INTRODUCTION
Decompressive neuroplasty (DN) is an effective treatment for low back pain or lower limb pain caused by adhesions in the epidural space that are refractory to conservative treatment [
1]. Adhesions in the epidural space play an important role in chronic lumbar pain syndrome [
2–
4]. These adhesions occur due to fibrosis of the epidural tissue that is induced during the healing process following tissue damage from spinal surgery or spine degeneration-induced inflammation [
3]. Multiple systematic reviews have confirmed that DN is an effective treatment for failed back surgery syndrome, spinal stenosis, and radiculopathy [
5–
7]. Several drugs, in appropriate volumes, may be administered through the catheter to achieve physical and chemical adhesiolysis.
Among the various drugs used for DN, hypertonic saline (HS) is known to reduce adhesions by removing water from the epidural space through osmosis [
8]. Furthermore, HS has cytotoxic effects on fibrous tissue, which might aid in the lysis of epidural fibrosis [
9]. Manchikanti et al. [
10] showed significant clinical improvement with the epidural administration of HS for adhesiolysis. However, the epidural administration of HS can result in pain during injection [
11]. Indeed, it has been reported that the administration of HS stimulates C-fiber afferents [
12], and HS is widely used as an experimental model of muscle or other connective tissue pain in humans [
13,
14]. Such findings suggest that HS may stimulate nociceptive afferents in the epidural space, thus causing pain.
To reduce injection-related pain and discomfort, local anesthetics are administered epidurally prior to the administration of HS [
11]. However, our previous study using different concentrations of HS revealed that even at lower HS concentrations (5% NaCl), mild to moderate injection-related pain was observed, despite the prior administration of a local anesthetic solution into the epidural space [
15]. Therefore, in addition to administering a local anesthetic, Jankovic and Peng [
16] recommended that HS be administered as an epidural infusion over the course of 15 to 30 minutes.
Although the pain caused by epidural injections of HS is well established, no studies to date have evaluated the effectiveness of various methods for reducing injection-related pain. Therefore, the aim of this study was to investigate whether administering a continuous infusion of HS through an infusion pump would reduce the pain compared with that experienced during repeated HS bolus injections. We expected that, compared with bolus injections, continuous infusion of HS through an infusion pump would reduce injection-related pain by preventing a rapid increase in epidural pressure and reducing the spread of HS.
Go to :

MATERIALS AND METHODS
1. Study participants
This study was approved by the Seoul National University Bundang Hospital Institutional Review Board of (IRB No: B-1708/415-303) and registered at cris.nih.go.kr (KCT 0003321). The study participants included consecutive outpatients scheduled for DN at our pain center between October and December 2017. In our center, DN is a common procedure performed in patients with back pain or lower limb pain in whom conservative therapies, including epidural steroid injection, have failed.
The main indications for DN that result in such discomfort are spinal stenosis, disc herniation, and failed back surgery syndrome. The inclusion criteria were as follows: (1) age between 18 and 80 years; (2) presence of chronic low back pain, lower extremity radiating pain, or both, for at least 6 months; (3) treatment involving DN using a 1-day protocol including HS infusion; and (4) a pain score of 6 or more on the 11-point numerical rating scale (NRS) after appropriate conservative treatment, including oral analgesics, physical therapy, and epidural steroid injection. The exclusion criteria were as follows: (1) lack of correlation between neuropathic back pain with radicular symptoms and magnetic resonance imaging (MRI) findings; (2) lumbar surgical intervention in the previous 6 months; (3) coagulopathy, chronic infection, or skin infection at the procedure site; (4) poorly controlled psychiatric disorders or acute medical illness, or underlying systemic diseases that could interfere with the interpretation of the outcome assessments; (5) pregnancy or lactation; (6) history of adverse reaction to local anesthetics or steroids; (7) inability to understand informed consent and the study protocol; and/or (8) inability to lie prone for the procedure.
After obtaining written informed consent, eligible patients were randomly assigned to one of two groups, the bolus injection group (group B) or the continuous infusion group (group C), at the last outpatient session before the procedure. Because the target number of subjects was fewer than 100, block randomization (block size: two or four) was performed using a computer-generated randomization program, which was operated by a clinician who was not involved in the study [
17].
