Abstract
Chronic perineal pain is an often encountered problem, which produces a great degree of functional impairment and frustration to the patient and a challenge to the treating physician. The reason for this problem is that the region contains diverse anatomic structures with mixed somatic, visceral and autonomic innervations affecting bladder and bowel control and sexual function. A blockade of nociceptive and sympathetic supply to the perineal region, supplied through the ganglion impar has been shown to benefit patients with chronic perineal pain. Several options to this block have been described that chemical neurolysis, radiofrequency ablation etc. Although the analgesic effect of Botulinum toxin type A (BoNT-A) has long been considered secondary to its action for muscle relaxation, BoNT-A also affects the release of the neurotransmitters that are involved in pain perception. We describe a patient who was successfully given ganglion impar block with BoNT-A.
Go to : 
Chronic perineal pain is difficult to treat because the perineum consists of various anatomic structures, sympathetic nerves, and somatic nerve fibers [1]. For the treatment of perineal pain, blockage of the ganglion impar, which is located in the most inferior aspect to the sympathetic nervous system, has been introduced. Since then, blockage of the ganglion impar has been used to treat diseases, such as perineal or perianal malignant pain, excessive perianal sweating, rectal tenesmoid pain, and coccygodynia [1-3]. To achieve blockage of the ganglion impar, the following methods exist: local anesthetics, concomitant use of local anesthetics and steroids, neurolysis by the use of alcohol or phenol, and radiofrequency ablation [4]. We performed blockage of the ganglion impar using Botulinum toxin type A (BoNT-A). The mechanism of action of BoNT-A involves blockage of acetylcholine secretion by binding to the presynaptic nerve ending. BoNT-A has therefore been used to treat diseases associated with excessive muscle contraction [5]. In recent years, attempts have been made to use BoNT-A for the treatment of diseases, such as various types of headaches, lower lumbar pain, myofascial pain syndrome, and complex regional pain syndrome (CRPS), suggesting that the analgesic effect of BoNT-A has other mechanisms than the secondary effect due to muscle relaxation [6-8]. In a patient with perineal pain who did not have a satisfactory treatment outcome despite the use of drug therapy and other various types of nerve block, we achieved successful reduction of pain following blockage of the ganglion impar with the use of BoNT-A. Herein we report our case with a review of the literature.
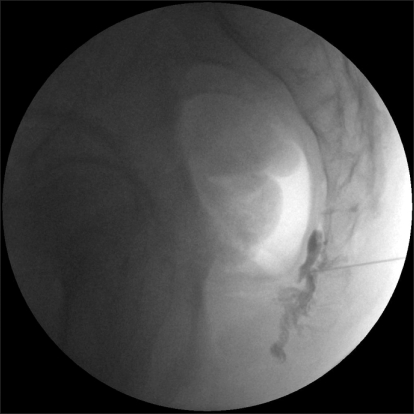
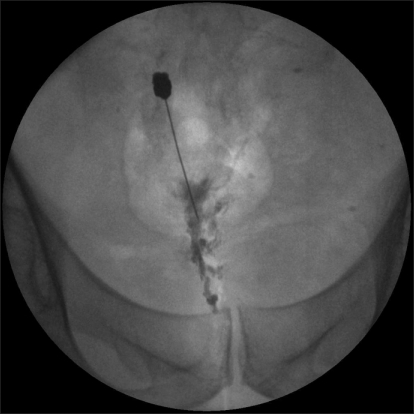
A 40-year-old man sought evaluation of a 4-year-history of perianal and perineal pain, including the testes and penis. In this patient, there was no notable history of trauma or disease. On MRI and CT scans, which included the abdomen and pelvis, there were normal findings. At the time of admission, the patient had a visual analog scale (VAS) of 8/10. The patient had the persistent presence of pain, which was characterized as bursting and explosive. Due to the presence of pain, the patient could not assume a sitting position for more than 5 minutes. The patient also stated that he could not perform work or household chores. Four years before the initial onset of pain, the patient had been working as aresearch staff in the US. At the time, the patient sought evaluation in a urology department under the assumption that the condition originated from the prostate gland. Following evaluation, the patient was considered normal without a discernible etiology for the pain. The patient was therefore transferred to a pain clinic, where the patient was given oral medications (gabapetin 1,800 mg/day and methadone) in an outpatient setting. The patient also received superior hypogastric, caudal, and T12-L1 epidural blocks. However, these procedures had no effect in reducing the pain. In early February 2007, the patient sought evaluation in an outpatient clinic in the US due to aggravation of the pain in a lying position, and underwent blockage of the ganglion impar; the VAS decreased from 8/10 to 4/10. Because the effect was sustained for approximately 1 day, the patient could not ambulate for 2 months. During this period, the patient remained in a lying position. Thereafter, the patient underwent blockage of the ganglion impar on two occasions. Following this, the