Abstract
Background
Chronic low back pain can be a manifestation of lumbar degenerative disease, herniation of intervertebral discs, arthritis, or lumbar stenosis. When nerve roots are compromised, low back pain, with or without lower extremity involvement, may occur. Local inflammatory processes play an important role in patients with acute lumbosciatic pain. The purpose of this study was to assess the value of erythrocyte sedimentation rate (ESR) and high sensitivity C-reactive protein (hsCRP) measurements in patients with chronic low back pain or radiculopathy.
Go to : 
Chronic low back pain can be a manifestation of failed back surgery, lumbar degenerative disc disease, herniation of intervertebral discs, arthritis, or spinal stenosis. When nerve roots exiting the spinal column are compromised, low back pain with radiation into the lower extremities may occur, also known as lumbar radiculopathy [1]. Inflammation plays a major role in radiculopathy [2,3]. Once inflammation has been established, the nerves become exquisitely sensitive to pressure, producing prolonged and pain-generating discharge with either gentle manipulation or pressure [4]. Such local inflammation is caused by inflammatory mediators such as interleukin-6 produced by macrophages and monocytes at the inflammatory site. High concentrations of inflammatory mediators may cause a systemic inflammatory reaction [2,5,6]. Therefore, it is believed that the levels of high sensitivity C-reactive protein (hsCRP) are increased by low back pain.
Laboratory measurement of acute phase protein is a valuable indicator of the presence and extent of inflammation and its response to treatment. Among acute phase proteins, C-reactive protein is the first to appear. It is a sensitive systemic marker of inflammation and tissue damage [5] and it also appears 6-8 hours after infection. A more non-specific measure of inflammation is the erythrocyte sedimentation rate (ESR), a common hematology test that tracks the rate of red blood cell precipitation for 1 hour.
There are several reports in the literature regarding hsCRP levels in patients with lumbosciatic pain. In general, hsCRP appears to be higher in patients with acute lumbosciatic pain [7,8]. However, little is known regarding either ESR levels or hsCRP levels in patients with chronic low back pain. There are also no clinical studies that have examined any correlation between ESR and age in chronic low back pain patients. The aim of the present study was to assess the value of ESR and/or hsCRP measurements for determination of inflammation in patients with chronic low back pain or radicular pain.
Go to : 
The study subjects consisted of 273 patients who were scheduled for nerve block due to their low back pain and/or radiating pain. Their ages ranged from 30 to 77 years. The Institutional Review Board approved the review of this study. Data were collected prospectively on the 273 patients during a 6 month period beginning on September 1, 2008. All patients had magnetic resonance imaging or computerized tomography and a conventional neurological examination was carried out. Inclusion criteria for this study were a history of over 2 month's low back pain with no motor deficit. Exclusion criteria included: 1) acute symptoms with an immediate onset of less than 8 weeks, 2) compression fracture, 3) malignancy, 4) inflammatory diseases including rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, Reiter disease etc, 5) use of steroids or other medications, and 6) body temperature > 36.5℃.
A blood sample was taken from each patient, 2 hours before the interventional procedure, for measurement of hsCRP and ESR values. The hsCRP was measured by a Hitachi 7080 (TIA method) and ESR by the Westergren method. The following parameters were recorded: patient age, sex, diagnosis, and serum levels of ESR and hsCRP. The correlation between age and ESR or hsCRP was analyzed by Pearson's correlation coefficient. A Mann Whitney U test was used for statistical analysis. P values less than 0.05 were considered statistically significant.
Go to : 
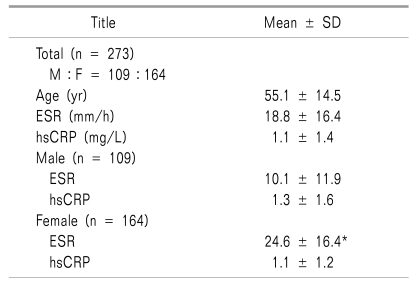
The clinical data and results of laboratory tests are shown in Table 1 and 2. The mean ESR was 18.8 mm/hr and mean hsCRP was 1.1 mg/L. An age-related elevation of ESR was observed (P = 0.000) (Fig. 1) and mean ESR levels were significantly higher in female than in male subjects (24.6 vs 10.1 mm/h; P = 0.000).
The hsCRP levels showed no correlation with age (P = 0.113) and there was no statistical difference between the two sexes (P = 0.189)(Table 2).
Go to : 
It appears that most individuals have little inflammatory response as a result of chronic low back pain. Our results differ from those of Le Gars et al. [9], who studied 35 patients with sciatica for ultrasensitive CRP levels.
