INTRODUCTION
Postoperative pain hinders the recovery from an operation and causes various stress reactions [
1]: it can negatively affect the cardiovascular system and the respiratory system by stimulating the sympathetic nervous system and causing hypoxia in body organs, as well as pneumonia and other pulmonary complications resulting from respiratory failure. The reactions in the cardiovascular system include an increase of heart rate, stroke volume, myocardial oxygen consumption, and peripheral vascular resistance [
2]. In addition, it can bring about complications such as phlebothrombosis, pulmonary embolism, respiratory failure, and myocardial infarction [
3].
In addition to opioids, non-steroidal anti-inflammatory drugs (NSAIDs) are frequently used in postoperative pain control [
4,
5]. NSAIDs such as ketorola are able to effectively control mild and moderate postoperative pain [
6,
7], but long-term administration of them may cause hemorrhaging at the operated part, gastrointestinal bleeding, and renal insufficiency [
8]. Recently, paracetamol, an acetaminophen venous drug, was developed (Perfalgan®, BMS Pharmaceutical Korea), and it has a similar analgesic effect as that of NSAIDs, but with less side effects [
9,
10]. Paracetamol is a useful pharmaceutical that can be applied for the treatment when immediate venous injection is required due to the pain or high fever, or when the side effects of NSAIDs concerned, and when venous injection is the only possible treatment for mild and moderate pain, especially for postoperative pain and fever. In addition, since paracetamol has an opioid-sparing effect when it is used together with a small quantity of opioid, it can show good analgesic effect while minimizing the side effects of opioid, such as respiratory depression, bradycardia, and hypoxia.
Therefore, to study the effects of paracetamol, which might be substituted for NSAIDs or opiods but with less side effects, we compared the postoperative analgesic effects and the side effects by medicating paracetamol 1 g or ketorolac 30 mg, each of the drugs independently, or by medicating paracetamol 700 mg and morphine 3 mg together for thyroidectomy patients.
Go to :

MATERIALS AND METHODS
Conforming to the ethics regulations of the hospital ethics committee, this study was conducted after giving an explanation to the patients and obtaining their consent. The subjects of this study were the 80 female patients, aged between 20 and 60, who were scheduled to undertake regular surgery under general anesthesia, had already undergone a total thyroidectomy without endoscopy and robot-aided procedure, and were at the Class 1 and 2 of the American Society of Anesthesiologists Physical Status classification. Those who had a history of drug abuse, hypersensitivity to drugs, diseases in the cardiovascular system, respiratory system, kidneys or liver, those who had been medicated with drugs that could affect the analgesic effect before the operation, and those who were regarded inappropriate for the clinical test for other reasons were excluded from the subjects. There was no significant difference among the subjects in the 4 groups of age, height, weight, and the duration of anesthesia (
Table 1).
Table 1
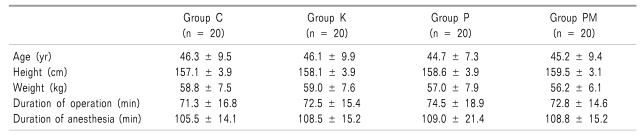