pain decreased to a VAS of 4/10. When the pain was severe, however, it was a VAS of 8/10. Every 3 months, the patient underwent blockage of the ganglion impar 4 times with the use of steroids 40 mg each, 4 times. In September 2008, the patient returned to Korea. At the time of his initial outpatient visit, the patient was recommended to undergo the blockage of the ganglion impar by radiofrequency ablation. A diagnostic blockage was first performed using 0.5% bupivacaine 2 ml because the patient was concerned about the destruction of the nerve ganglion. The patient was monitored clinically, and 3 months later, the VAS of 8/10 had decreased to 5/10. Approximately 1 week later, however, the pain recurred with a VAS of 7-8/10. The severity of pain was not significantly different from the pain which existed prior to treatment. The patient was therefore motivated to have blockage of the ganglion impar using BoNT-A. For blockage of the ganglion impar, the patient was placed in a prone position. A subcutaneous infiltration was performed in the superior area of the anococcygeal ligament. This area was chosen as a puncture point. Using a C-shaped image intensifier, a 22 G, 10 cm block needle in which the terminal part was bent at an angle of 30 degrees was advanced to a distance of 6 cm. Thus, attempts were made at the sacrococcygeal junction to reach the anterior surface. Following infusion of 2 ml of contrast media, the lateral and anterior-posterior views were evaluated (Fig. 1, 2). Based on the spread pattern of the contrast media, the infusion was performed with a concomitant use of 0.5% bupivacaine 1 ml and BoNT-A 80 U. The VAS of 7-8/10 decreased to 3/10, but the VAS increased to 5/10 two months following the blockage. Accordingly, with the use of BoNT-A, 2 months following the blockage, the ganglion impar was blocked using 0.5% bupivacaine 2 ml and BoNT-A 100 U. When the patient underwent blockage using BoNT-A again, the VAS decreased to 2/10 and this was maintained for 6 months. The patient perceived the presence of perineal pain (a VAS of 5/10) during the sexual intercourse, which led to the third session of blockage of the ganglion impar at the same dose, which diminished the VAS to 2/10. At present, 3 months following the last treatment, other than perineal pain (a VAS of 4/10), the patient has had no problems in performing activities of daily living and work, including sitting and walking. The patient has maintained a VAS of 2/10. To evaluate the degree of disability due to chronic pain during activities of daily living, a pain disability index (PDI) [9] was administered. The PDI has seven categories, such as activities associated with family/home responsibilities, recreation, social activities, occupation, sexual behaviors, self-care, and life-support activities, and scores are given with a scale ranging from 0 (no disability) to 10 (total disability). According to the PDI, scores were 10, 9, 10, 10, 10, 7, and 1, respectively, prior to treatment. Following the third session of blockage of the ganglion impar using BoNT-A, the PDI scores were greatly improved to 2, 2, 2, 2, 4, 3, and 0, respectively. These results indicate that the patient's condition had clearly improved. Currently, the patient has had a satisfactory outcome and is receiving follow-up observation.
Go to : 
The etiology for CPP may include benign causes, such as chronic prostatitis and chronic proctitis, as well as malignant causes, such as carcinoma of the pelvic organs. Infrequently, the cause of pain may be idiopathic. Chronic perineal pain is difficult to treat. The ganglion impar is a solitary retroperitoneal structure at the level of the sacrococcygeal junction and it marks the termination of the paravertebral sympathetic chain [1,4]. The ganglion impar receives the nerve fibers from the sympathetic and parasympathetic nervous system, which are present in the lumbar and sacral regions, and it is responsible for the distribution of sympathetic nerve fibers in the organs around the pelvis or reproductive organs [1,4]. The sympathetic nervous system is associated with myriads of pain syndromes. A blockage of the sympathetic nerve fibers has therefore been used to alleviate pain [1]. A ganglion impar block can be used to treat acute or chronic perineal pain [1,4]. In addition, blockage of the ganglion impar has been used extensively to treat anal or perianal sweating, rectal tenesmoid pain, and coccygodynia [1-3]. There exist various methods to block the ganglion impar, such as local anesthetics, concomitant use of local anesthetics and steroids, alcohol or phenol, and neurolysis by radiofrequency ablation [4].
