INTRODUCTION
Patients suffering from intense, chronic pain anticipate the development of new medicines that have little to no side effects; meanwhile, relying on numerous alternative medicines. Inhaling the fragrance of essential oils is considered to be an alternative medicine to achieve psychological and physical relaxation. Fragrances are used in cosmetics, foods, and in health-promoting agents. The utilization of fragrances dates back to at least 3000 B.C., where essential oils were used for mummification and in beauty products. There are even records of Hippocrates using fragrances to cure diseases. Scientific experiments have shown that when aromatic fragrances are inhaled, pain is sensed differently, unlike when unpleasant odors are inhaled [
1].
Eucalyptus has been used in Brazilian traditional medicine as an anti-inflammatory, analgesic, and antipyretic agent, and to treat flu, common cold, and nose bleeds [
2–
4]. Modern science has also demonstrated such beneficial effects of
Eucalyptus. However, further studies are necessary to confirm the precise pathways involved in the analgesic effects of
Eucalyptus.
In this study, two methods were used to observe the analgesic effects of Eucalyptus in various pain models of mice: 1) inhalation of the essential oil of Eucalyptus (EOE) and 2) intraperitoneal injections for systemic administration. Pain models included formalin-induced, acetic acid-induced writhing, and thermal-stimulated pain. Behavioral experiments utilizing antagonists of pain-killers have been used to investigate which pain pathway is related to such analgesic effects. Moreover, drowsiness and dizziness may appear as side-effects after a medicine is injected. Certainly, side-effects need to be reduced for the medicine to be used clinically. Therefore, in this study, the rotarod test was used to investigate potential side effects caused by the drug.
Go to :

MATERIALS AND METHODS
1. Experimental animals
Prior to the experiment, male C57BL/6 mice aged 8 weeks (Samtaco Inc., Osan, Korea) were maintained under a 12 h light-dark cycle (lights on from 7 AM to 7 PM) at 21 ± 2°C. The animals had access to sterilized food and tap water as they desired and were subjected to the following tests: the formalin test, acetic acid test, rotarod test, and plantar test. Animals were used independently for each experiment. The guidelines of the Institutional Animal Research Ethics Committee of Korea University (approval no. KUIACUC-2011-84) were followed for the care of the experimental animals in all experimental measures.
2. Injection of EOE
Animals were injected with EOE (Aromarant Co. Ltd, Rottingen, Germany) dissolved in almond oil at 11.25, 22.5, 45, 90, and 180 mg/kg intraperitoneally in an injection volume of 0.1 ml/100 g body weight. Normal saline (0.9% w/v) was administered to the control group, whereas almond oil alone was injected to the vehicle group. The control, vehicle, and essential oil groups were injected 30 min before the formalin, thermal plantar, and rotarod tests, and 35 min before the acetic acid-induced writhing test.
