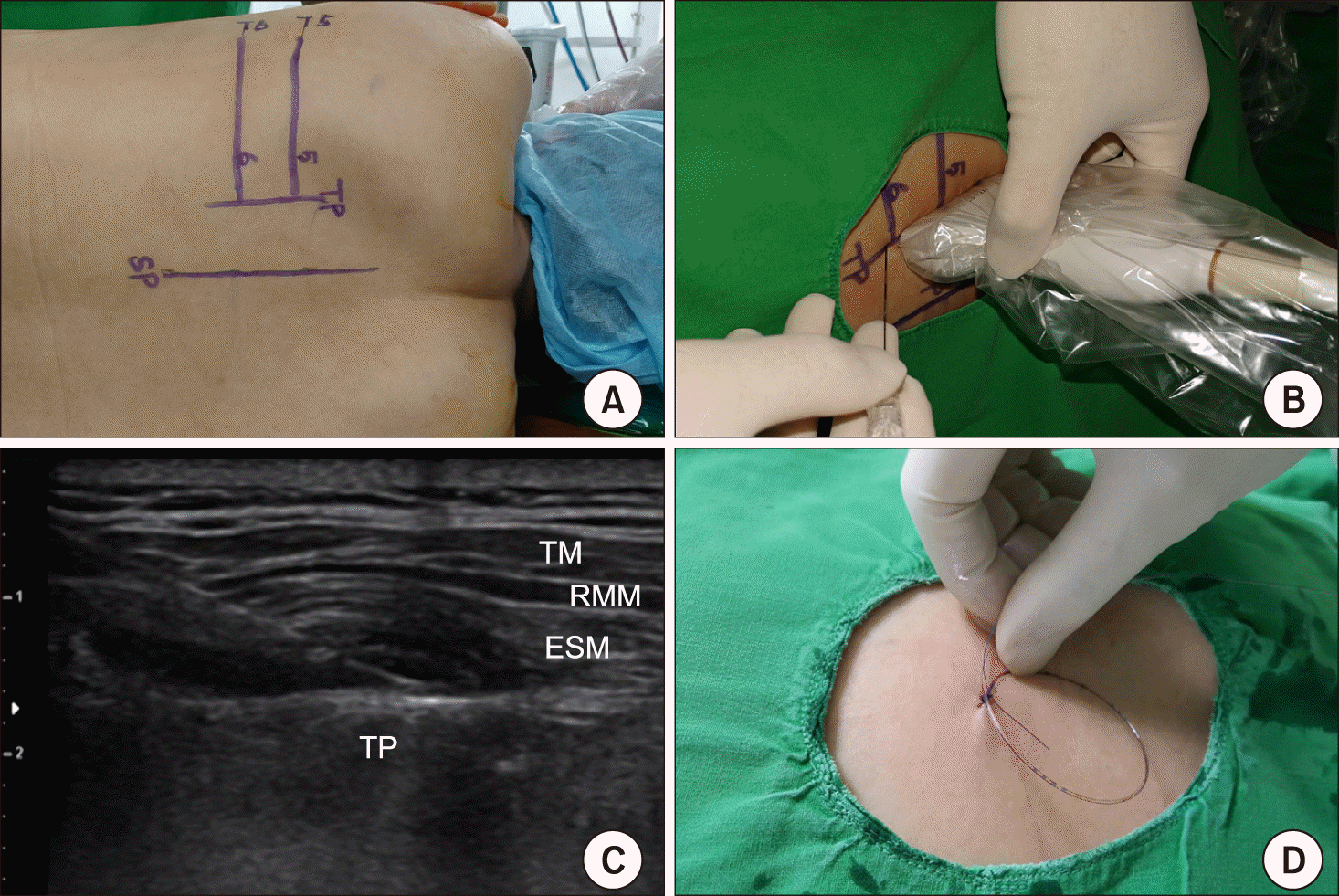
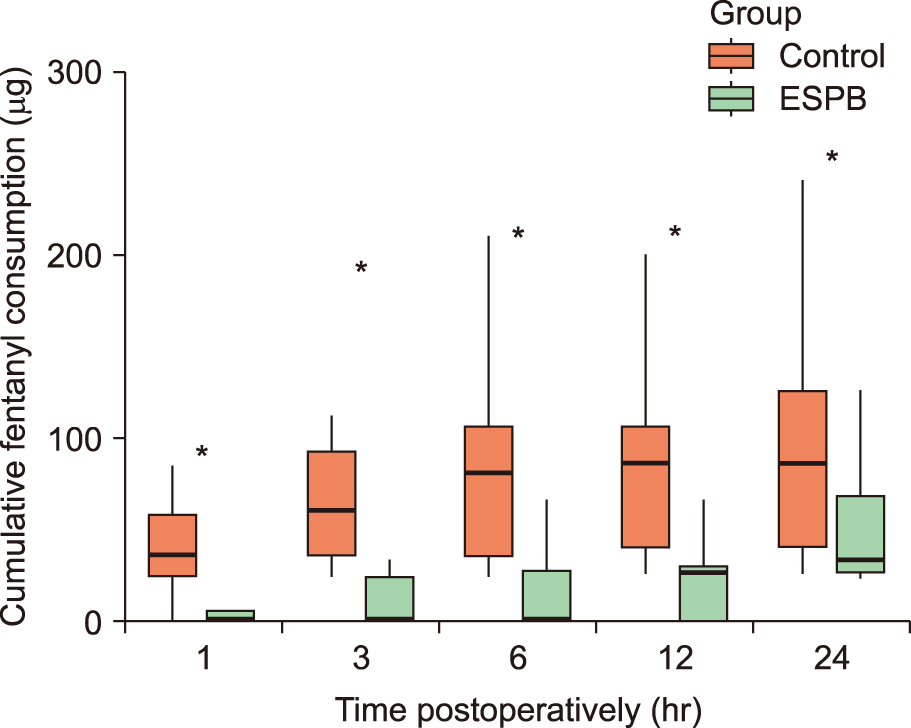