We recorded the demographic and clinical characteristics of the patients, including age, sex, height, weight, pain scores relating to low back or lower limb pain based on the NRS, duration of pain, the Oswestry Disability Index (ODI) (score range, 0–100), history of lumbar spine surgery, diagnosis based on patient history, pre-procedural MRI findings, current medications, target level of the lumbar spine, and underlying diseases (hypertension, diabetes mellitus, and major depressive disorders).
2. Procedures
All procedures were performed by three pain specialists, each with more than 5 years of experience in pain medicine, in an operating room under sterile conditions using fluoroscopic guidance. An intravenous line was established, and cefazolin (1 g) was administered prior to the start of the procedure. After MRI confirmation of the correlation between the location of the pathology and the radicular pain, 5 mL of 1% lidocaine was injected around the sacral hiatus, with the patient lying in the prone position. An 18-gauge Tuohy needle (Sewoon Medical Co., Ltd., Seoul, Korea) was inserted through the sacral hiatus and advanced to the mid-body of the third sacral vertebra under fluoroscopic guidance. After confirming that the needle was properly positioned in the epidural space, lumbar epidurography was performed, with approximately 2 to 5 mL of Omnipaque
®300 (iohexol, 300 mg iodine per mL; GE Healthcare, Piscataway, NJ) as the contrast medium, and filling defects were identified by fluoroscopic imaging [
18]. We determined the target of the intervention by correlating the patient’s symptoms and MRI or computed tomography findings with the filling defect identified on the epidurogram. If symptoms were present on both right and left sides, the treatment area was designated as the area where the patient had more severe symptoms.
Following epidurography, a 19-gauge epidural catheter (EpiStim®; Sewoon Medical Co., Ltd.) with a radiopaque guidewire was slowly passed through a Tuohy needle to the target area. The guidewire was removed and the location of the tip of the epidural catheter was confirmed by injecting contrast medium into the anterolateral epidural space. Mechanical adhesiolysis was performed with 10 to 20 mL of normal saline. After adhesiolysis, adequate filling of the target nerve roots and epidural space was confirmed. We also confirmed that no accidental intravascular, subarachnoid, or extra-epidural injection had occurred. The final position of the catheter tip was determined via the slow injection of a contrast medium and 6 mL of 0.25% ropivacaine (Ropiva injection®; Hanlim Pharm Co., Seoul, Korea) containing 1,500 units of hyaluronidase (H-lase®; Kuhnil Pharm Co., Seoul, Korea). Following completion of the injection, the catheter was taped utilizing a bio-occlusive dressing, and the patient was placed in a supine position and transferred to the recovery room.
In the recovery room, patients were monitored for any potential complications, including motor weakness over the lower extremities and reduced sphincter tone. After confirming the absence of complications, 4 mL of 5% NaCl solution (Sodium chloride injection
®; Choongwae Co., Seoul, Korea) [
15] was injected according to the following methods. In group B, four bolus injections containing 1 mL of 5% NaCl solution each were administered
via the epidural catheter over 5–10 seconds, at 15-minute intervals. In group C, 4 mL of 5% NaCl solution was administered as an infusion over a period of 60 minutes
via an infusion pump connected to the epidural catheter. We used a piezoelectric actuated infusion pump (HPMF
®; Hyun Medics Co., Bucheon, Korea) [
19]. This device is used to automatically inject a liquid chemical using a microcomputer, with a piezoelectric actuator as the driving source. Using this device, the drug can be infused over a fixed time interval; a piezoelectric actuated infusion pump is simpler in structure, less faulty, and cheaper than a motor-operated pump, and can be controlled to improve the reliability of the infusion pump with a microcomputer [
19]. In addition, hydrophobic filters may be used to prevent the air remaining inside the fluid bag from flowing into the body.
The researchers who performed the procedures, and outcome assessments were blinded to the patients’ group assignments. The HS was administered by an anesthesiology resident who was not involved in the study; the anesthesiology resident also measured the residual volume of HS to confirm precise administration of HS into the epidural space through the infusion pump. After the injection of HS, 2 mL of 0.9% NaCl solution containing 5 mg of dexamethasone was injected through the epidural catheter, and the catheter was removed. All patients enrolled in this study received a one-day regimen of DN, as described previously [
10]. Patients were hospitalized on the day of the procedure, given an ambulatory protocol, and discharged after removal of the epidural catheter. Acetaminophen or non-steroidal anti-inflammatory drugs were prescribed as appropriate for pain control during the post-procedure follow-up period.