3. Inhalation of EOE
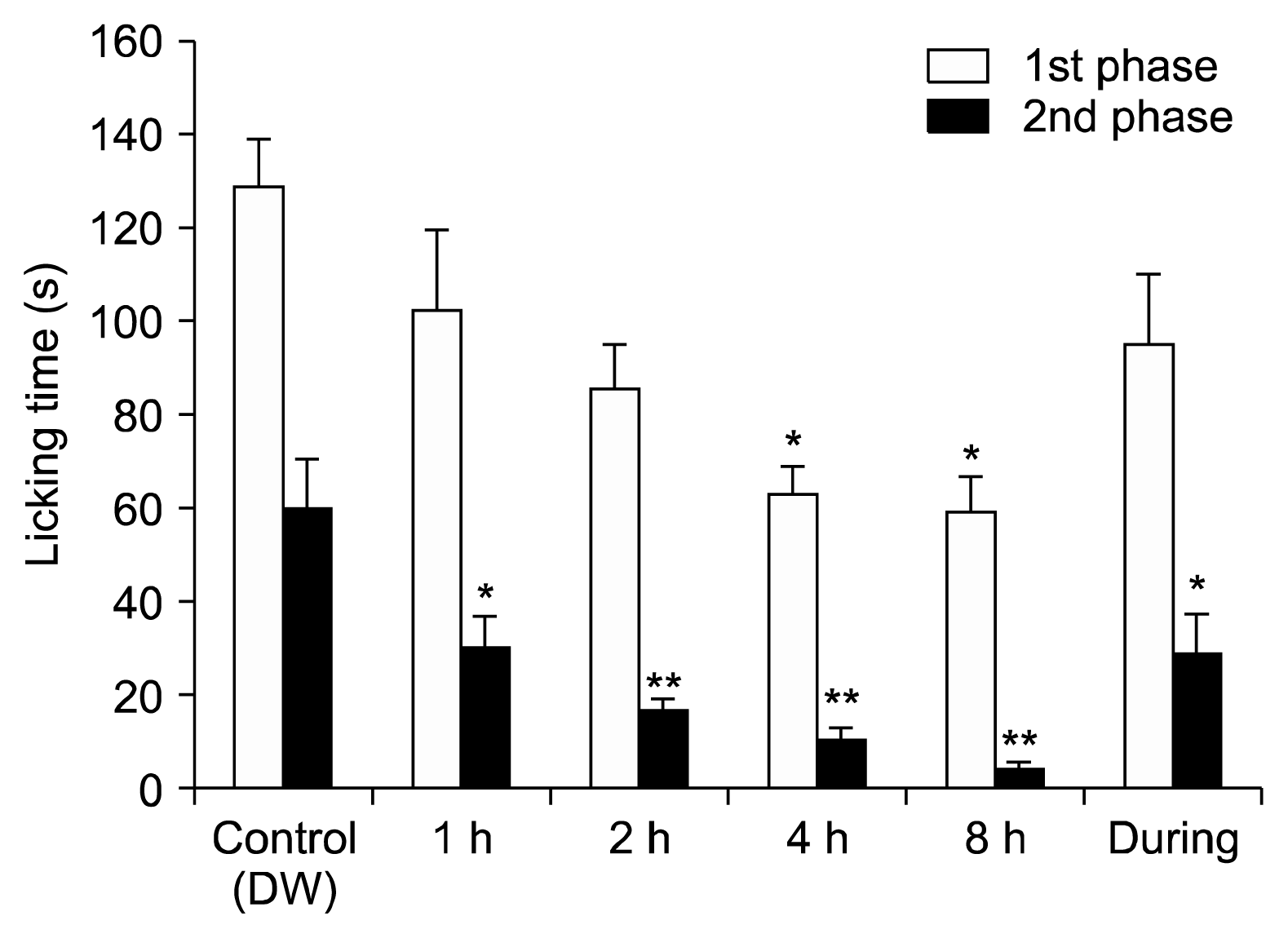
The hold-board apparatus consisted of a square transparent plexiglas cage (50 cm × 50 cm × 30 cm) with 4 holes in the bottom which were prepared for inhalation of the essential oils. The EOE was first combined with the same volume of distilled water and then absorbed in cotton that was set on the upper side of the inhalation box to ensure vaporization. A 1 ml aliquot of EOE was vaporized in a petri dish (90 × 15 mm) in the EOE group, while only distilled water was given to the control group. Before the formalin test, mice were exposed to the translucent Plexiglas cage for 1, 2, 4, and 8 h, except for the group exposed to EOE.
4. Drugs
All drugs and vehicles were injected intraperitoneally 15 min prior to the EOE injection, except in the case of the inhalation tests, at a rate of 0.1 ml/100 g of body weight and included morphine (4, 8 mg/kg), indomethacin (10 mg/kg), naloxone (8 mg/kg), 5′-guanidinonaltrindole (0.3 mg/kg), and glibenclamide (2 mg/kg).
5. Formalin test
The formalin test was similar to the one reported by Hunskaar and Hole [
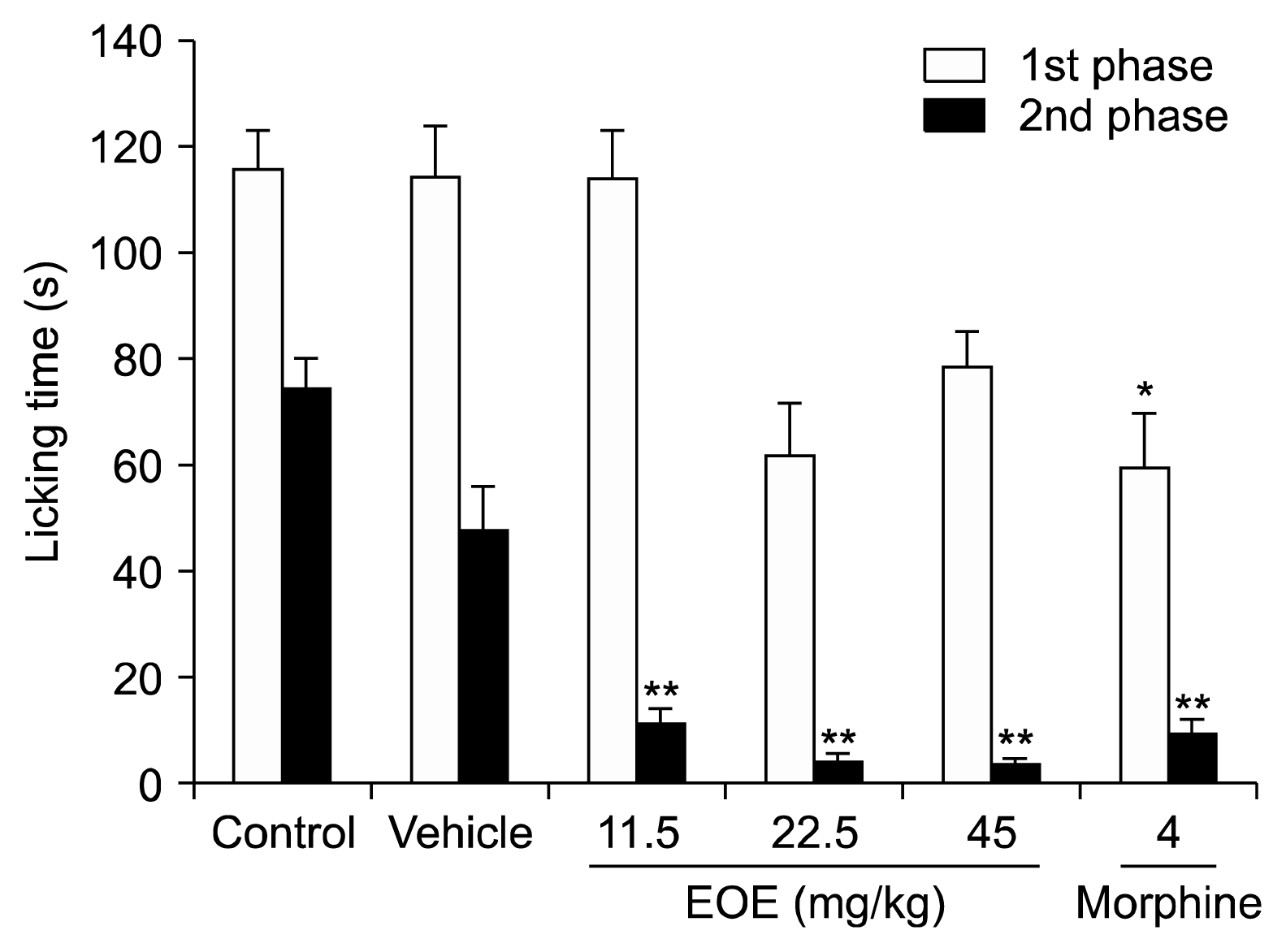