The subject patients were divided into 4 groups, 20 patients in each. An intravenous injection of paracetamol 1 g was performed for 15 minutes, 30 minutes before the end of the operation to P Group (n = 20), ketorolac 30 mg to K Group (n = 20), paracetamol 700 mg and morphine 3 mg to PM Group (n = 20), and normal saline to C Group (n = 20).
All the patients underwent alimentary abstinence for 8 hours before the operation, and midazolam 2 mg and glycopyrrolate 0.2 mg were injected through the muscle 30 minutes before the operation as the preanesthetic medication. The induction was performed by the intravenous injection of propofol 2 mg/kg. After loss of consciousness and sufficient muscle relaxation with rocuronium 0.8 mg/kg, the endotracheal intubation followed. The anesthesia was maintained with 2 L/min of O2 and N2O, as well as sevoflurane 1.5-3.0 vol%. The tidal volume and respiratory rate were regulated to keep the positive end expiratory carbon dioxide pressure at 30-35 mmHg. Opiods that could affect the postoperative analgesic effect were not used. Ramosetron (Nasea®, Astellas Pharma Korea) 0.3 mg was intravenously injected just before finishing the operation to prevent nausea and vomiting. The effect of muscle relaxant was reversed by using pyridostigmine 15 mg, glycopyrrolate 0.2 mg, and atropine 0.5 mg after the end of the operation.
The duration of the anesthesia was recorded as the time from the induction to the removal of the endotracheal tube, and the duration of the operation was recorded as the time from the initiation of the skin incision to the end of the skin suture. The anesthesiologists, who were blinded to the patients' groupings, visited the patients after 30 minutes and 1 hour in the postoperative recovery room, and after 2, 4, and 6 hours at the ward; they surveyed the degree of pain (VAS, visual analogue scale: 10 cm), side effects, such as nausea, vomiting, headache, sedation, dizziness, and respiratory depression, and the patients' overall degree of satisfaction regarding the pain control. The patients' overall degree of satisfaction regarding the pain control was evaluated for a comparison among the groups on a 4-point scale: 1 point for poor, 2 points for fair, 3 points for good, and 4 points for excellent. When the patients wanted treatment because the pain was not regulated and the patient could not endure the pain, an intravenous injection of pethidine hydrochloride 25 mg was performed and a repeated injection was allowed for the same patient who wanted more pain control.
The statistical process was conducted with SPSS version 13.0 (SPSS Inc. Chicago, IL, USA) and the comparison among the groups were analyzed by an ANOVA test and a Pearson chi-square test. All the measurements were expressed as "mean ± standard deviation" and the cases with the P value less than 0.05 were judged to be statistically significant.
Go to :

RESULTS
The VASs of C Group were 6.2 ± 1.2 and 5.9 ± 1.0 at 30 minutes and 1 hour after the operation, respectively. The VASs of K Group, P Group, and PM Group were 4.0 ± 1.2, 4.1 ± 1.1, and 4.1 ± 1.2, respectively, at 30 minutes after the operation, and 3.8 ± 1.2, 3.9 ± 1.3, and 3.9 ± 1.1, respectively, at 1 hour after the operation. The VASs of C Group were significantly higher than those of K Group, P Group, and PM Group (
P < 0.05). There was no significant difference among the groups in the VAS at 2 hours, 4 hours, and 6 hours after the operation. The VAS tended to decrease in all the 4 groups as the time passed after the operation (
Table 2,
Fig. 1).
 | Fig. 1Comparison of postoperative VAS for pain. VAS at 0.5 and 1 hr after the end of surgery were significantly lower in group K, group P, and group PM than in group C. Value are mean ± SD. Group C: normal saline, Group K: ketorolac 30 mg, Group P: paracetamol 1 g, Group PM: paracetamol 700 mg + morphine 3 mg. *P < 0.05 compared with group C. 
|
Table 2
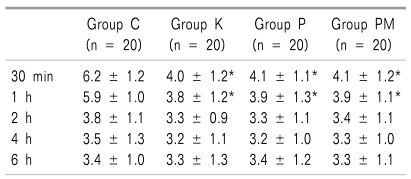

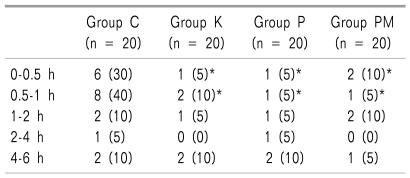
The number of cases where pethidine hydrochloride, the additional analgesic, was used was 6 (30%) and 8 (40%) at 30 minutes and 1 hour after the operation, respectively, in C Group. The number of additional analgesic injection in K Group, P Group, and PM Group was 1 (5%), 1 (5%), and 2 (10%) at 30 minutes, and 2 (10%), 1 (5%), and 1 (5%), respectively, at 2 hours after the operation. Thus, the use of pethidine hydrochloride was significantly (
P < 0.05) more in C Group than K Group, P Group, and PM Group (
Table 3).
Table 3
Incidences of Uses of Rescue Drugs During Each Period

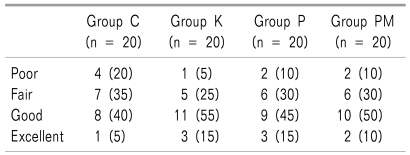
The patients' overall degree of satisfaction in C Group, K Group, P Group, and PM Group was evaluated as "poor (1 point)" by 4 (20%), 1 (5%), 2 (10%), and 2 (10%), respectively, "fair (2 points)" by 7 (35%), 5 (25%), 6 (30%), and 6 (30%), respectively, "good (3 points)" by 8 (40%), 11 (55%), 9 (45%), and 10 (50%), respectively, and "excellent (4 points)" by 1 (5%), 3 (15%), 3 (15%) and 2 (10%), respectively. Although the overall degree of satisfaction was lower in C group than in K Group, P Group, and PM Group, it was not significantly different among the 4 groups (
Table 4).
Table 4
Patient Satisfaction at Six Hours after Surgery Using a Verbal Rating Scale