3. Outcome measures and follow-up
All baseline and post-procedure outcome data were collected by a physician at the pain center who was not involved with the study and who was blinded to the study design. The primary outcome of this study was the difference in the intensity of HS-induced pain between the two groups, as scored according to the 11-point NRS (0 = no pain, 10 = unbearable pain). Infusion-related pain was defined as pain experienced during the epidural HS administration, which was perceived by the patient as different from the baseline lower back or leg pain. Pain assessments were conducted in the recovery room according to the 11-point NRS. In group B, pain scores were recorded during each of the four bolus injections (15-minute intervals); in group C, pain scores were recorded every 15 minutes on four occasions during the 1-hour infusion period. Pain scores were also recorded at 1 and 3 months after the procedure using the NRS for lower back pain or lower extremity pain during outpatient follow-up. Moreover, the ODI was recorded at the 3-month follow-up examination. Patients were instructed to report any adverse events, including paresthesia, neuralgia, numbness, and motor weakness, to the physician during the procedure and at each follow-up visit.
4. Statistical analysis
A two-arm pilot study that included 19 patients in group B and 13 patients in group C was performed before this study. The sample size for the present study was calculated based on the results of the pilot study (group B: 5.11 ± 3.01, group C: 2.31 ± 2.84). The common standard deviation used was 3.01, the standard deviation of the bolus group, to ensure a conservative calculation. The formula used to calculate the number of subjects for the study was as follows:
(k: matching ratio, σ: standard deviation, α: Type I error, β: Type II error, ϕ: standard normal distribution function)
Based on the pilot study, for 80% power and a two-tailed significance level of 5%, 19 patients were required in each study group, for a total sample size of 40 participants. Assuming 20% dropout, 25 patients were required in each group.
All statistical analyses were performed using R statistical software (ver. 3.2.3; R Foundation for Statistical Computing, Vienna, Austria). Data are presented as the mean ± the standard deviation, or as the number and percentage of total participants. To compare the demographic and clinical characteristics between the two groups, t-tests, χ2 tests, and Fisher’s exact tests were used. As the primary outcome measures were obtained at four points in time, a repeated measures analysis of variance was performed with post hoc analyses using t-tests. The NRS and ODI scores, which were secondary outcomes, were also analyzed using repeated measures analyses of variance, and t-tests were used for post hoc analyses. Within each group, time-to-time comparisons were performed with pairwise comparisons of the predictive margins. The level of statistical significance was set at P < 0.05.
Go to :

RESULTS
Fifty patients were enrolled in the study; 44 completed the full follow-up protocol (
Fig. 1). Four patients from group B and two patients from group C refused the procedure or withdrew consent to participate in the study. No patients withdrew during the follow-up period. Thus, data from 44 participants (21 in group B and 23 in group C) were included in the final analysis.
Table 1 describes the demographic and clinical characteristics of the patients. No significant differences in the demographic and clinical variables were identified between the two groups.