5]. Twenty μl of formalin (2 % v/v) was injected into the mice (n = 7–12) dorsal surface of the left hind paw of the mice. The mice licking their injected paw were instantly timed. Their response was considered, based on the following: 1) 0–5 min after the formalin injection was the nociceptive pain response and 2) 20–25 min after was the inflammatory pain response.
6. Acetic acid-induced abdominal writhing test
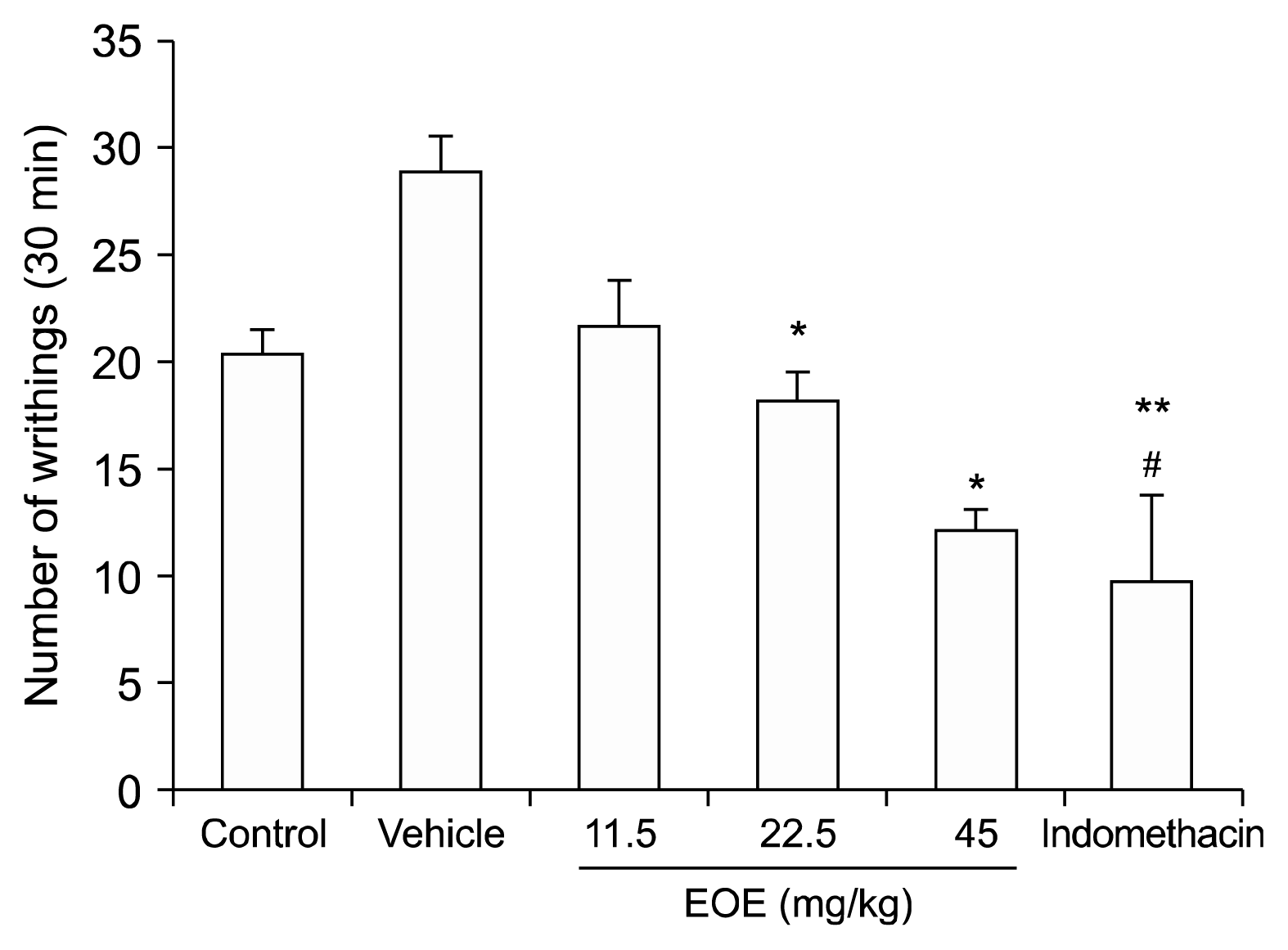
In accordance to Quintans-Júnior et al. [
6], abdominal writhing was assessed in mice (n = 8–10). Acetic acid was injected intraperitoneally as a 0.5% solution (10 ml/kg). Total writhing movements were counted during a 30-min period starting 5 min after the acetic acid injection.
7. Thermal plantar test
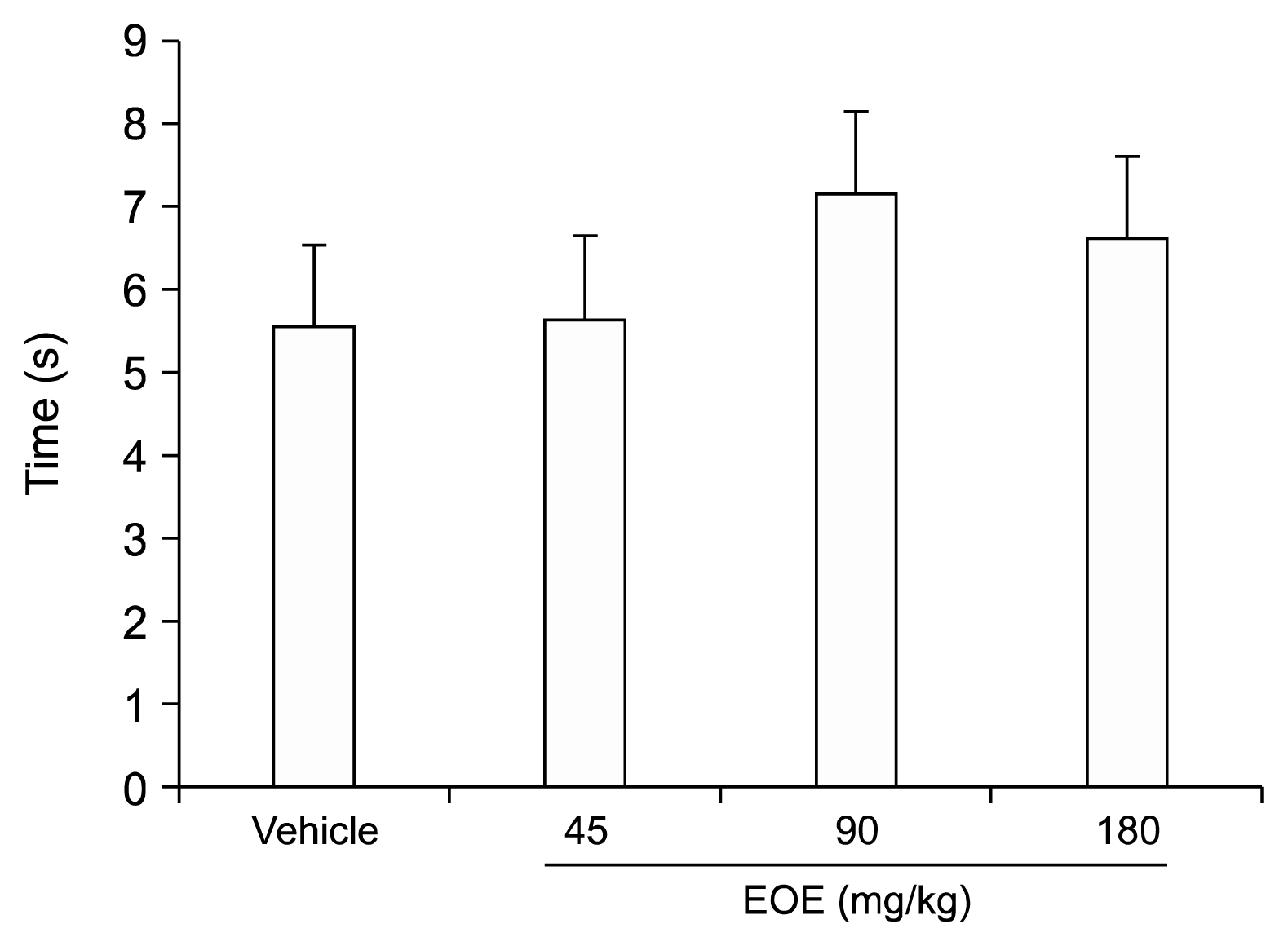
To assess paw withdrawal latency (PWL) to a thermal nociceptive stimulus, the Hargreaves method [
7] was utilized using intraperitoneal administrations of 0.9% saline (control group), vehicle, or EOE (45 and 90 mg/kg) (n = 10 per group). The plantar surface of the hind paw received the center of a focused beam of heat. To avoid tissue damage, the application of the beam was halted at 20 s. With at least 1-min intervals, 4 measurements were obtained on each hind paw. The mean PWL of each group was evaluated using previously determined standard values for each hind paw.
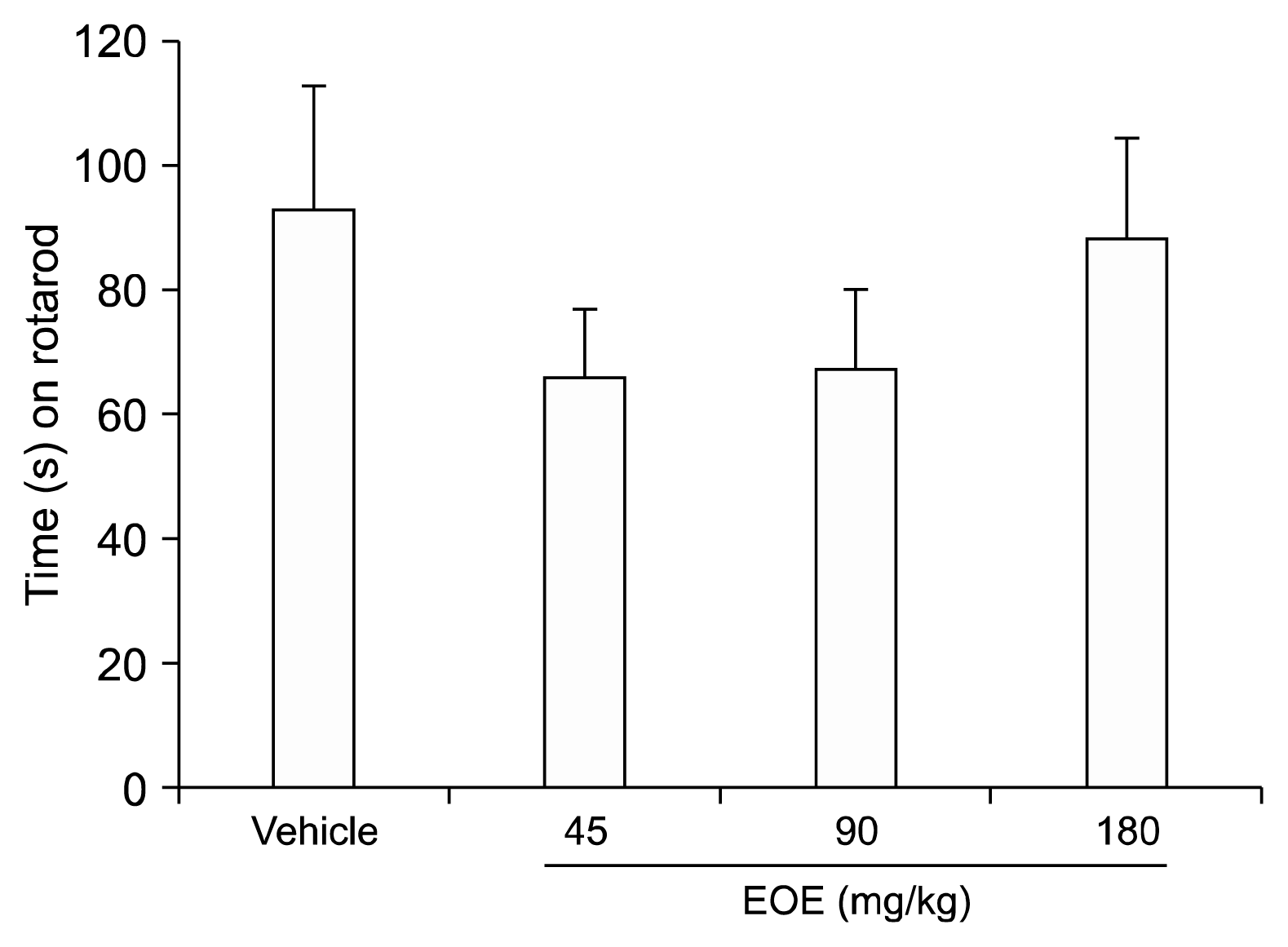
8. Rotarod test