We performed blockage of the ganglion impar using BoNT-A. Botulinum toxin is a potent neurotoxin which is extracted from the exotoxin of Clostridium botulinum, which proliferates in contaminated foods and triggers the occurrence of food poisoning. Immunologically, there are seven types of antigens. Of these, types A and B have been used to treat human diseases [6]. BoNT-A binds to the presynaptic nerve endings and blocks the secretion of acetylcholine, thus causing a flaccid muscle paralysis. This chemodenervation transiently occurs, and while minimizing the systemic side effects, it persistently reduces or abolishes the activities of muscles, sweat glands, or muscles of contraction for several months [6]. Accordingly, the use of BoNT-A is effective in treating diseases associated with an excessive contraction of the muscles, including strabismus, blepharospasm, unilateral blepharospasm, and abnormal muscle tension in the neck [5]. Attempts have also been made to treat various types of headaches, lower lumbar pain, myofascial pain syndrome, and CRPS with BoNT-A [6,10-12]. Recent studies have suggested that BoNT-A has an effect on the secretion of neurotransmitters which are involved in the recognition of pain secondary to the analgesic effect due to muscle relaxation [6-8].
Analgesic mechanisms of BoNT-A can mainly be divided into peripheral, spinal, and cerebrocortical mechanisms. According to Cui et al. [8], the peripheral mechanisms are of interest based on the finding that the subcutaneous solar administration of BoNT-A had a significant analgesic effect in an experimental model of pain induced using formalin in rats. In cases of neurogenic inflammation, with the initiation of secretions of substances, such as substance P, CGRP, and glutamate, local vasodilation, plasma leakage, and the destruction of mast cells occur. Owing to these inflammatory events, bradykinin, ATP, histamine, and serotonin, which are known to provoke hypersensitivity of peripheral nociceptors, accumulate. It has been reported that BoNT-A blocks the early stage of these chain reactions of neurogenic inflammation and thereby reduces pain [8]. Park et al. [13] clarified that BoNT-A effectively reduced mechanical, cold allodynia in an animal experimental model of neuropathy. Subsequently, according to Ranoux et al. [14], an intradermal injection of BoNT-A had a direct analgesic effect in patients with local chronic neuropathy associated with allodynia. These reports provide a theoretical basis for the availability of BoNT-A for the treatment of neuropathies.
The activity of central pain receptors immediately after pain stimulation can be measured based on the expression of c-fos, genes associated with the early expression of pain. BoNT-A blocks the secretion of neurotransmitters which are involved in the pathophysiology of neurogenic inflammation, such as glutamate, secreted from the primary afferent pain receptor fibers. BoNT-A therefore reduces the extensive activity of dorsal horn neurons of the spinal cord, which has been confirmed based on the decreased expression of c-fos [6]. BoNT-A is involved in the blockage of secretion of the above-mentioned inflammatory mediators and suppresses the expression of c-fos occurring immediately after pain sensation. It can therefore be concluded that BoNT-A suppresses peripheral sensitization, and this leads to central desensitization [7].
As described herein, in addition to the peripheral and spinal cord effects of BoNT-A, the effects on the central nervous system have also been examined in many studies. With respect to the finding that BoNT-A causes alterations in the sensory pattern via the neural axis, including the cerebral cortex, it has been reported that major mechanisms altering the overall recognition of pain by BoNT-A originate from the neuroplastic reorganization of excitatory and inhibitory balances [15]. Through experimental results that BoNT-A has an anti-nociceptive effect via axonal transport to the central nervous system following peripheral injection [16], it has been demonstrated that the central nervous system is involved in the mechanisms by which BoNT-A reduces pain.