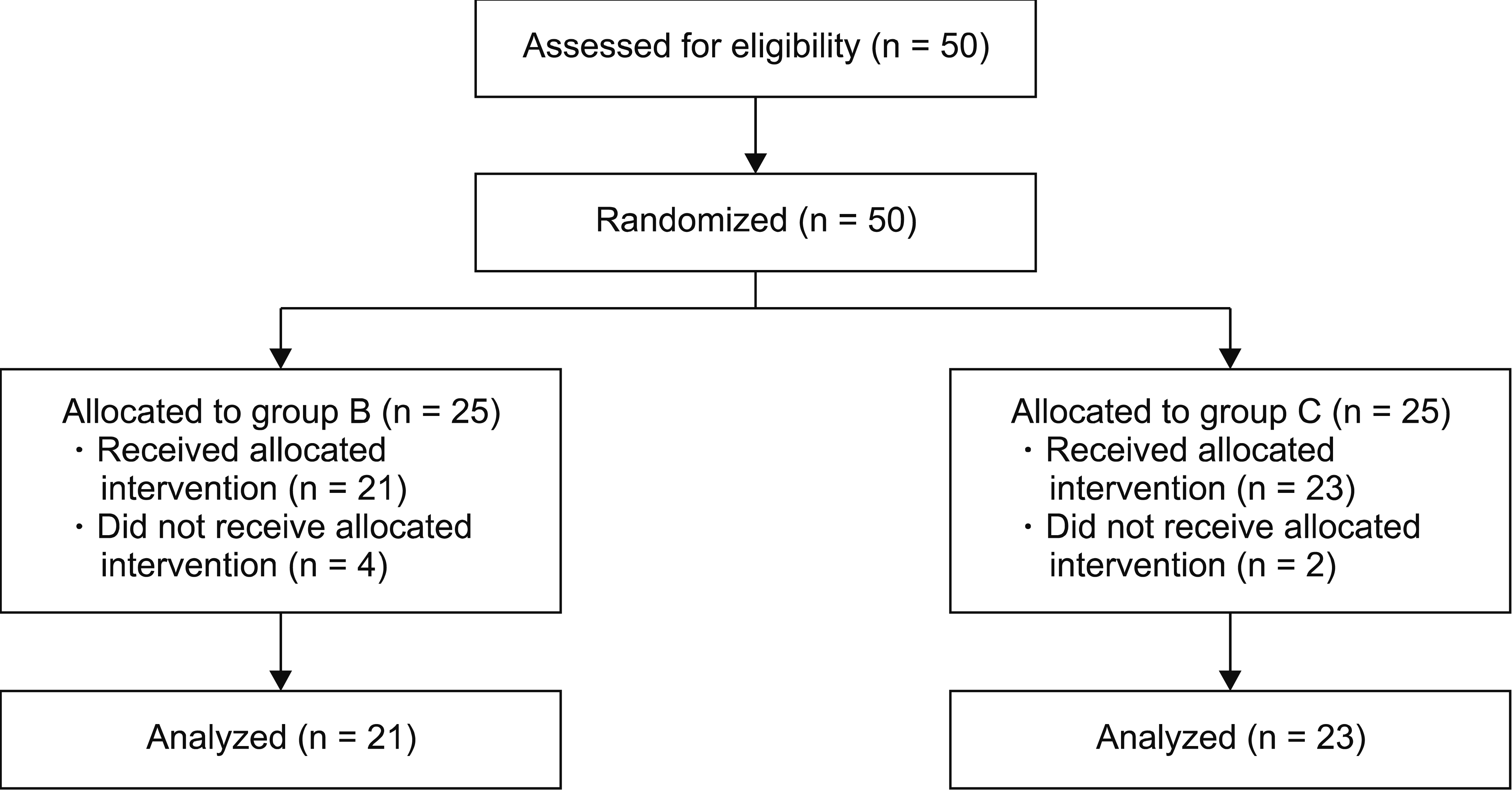 | Fig. 1Flow diagram of patients in this study. At 3 months post-procedure, 21 and 23 patients remained in each arm, respectively. 