To measure whether the EOE could influence motor coordination, a rotarod test was applied. EOE (45, 90, and 180 mg/kg) and vehicle (almond oil) was administered intraperitoneally. Animals were subjected to spinning at 4 rpm (n = 10 per group) using a rotating rod (Scitech Korea, Seoul, Korea). The rotation was progressively augmented at a rate of 1 revolutions per minute (rpm) after mouse stabilization. Infrared sensors recorded the time that the mice managed to remain on the rod and the speed at which they fell off. The average of three trials was used for each mouse.
9. Statistical analysis
Mean ± standard error was used. One-way ANOVA, followed by Tukey’s post hoc test was employed to evaluate differences among treatment groups. A P value of < 0.05 was considered statistically significant in all cases.
Go to :

DISCUSSION
In Brazilian folk medicine, various Eucalyptus species from the Myrtaceae family are used to treat numerous medical conditions. Traditionally, for example, hot water extracts of the dried leaves of E. citriodora are used in cosmetics and for culinary purposes. Moreover, the extracts are also used to treat common cold, flu, and sinus congestion. However, as various essential oils have different active chemical components, they should be used appropriately according to their physiological effects. More interestingly, diverse analgesic effects have been shown among various species of Eucalyptus. Therefore, individual assessments of each species, assessing their analgesic effects, mechanism of action, and side effects are necessary for proper aromatherapy. Therefore, the purpose of this study was to elucidate the analgesic effects, pain pathways, and motor coordination effects of EOE on somatic, visceral, thermal, and inflammatory nociception.
The formalin test was modified to evaluate inflammatory pain, nociceptive pain, and the central sensitization effects of EOE. Analgesic effects on inflammatory pain were observed through evaluation of the first and second phases after intraperitoneal injection or inhalation of EOE followed by hind-paw injection of 20 μL of formalin (2% v/v). An analgesic effect of EOE was observed for nociceptive pain and for inflammatory pain. Moreover, compared to the analgesic effect of morphine (licking times: 9.6 s), the analgesic effect of EOE was greater (licking times: 4.4 and 4.1 s for 22.5 and 45 mg/kg, respectively). Although there was no analgesic effect in the first phase of the formalin test in conjunction with inhalation of EOE, dose-dependent (1, 2, 4, and 8 h) analgesic effect was observed during the second phase. In other words, a higher analgesic effect was observed in the group that inhaled EOE 2 h prior to the formalin test than the group that inhaled EOE only during the formalin test. Therefore, the longer the inhalation of EOE, the greater the analgesic effect. In conclusion, although EOE does not have an analgesic effect on somatic pain, there was an analgesic effect and central analgesic effect on inflammatory pain.