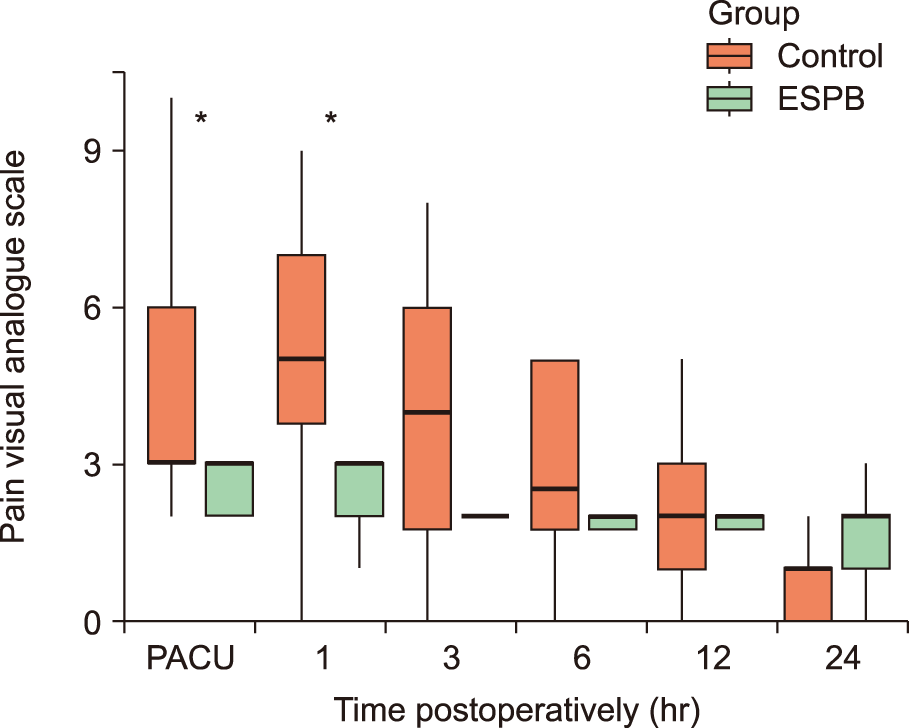




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download