|
Table 1
Comparison of the Demographic and Clinical Variables between the Bolus Injection and Continuous Infusion Groups
|
Variable |
Bolus injection group (n = 21) |
Continuous infusion group (n = 23) |
P value |
|
Age (yr) |
70.7 ± 6.6 |
68.0 ± 11.3 |
0.339 |
|
Sex (M/F) |
10 (47.6)/11 (52.4) |
11 (47.8)/12 (52.2) |
1.000 |
|
Height (cm) |
159.1 ± 10.7 |
160.9 ± 9.9 |
0.580 |
|
Weight (kg) |
63.6 ± 9.3 |
67.2 ± 15.2 |
0.345 |
|
Duration of pain (mo) |
82.1 ± 99.8 |
80.8 ± 115.4 |
0.968 |
|
HTN (yes) |
11 (52.4) |
16 (69.6) |
0.390 |
|
DM (yes) |
6 (28.6) |
6 (26.1) |
1.000 |
|
MDD (yes) |
1 (4.8) |
2 (8.7) |
1.000 |
|
Current medication |
|
|
0.794 |
|
No analgesic use |
3 (14.3) |
4 (17.4) |
|
|
Strong opioid analgesic usea
|
4 (19.0) |
2 (8.7) |
|
|
Weak opioid analgesic use onlyb
|
3 (14.3) |
4 (17.4) |
|
|
Non-opioid analgesic use onlyc
|
11 (52.4) |
13 (56.5) |
|
|
Number of previous interventionsd
|
2.1 ± 2.3 |
3.1 ± 2.6 |
0.169 |
|
Diagnosis |
|
|
0.279 |
|
HIVD |
10 (47.6) |
6 (26.1) |
|
|
Spinal stenosis combined |
8 (38.1) |
14 (60.9) |
|
|
FBSS |
3 (14.3) |
3 (13.0) |
|
|
Stenosis severitye
|
|
|
0.325 |
|
None or mild |
10 (47.6) |
6 (26.1) |
|
|
Moderate |
1 (4.8) |
2 (8.7) |
|
|
Severe |
10 (47.6) |
15 (65.2) |
|
|
Target level |
|
|
0.129 |
|
L3/4 |
0 (0) |
1 (4.3) |
|
|
L4/5 |
5 (23.8) |
11 (47.8) |
|
|
L5/S1 |
16 (76.2) |
11 (47.8) |
|
|
Bilateral symptom |
2 (9.5) |
1 (4.3) |
0.935 |
|
NRS (0–10) |
7.8 ± 1.0 |
7.4 ± 1.1 |
0.247 |
|
ODI (0–100) |
39.6 ± 13.3 |
40.4 ± 11.7 |
0.830 |

No statistically significant difference in the injection-related pain scores was found between the two groups for the initial administration of HS (
Table 2,
Fig. 2;
P = 0.836). However, there was a statistically significant reduction in injection-related pain in group C compared with that in group B from the second bolus injection of HS (
P = 0.004,
< 0.001, and
< 0.001, respectively). According to the within-group post hoc analyses, group C did not show a significant change in the pain scores between the first and subsequent assessments. However, in group B, compared with the first administration, increased pain was noted during subsequent injections (
Table 2,
P = 0.001,
< 0.001, and
< 0.001, respectively). In addition, within-group comparisons for group B demonstrated a significant increase in pain from the second administration to the fourth administration (
P = 0.01).
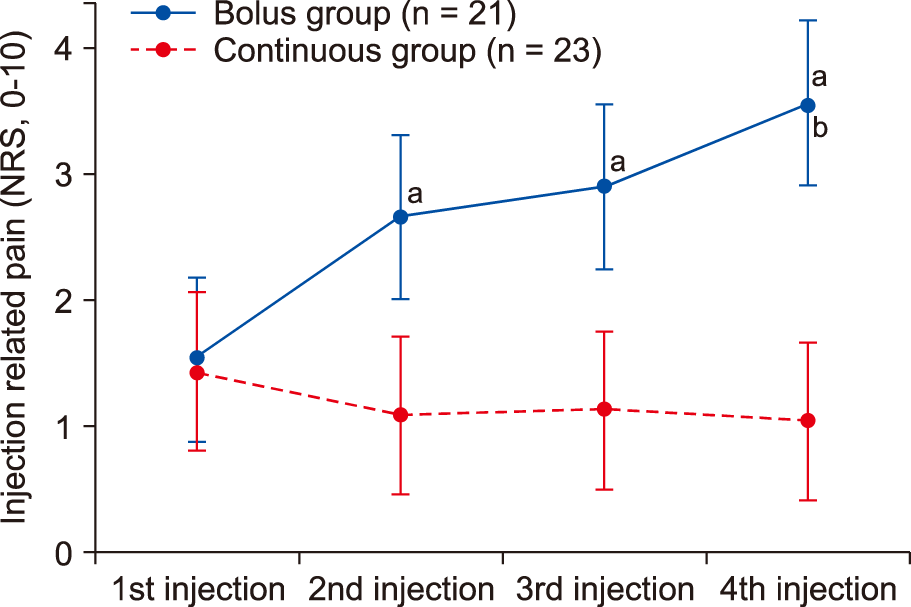 | Fig. 2The 11-point numeric rating scale (NRS) scores (0 = no pain, 10 = unbearable pain) of injection-related pain induced by hypertonic saline infusion. Data are shown in a box plot with the 95% confidence intervals (whiskers). aSignificant at P < 0.05, compared to the 1st injection NRS. bSignificant at P < 0.05, compared to the 2nd injection NRS. 