In our study, we first performed an intraperitoneal injection of EOE and then administered formalin 30 min later, referring to previous papers [
8–
11]. However, since EOE is a mixture of various ingredients, it is unclear when the maximum action will take place after intraperitoneal administration. However, the main component of EOE is 1,8-cineole which is lipophilic and passes easily through the cell membrane. Therefore, we think that the reason for not showing the effect in the first phase of the formalin test is that there may be no drug effect. Essential oils, composed of many different molecules, enter the olfactory bulb via the nose where they can exert effects on the olfactory cortex, which is connected to various regions of the brain. Many studies on pain have shown that the limbic system is connected to the amygdala and hippocampus [
11–
13], which control emotion and memories.
After injecting EOE (11.5, 22.5, or 45 mg/kg each) and indomethacin (10 mg) intraperitoneally, an anti-nociceptive effect was observed during the writhing test. A statistically significant analgesic effect at 45 mg/kg of EOE was observed compared to that of the control group (12.1 vs. 20.3 writhing movements/30 min), and a similar effect was observed for the indomethacin group (9.63 writhing movements/30 min). Visceral pain involves different pain expression pathways from those of somatic pain. Sensory innervation of the viscera not only includes bilateral spinal, thoracic, and most abdominal organs, but also includes vagal afferents. Spinal afferents conduct noxious stimuli and vagus afferents conduct chemonociception, autonomic, and emotion responses [
14,
15]. Such stimulation to the viscera is amplified and modulated by passing through voltage-gated and ligand-gated ion channels of the spinal cord and supraspinal area. Through these processes, the message is diffused and poorly localized, and is specifically shown as referred patterns. Moreover, in central levels, the analgesic mechanism is shown in relation to typical emotional and autonomic responses [
16]. Therefore, visceral pain is a complex pathway in which the receptive ending, ion channel, chemical substance, nerve transmission, and modulation are related. In this study, we can expect that, although EOE did not have analgesic effects on somatic and thermal nociception, the analgesic effect observed against visceral pain may involve interactions in diverse parts the body. Similarly, intraperitoneal injection (5 and 10 mg/kg) of essential oils of
C. citratus followed by the acetic acid-induced writhing test showed inhibition (48 and 87%) [
17].
A plantar test was used to detect the thermal anti-nociceptive effect of intraperitoneally injected EOE. Although the concentrations of injected EOE (45, 90, and 180 mg/kg each) were increased, there was no statistically significant result, suggesting that EOE does not affect normal thermal sensation.
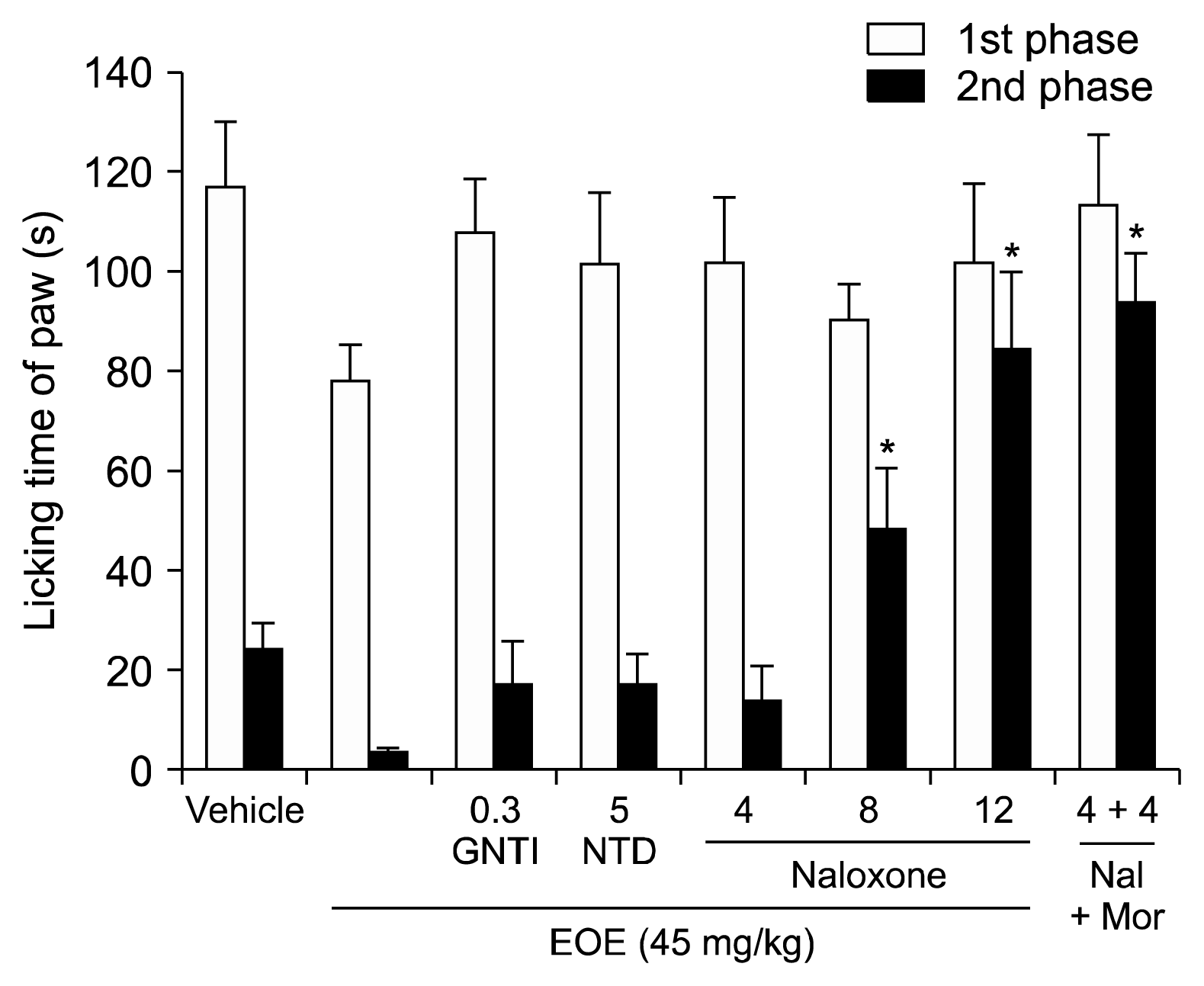
A well-known analgesic effect of EOE is that it acts as a potent inhibitor of the inflammatory response through the arachidonic acid metabolic pathway by inhibiting the production of leukotriene B2, prostaglandin E2, and other arachidonic acid metabolites such as eucalyptol [