In general, the normal range of CRP for all ages is 0-10 mg/L, but these levels may be affected by several factors. Some studies have reported that hsCRP was increased in the acute phase of inflammation or during severe pain [5,6,9,10]. Sugimori et al. [11] demonstrated that the mean serum hsCRP concentration in patients with herniation of lumbar intervertebral discs was higher than that found in a group of normal volunteers. They suggested that the significantly higher concentration of serum hsCRP might indicate a systemic inflammatory response to impingement of the nerve root caused by disc herniation. However, although there were significant differences between the pain group and control group, the measured hsCRP values, with a mean of 0.056 mg/dl for the pain group, were within the normal reference limit in both groups. Ackerman and Zhang [12] reported that lumbar disc protrusion, prolapse, extrusion, and sequestered groups showed elevations in hsCRP levels of 0%, 20%, 80%, and, 73% respectively. Numerous studies have shown that inflammatory responses occur in the area surrounding a disc herniation. The response to such a reaction may be a stimulation of the liver, resulting in an increase in the concentration of CRP.
In our study, hsCRP values were within normal reference values. Gebhardt et al. [7] evaluated the course of hsCRP levels over a 6 month period and reported that the mean hsCRP remained at constant levels in patient with chronic low back pain. They indicated that significant systemic inflammatory reactions did not arise in patients with chronic low back pain.
Pain severity has been reported to affect hsCRP levels. Stümer et al. [8] observed a strong association between pain severity and hsCRP levels in patients with acute sciatic pain but not in those with chronic low back pain. The higher hsCRP levels were associated with higher pain levels. However, in chronic low back pain patients, pain severity might be more strongly related to overweight and psychological factors rather than to pathophysiological changes resulting from low grade systemic inflammation, which would be the cause in patients with acute sciatic pain [8].
Our study has shown a mean ESR of 18.8 mm/hr. The normal range for ESR is more debatable. In healthy persons less than 40 years old, ESR is 10 mm/h and for those over 60 years old, it is 18 mm/h on average. The normal range can be as high as 25 mm/h [13]. ESR is also affected by the pathogenesis of inflammatory processes. The ESR will increase as a result of any cause or focus of inflammation. When an inflammatory process is present, a high proportion of fibrinogen in the blood causes red blood cells to stick to each other, which raises the ESR. In this study, ESR levels were not significantly raised. We postulate that the observation of normal ESR levels indicated that inflammatory processes were not occurring in low back pain patients.
Some studies have demonstrated an age-related elevation in ESR in healthy populations [13,14]. Griffiths et al. [14], in their study on 200 patients in the age range 60-80 years, reported that ESR increased with increasing age in all male and female patients, and that ESR exceeded 19 mm/h in men and 22 mm/h in women aged 60-89 yrs. In our study, when ESR was compared based on 50 years of age, the mean ESR for patients over 50 years old showed higher values compared to that for patients less than 50 years old and there was an age-related elevation of ESR. These results were in close agreement with those previously reported [13,14]. The results obtained in our study indicate that ESR is influenced by age in low back pain patients, and that evaluation of these measurements needs to consider the patient's age.
In our study, ESR values were higher for women than men. There have not yet been any studies on the association between ESR and gender. However, according to the study by Griffiths et al. [14], in elderly patients, ESR was 19 mm/hr in men and 22 mm/hr in women. This aspect also requires further study.
Our study had some limitations. We did not carry out the study according to diagnosis or pain severity. Secondly, our study did not explain why ESR was higher in women. Despite the limitations of our study, this study is the first to evaluate ESR levels in patients with chronic low back pain.
In conclusion, in low back pain patients, CRP or ESR levels were expected to increase much more than we observed due to inflammatory reactions. However, neither ESR nor hsCRP values were elevated above the normal reference values. Therefore, in cases where ESR or hsCRP values are significantly raised above normal levels, further investigation should be carried out to ascertain the possibility of underlying systemic infection/inflammation.
Go to : 
References
1. Howe JF, Loeser JD, Calvin WH. Mechanosensitivity of dorsal root ganglia and chronically injured axons: a physiological basis for the radicular pain of nerve root compression. Pain. 1977; 3:25–41. PMID: 195255.