|
Table 2
Procedure-related Pain during Hypertonic Saline Injection and Clinical Outcome after Decompressive Neuroplasty
|
Variable |
Bolus injection group (n = 21) |
Continuous infusion group (n = 23) |
P valuea
|
|
Injection NRS (0–10) |
1st injection |
1.52 (0.87–2.18) |
1.43 (0.81–2.06) |
0.836 |
|
2nd injection |
2.67c (2.01–3.32) |
1.09 (0.46–1.71) |
0.004 |
|
3rd injection |
2.90c (2.25–3.56) |
1.13 (0.50–1.76) |
<0.001 |
|
4th injection |
3.57c,d (2.97–4.23) |
1.04 (0.48–1.67) |
<0.001 |
|
Group effect P valueb
|
|
|
0.0001 |
|
NRS (0–10) |
Baseline |
7.81 (6.92–8.70) |
7.43 (6.58–8.29) |
0.548 |
|
1 month |
5.57c (4.68–6.46) |
5.65c (4.80–6.50) |
0.897 |
|
3 months |
5.38c (4.49–6.27) |
5.70c (4.85–6.56) |
0.614 |
|
Group effect P valueb
|
|
|
0.9892 |
|
ODI (0%–100%) |
Baseline |
39.62 (34.46–44.77) |
40.43 (35.51–45.36) |
0.821 |
|
3 months |
25.33c (20.18–30.49) |
25.57c (20.64–30.49) |
0.949 |
|
Group effect P valueb
|
|
|
0.8768 |

During the follow-up period, the NRS scores decreased significantly until 3 months after the procedure compared with the baseline values in both groups (
Fig. 3; group B:
P < 0.001, group C:
P < 0.001). However, no significant differences in the NRS scores were observed between the two groups at the 1- and 3-month follow-up evaluations (
P = 0.897 and 0.614, respectively).
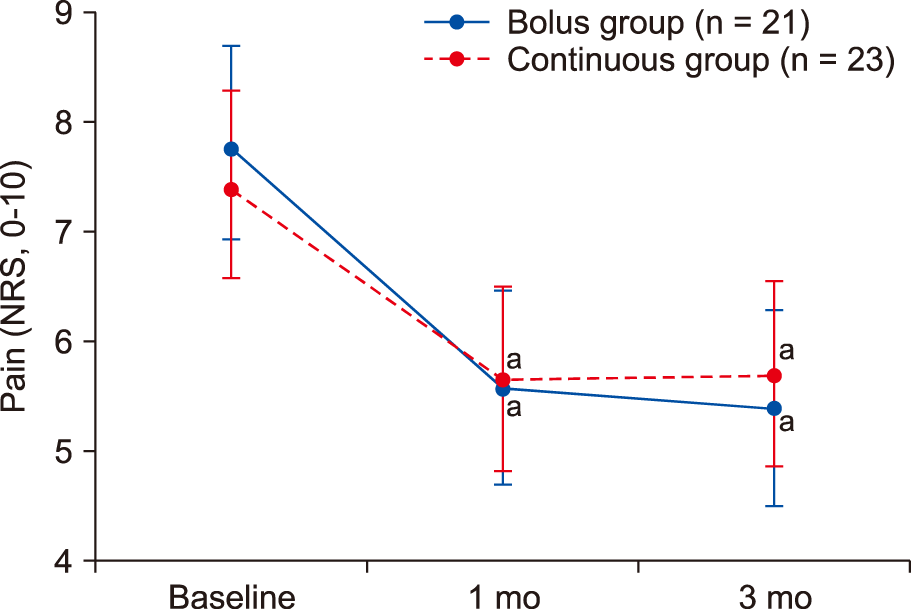 | Fig. 3The 11-point numeric rating scale (NRS) scores (0 = no pain, 10 = unbearable pain) for the leg and lower back pain in patients receiving lumbar epidural adhesiolysis. Data are shown in a box plot with the 95% confidence intervals (whiskers). aSignificant at P < 0.05, compared to the baseline NRS. 
|
The ODI decreased significantly compared with the baseline values until 3 months after the procedure in both groups (
Fig. 4; group B:
P < 0.001, group C:
P < 0.001). However, no significant difference in the ODI was identified between the two groups after 3 months (
P = 0.949).