Alcohol and phenol, which have been commonly used for neurolysis, cannot accurately predict drug spread, and therefore these agents cannot block nerves selectively. Alcohol and phenol cause irreversible destruction. Furthermore, alcohol and phenol have been reported to induce novel pain [17]. In cases of pain in which radiofrequency ablation is used, there exist specialized equipment, such as an electrostimulating device and a minute controller. Accordingly, in these cases the selective destruction of nerve fibers is possible. There is a lower possibility that complications might occur in cases in which neurolysis is performed. The size and location of lesions can be controlled. Of the methods for blocking ganglion impar, radiofrequency ablation is excellent [4]. In cases in which ganglion impar is blocked, however, an approach is commonly made via the anococcygeal ligament. Because a cannula has a linear form, an accurate approach cannot be made to the ganglion impar. The patient presented herein was apprehensive about neurolysis using radiofrequency ablation . Accordingly, as an alternative method to radiofrequency ablation, blockage of the ganglion impar was performed using BoNT-A. Carroll et al. [11] performed blockage of lumbar sympathetic ganglia in patients with CRPS, and compared treatment outcomes between the group in which 0.5% bupivacaine 10 ml was used alone and a group in which concomitant use of 75 U BoNT-A and 0.5% bupivacaine was attempted. As a result, the decrease in VAS was markedly greater and the analgesic period was prolonged in the group in which bupivacaine 10 ml and 75 U BoNT-A was concomitantly used for blockage of the ganglion impar. This is an example demonstrating the sympathetic nerve block effect of BoNT-A based on clinical studies rather than animal experiments, which provides a basis for the sustained effect and the decrease in VAS as compared with cases in which the patient underwent blockage of the ganglion impar using conventional types of local anesthetics. Accordingly, as shown in animal experiments in which formalin was used [8], the mechanism of BoNT-A is not referred to as direct destruction of nerve fibers, rather based on blockage of pain-controlling neurotransmitters. A reversible, transient analgesic effect is considered one of the advantages in the current case, and. in agreement with an experimental animal reports by Kim et al. [18] in which BoNT-A caused no marked histopathologic changes as compared with cases in which blockage was done using alcohol or phenol, and the effects were persistently present for > 1 month.
In the current case, the use of BoNT-A for blockage of the ganglion impar was safer than neurolysis based on the previous types of chemical or high-frequency ablation. The effects were superior to cases in which local anesthetics were solely used, and the analgesic period was prolonged. Based on these advantages, BoNT-A is proposed as a new method for blocking the sympathetic nervous system. Aside from these advantages, there are also disadvantageous in that BoNT-A can pose a safety issue. BoNT-A is rather expensive and requires serial use. In association with this, in adults weighing 70 kg in which the LD50 of BoNT-A amounts to 3,000 U, the common clinical dose is at most 25-100 U and it can therefore be considered very safe. BoNT-A is used at 3-6 month intervals, and the proportion of antibody formation has been reported to be < 4% [5,19]. The problem that the drug effect is lost due to the formation of antibodies following repeated use can also be resolved [5,19]. It it has been reported that the use of BoNT-A reduces the amount of analgesic drugs, the need for treatment, and the length of hospital stay associated with the side effects of drugs [20]. Together, these features may overcome the cost associated with BoNT-A. Because the number of cases in which BoNT-A has been used for the blockage of ganglion impar is limited, further clinical studies and application are needed.
Go to : 
References
1. Toshniwal GR, Dureja GP, Prashanth SM. Transsacrococcygeal approach to ganglion impar block for management of chronic perineal pain: a prospective observational study. Pain Physician. 2007; 10:661–666. PMID: 17876362.
2. Lee HK, Yang SK, Lee HJ, Lee SY, Kim SM, Kim BS, et al. The effect of ganglion impar block for excessive perianal sweating. Korean J Pain. 1995; 8:363–366.
3. Kim SK, Ahn CS, Cho YR, Lim SY, Shin KM, Hong SY, et al. Ganglion impar block in the management of rectal tenesmoid pain. Korean J Pain. 1996; 9:226–228.
4. Reig E, Abejón D, del Pozo C, Insausti J, Contreras R. Thermocoagulation of the ganglion impar or ganglion of Walther: description of a modified approach. Preliminary results in chronic, nononcological pain. Pain Pract. 2005; 5:103–110. PMID: 17177756.