2. Saal JS, Franson RC, Dobrow R, Saal JA, White AH, Goldthwaite N. High levels of inflammatory phospholipase A2 activity in lumbar disc herniations. Spine. 1990; 15:674–678. PMID: 2218714.

3. Grönblad M, Virri J, Tolonen J, Seitsalo S, Kääpä E, Kankare J, et al. A controlled immunohistochemical study of inflammatory cells in disc herniation tissue. Spine. 1994; 19:2744–2751. PMID: 7899973.

4. Refshauge KM, Maher CG. Low back pain investigations and prognosis: a review. Br J Sports Med. 2006; 40:494–498. PMID: 16720885.

5. Kang JD, Georgescu HI, McIntyre-Larkin L, Stefanovic-Racic M, Donaldson WF 3rd, Evans CH. Herniated lumbar intervertebral discs spontaneously produce matrix metalloproteinases, nitric oxide, interleukin-6, and prostaglandin E2. Spine. 1996; 21:271–277. PMID: 8742201.

6. Nygaard OP, Mellgren SI, Osterud B. The inflammatory properties of contained and noncontained lumbar disc herniation. Spine. 1997; 22:2484–2488. PMID: 9383853.

7. Gebhardt K, Brenner H, Stürmer T, Raum E, Richter W, Schiltenwolf M, et al. The course of high-sensitive C-reactive protein in correlation with pain and clinical function in patients with acute lumbosciatic pain and chronic low back pain - a 6 months prospective longitudinal study. Eur J Pain. 2006; 10:711–719. PMID: 16403662.
8. Stürmer T, Raum E, Buchner M, Gebhardt K, Schiltenwolf M, Richter W, et al. Pain and high sensitivity C reactive protein in patients with chronic low back pain and acute sciatic pain. Ann Rheum Dis. 2005; 64:921–925. PMID: 15897311.
9. Le Gars L, Borderie D, Kaplan G, Berenbaum F. Systemic inflammatory response with plasma C-reactive protein elevation in disk-related lumbosciatic syndrome. Joint Bone Spine. 2000; 67:452–455. PMID: 11143913.
10. Pepys MB, Baltz ML. Acute phase proteins with special reference to C-reactive protein and related proteins (pentaxins) and serum amyloid a protein. Adv Immunol. 1983; 34:141–212. PMID: 6356809.

11. Sugimori K, Kawaguchi Y, Morita M, Kitajima I, Kimura T. High-sensitivity analysis of serum C-reactive protein in young patients with lumbar disc herniation. J Bone Joint Surg Br. 2003; 85:1151–1154. PMID: 14653598.

12. Ackerman WE 3rd, Zhang JM. Serum hs-CRP as a useful marker for predicting the efficacy of lumbar epidural steroid injections on pain relief in patients with lumbar disc herniations. J Ky Med Assoc. 2006; 104:295–299. PMID: 16886882.
13. Osei-Bimpong A, Meek JH, Lewis SM. ESR or CRP? A comparison of their clinical utility. Hematology. 2007; 12:353–357. PMID: 17654065.

14. Griffiths RA, Good WR, Watson NP, O'Donnell HF, Fell PJ, Shakespeare JM. Normal erythrocyte sedimentation rate in the elderly. Br Med J. 1984; 289:724–725. PMID: 6434056.

Go to : 




 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download