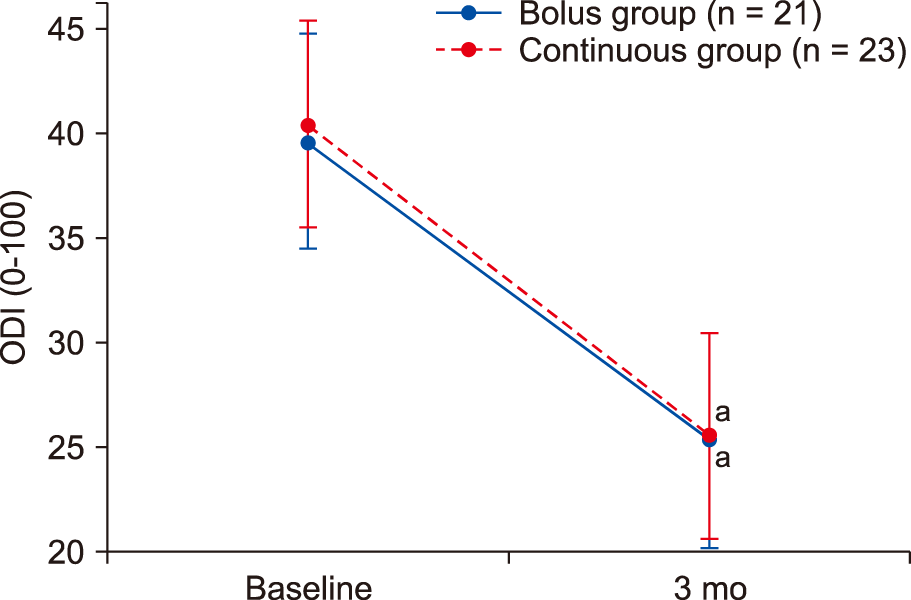 | Fig. 4Oswestry Disability Index (ODI) scores in patients receiving lumbar epidural adhesiolysis. Data are shown in a box plot with the 95% confidence intervals (whiskers). aSignificant at P < 0.05, compared to the baseline ODI. 
|
No serious complications during injection or cases of inappropriate drug delivery were reported. No post-procedure complications (hypotension, paresthesia, motor weakness, or headache) were reported during the follow-up period, and no other complications, such as infection, sensory deficits, and deterioration of motor function, were reported throughout the study period. No patients withdrew from the study due to adverse effects.
Go to :

DISCUSSION
To the best of our knowledge, this is the first study to investigate two different methods of administering HS for DN and their effects on injection-related pain. The present study demonstrated a statistically significant reduction in the pain experienced by patients who received the HS through an infusion pump relative to those who received the HS through repeated bolus injections for DN.
In this study, patients experienced less pain when HS was infused over 60 minutes through an infusion pump than when HS was administered through four bolus injections. This suggests that the rate of HS administration influenced the severity of injection-related pain. Although the relationship between the rate of HS infusion and pain has not been determined, a study investigating the relationship between local anesthetic-induced pain and the rate of administration reported that the rate of administration had a greater effect on perceived pain during lidocaine infiltration than did buffering [
20]. The authors speculated that a slower injection rate was associated with decreased pain levels due to the slower distention of local tissue and the activation of fewer nerve endings. Although we could not measure the actual spread of HS, the slow infusion of HS may prevent it from spreading beyond the segment in which the local anesthetics had previously been applied, reducing nociceptor irritation.