5. Mahant N, Clouston PD, Lorentz IT. The current use of botulinum toxin. J Clin Neurosci. 2000; 7:389–394. PMID: 10942658.

6. Welch MJ, Purkiss JR, Foster KA. Sensitivity of embryonic rat dorsal root ganglia neurons to Clostridium botulinum neurotoxins. Toxicon. 2000; 38:245–258. PMID: 10665805.

7. Aoki KR. Evidence for antinociceptive activity of botulinum toxin type A in pain management. Headache. 2003; 43(Suppl 1):S9–S15. PMID: 12887389.

8. Cui M, Khanijou S, Rubino J, Aoki KR. Subcutaneous administration of botulinum toxin A reduces formalin-induced pain. Pain. 2004; 107:125–133. PMID: 14715398.

9. Tait RC, Chibnall JT, Krause S. The pain disability index: psychometric properties. Pain. 1990; 40:171–182. PMID: 2308763.

10. Ailani J, Young WB. The role of nerve blocks and botulinum toxin injections in the management of cluster headaches. Curr Pain Headache Rep. 2009; 13:164–167. PMID: 19272284.

11. Carroll I, Clark JD, Mackey S. Sympathetic block with botulinum toxin to treat complex regional pain syndrome. Ann Neurol. 2009; 65:348–351. PMID: 19334078.

12. Jin L, Kollewe K, Krampfl K, Dengler R, Mohammadi B. Treatment of phantom limb pain with botulinum toxin type A. Pain Med. 2009; 10:300–303. PMID: 19207237.
13. Park HJ, Lee Y, Lee J, Park C, Moon DE. The effects of botulinum toxin A on mechanical and cold allodynia in a rat model of neuropathic pain. Can J Anaesth. 2006; 53:470–477. PMID: 16636031.

14. Ranoux D, Attal N, Morain F, Bouhassira D. Botulinum toxin type A induces direct analgesic effects in chronic neuropathic pain. Ann Neurol. 2008; 64:274–283. PMID: 18546285.

15. Arezzo JC. Possible mechanisms for the effects of botulinum toxin on pain. Clin J Pain. 2002; 18(6 Suppl):S125–S132. PMID: 12569959.

16. Bach-Rojecky L, Lacković Z. Central origin of the antinociceptive action of botulinum toxin type A. Pharmacol Biochem Behav. 2009; 94:234–238. PMID: 19732788.

17. Mailis A, Furlan A. Sympathectomy for neuropathic pain. Cochrane Database Syst Rev. 2003; 2:CD002918. PMID: 12804444.
18. Kim HJ, Seo K, Yum KW, Oh YS, Yoon TG, Yoon SM. Effects of botulinum toxin type A on the superior cervical ganglia in rabbits. Auton Neurosci. 2002; 102:8–12. PMID: 12492130.

20. Radensky PW, Archer JW, Dournaux SF, O'Brien CF. The estimated cost of managing focal spasticity: a physician practice patterns survey. Neurorehabil Neural Repair. 2001; 15:57–68. PMID: 11527280.

Go to : 




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download