4]. In this experiment, 5′-guanidinonaltrindole (κ-opioid antagonist, 0.3 mg/kg) and naltrindole (δ-opioid antagonist, 5 mg/kg) could not antagonize
Eucalyptus, but naloxone (non-selective opioid antagonist, 4, 8, 12 mg/kg) could. Naloxone is a non-selective opioid antagonist and its binding affinity is highest for the μ-opioid receptor, then the δ-opioid receptor, and lowest for the κ-opioid receptor. In the present study, the analgesic effects of
Eucalyptus was not antagonized by the κ-opioid antagonist and δ-opioid antagonist. However, the analgesic effects of
Eucalyptus was antagonized by naloxone, a non-selective opioid antagonist. Therefore, the μ-opioid receptors are involved in the analgesic effects of EOE.
However, naloxone failed to modify the response of 1,8-cineole (cineole), suggesting a non-participation of μ-opioid receptors in the effects of cineole which is a principal component of EOE [
18]. Liapi et al. [
18] used the tail-flick and hot-plate tests, reflecting the spinal and supra-spinal levels, respectively. We used the formalin test, in which the response to formalin shows an early and a late phase. The early phase seems to be caused predominantly by C-fiber activation due to the peripheral stimulus, while the late phase appears to be dependent on the combination of an inflammatory reaction in the peripheral tissue and functional changes in the dorsal horn of the spinal cord. In the present study, naloxone could antagonize the analgesic effects of EOE in a dose dependent manner. It is therefore likely that μ-opioid receptors at the periphery are involved in the effect of EOE, unlike the findings of Liapi et al. [
18]. Further studies are needed on the deferent mechanism by which EOE acts in the central and peripheral regions.
The rotarod test evaluates the effect of a drug on motor the coordination of animals and is helpful to determine drug safety in humans. There was no statistically significant difference in the results between the treatment and control groups even when a high concentration of EOE (180 mg/kg) was utilized. This shows the possibility of EOE being developed as a new class of medicine, as it does not affect motor coordination.
In conclusion, the present study elucidated the antiinflammatory effects of EOE. Furthermore, its inherent medical specifications were reported in relation to species. This study also demonstrated the analgesic effects of EOE on nociception and inflammation, and that the anti-inflammatory effect of EOE likely affects the μ-opioid pathway. Moreover, EOE did not affect motor coordination. Thus, the combined results suggest that EOE has the potential to be developed into an analgesic agent to treat various types of pain. Further studies are necessary to improve our understanding of the pharmacology of EOE, and to elucidate the mechanism of action of these effects in other pain tests in addition to the formalin test using EOE’s isolated active components and purified ingredients for the treatment of pain.
Go to :





 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download