Another possible mechanism for HS-related pain is an increase in epidural pressure during the injection of HS. A human study reported that the injection speed is significantly correlated with the peak epidural pressure [
21]. The peak epidural pressure has also been directly correlated with the speed of injection in an animal study [
22]. In addition, it has been reported that the degenerative changes that occur with increasing age decrease the elasticity of the epidural space, leading to higher epidural pressures [
23]. The most common indications for DN include degenerative diseases, which are common in elderly patients, wherein the catheter is placed in the most stenotic region. Furthermore, HS is hyperosmolar and is hypothesized to increase the epidural pressure through the transfer of intracellular fluid to the extracellular space. Under these conditions, as HS is injected into the epidural space with reduced elasticity and structural narrowing, the pressure may temporarily increase, resulting in pain caused by compression of the surrounding tissues. Furthermore, the injection of large volumes of fluid can compress nerves and cause transient nerve damage [
24]. We think that administering HS through an infusion pump will help to avoid this complication by preventing a sudden increase in epidural pressure.
Another remarkable finding of the study was the increased incidence of injection-related pain associated with an increased number of doses in the bolus group. It was difficult to identify the mechanism underlying these phenomenon in the present study; we assumed that it was partially caused by central hyperexcitability due to repeated injections. A prior study of healthy adult men demonstrated that pain increased as 5% HS was injected repeatedly within muscles at regular intervals [
25]. Although the temporal summation caused by repetitive stimuli in the epidural space is unknown, we suspect that the two methods of administration (
i.e., bolus injection with a fixed time interval, and continuous infusion) may have had different effects on the sensitivities of nociception. Further research is needed regarding this phenomenon.
In the present study, we expected the HS to be more concentrated in the target area when infused continuously and slowly, thereby improving the effectiveness of DN. However, no differences in treatment outcomes were noted between the two groups in this study. Although the number of subjects in this study was sufficient to compare injection-related pain between the two administration methods, the sample size was too small to compare the treatment outcomes of DN, which were likely affected by several variables, such as the amount of spinal stenosis. Indeed, although there were no statistically significant differences between the two groups, more spinal stenosis was observed in group C than in group B. Spinal stenosis is reportedly a poor prognostic predictor of DN [
26]. Therefore, to compare the treatment outcomes between the two methods, additional studies that take into consideration the various factors that may affect the outcome of DN are needed.
Finally, it is less labor-intensive to administer HS as a pump-driven infusion than it is to administer HS as repeated bolus administrations. Bolus administrations need to be divided into several slow injections to reduce injection-related pain, making the procedure cumbersome. The reduction in required labor is particularly important if several patients require treatment on the same day. In our pain center, patients undergo HS administration through an infusion pump over the course of an hour. Physicians do not need to be present during the infusion, as nurses monitor the patients. Thus, in addition to reducing injection-related pain, the administration of HS as an infusion makes the procedure less labor-intensive.
Our study has several limitations. First, the study design precluded patient and doctor blinding. Therefore, we utilized an evaluator who was not involved with the study to assess infusion-related pain. However, we cannot completely exclude placebo effects due to differences in the methods of administration. Second, we did not measure epidural pressures or evaluate the drug distribution within the epidural space. Notably, we could not ignore differences in the epidural pressure and degree of local anesthetic concentration and spread that occurred as a result of the different volumes of contrast medium and normal saline that were administered to each patient. In addition, although the small sample size resulted in a lack of statistical differences in clinical variables between the two groups, we found differences in target level, diagnosis, and severity between the two groups. However, such differences support our findings, because there was greater stenosis in the continuous group, which could lead to increased injection-related pressure that limited drug distribution. Further studies are needed to determine the relationship between the infusion rate and epidural pressures, as well as the relationship between the infusion rate and drug distribution. Third, the infusion pump should reliably deliver a precise volume into the epidural space. In this regard, we also measured the residual volume of HS to assess whether a precise volume of the solution was administered into the epidural space through the infusion pump. Although no complications were reported in this study, larger studies are needed to ensure reliability of the infusion pump.
In conclusion, our study suggests that performing DN with a continuous HS infusion is an effective way of reducing injection-related pain. Although the outcomes at the 3-month follow-up evaluation did not reveal any advantages, the continuous infusion of HS using an infusion pump in DN is less painful and less labor-intensive than is the repeated bolus injection of HS.
Go to :








 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download