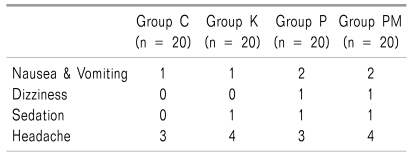
The overall occurrence of nausea and vomiting was not significantly different among the 4 groups. No significant difference was found among the 4 groups in the occurrence of side effects related to the medication, such as dizziness, sedation, respiratory depression, and headache (
Table 5).
Table 5
Incidences of Adverse Events

Go to :

DISCUSSION
Postoperative pain causes not only physical suffering but also mental fear and anxiety, affecting the recovery procedure of the patients by bringing about limited exercise, declined respiratory capability, and pulmonary complications [
11]. Hence, effective control of postoperative pain can provide a lot of advantages, including a reduction of the complications and early discharge from the hospital. Thyroidectomy is now an operation that is frequently performed thanks to the development of diagnosis technology, but it makes the patients uncomfortable, like other operations, due to the pain and the sequela caused by the pain.
The analgesics that are now used for postoperative pain control include opioids and NSAIDs. Ketorola, a NSAID, shows a powerful analgesic, anti-inflammatory, and antipyretic effect by inhibiting cyclooxygenase I and cyclooxygenase II. Since ketorola can be administrated in a parenteral manner, and it does not seriously suppress the respiratory and cardiovascular systems, it has been used for the purpose of postoperative pain control, independently or by mixing it with opioids through the patient-controlled analgesia equipment [
12,
13]. However, there have reports that intermittent intravenous injection or intramuscular injection of ketorolac elongated the bleeding time and reduced the platelet aggregation capability [
14,
15]. Thus, the effect of ketorolac on the intraoperative and postoperative hemorrhage is still a controversy. Splinter et al. [
16] reported that preoperative administration of ketorolac 1 mg/kg to the children undergoing tonsillectomy significantly increased the amount of intraoperative bleeding compared with the group to which codeine was administrated. In contrast, Power et al. [
17] reported that an intramuscular injection of ketorolac 30 mg during the induction of a patient undergoing cholecystectomy increased the duration of bleeding and the platelet aggregation capability, but not the quantity of bleeding. The potential risk of side effects by ketorolac is increased when it is medicated in a high dose, used for 5 days or longer, or when it is used on patients who are vulnerable to drugs, such as elderly patients [
18]. Thus, to prevent side effects, it should be used in an appropriate dose for a short period of time, or after checking whether the patients have a contraindication or not.
On the other hand, though acetaminophen has been universally used for pain treatment by oral administration, it could not be applied to patients who had to undergo postoperative alimentary abstinence. However, the recently-developed paracetamol is an acetaminophen for intravenous injection, and its use for postoperative pain control is gradually increasing. The modus operandi of paracetamol has not yet been clearly revealed, but it has been reported that paracetamol shows a powerful antipyretic and analgesic effect by inhibiting cyclooxygenase I and cyclooxygenase II, like NSAIDs, and inhibiting prostaglandin synthesis; additionally, it has weak anti-inflammatory effect and does not cause platelet function declination or gastric mucosal damage, and in such ways is different from NSAIDs [
19-
21]. Based on the functions of paracetamol that are different from those of NSAIDs, 1 hypothesis assumes that it might act on cyclooxygenase III and other mechanisms. Also, suppression of nitric oxide synthesis and supraspinal serotonin-dependent antinociceptive mechanism are studied at present [
22,
23].
Propacetamol, N-acetyl-paramino-phenol diethyl aminoacetic ester, the prodrug of paracetamol, is produced by the esterification of paracetamol and hydrolyzed by nonspecific plasma esterase. Thus, 2 g of propacetamol is pharmacokinetically equivalent to 1 g of paracetamol. Many studies regarding the effect of propacetamol on postoperative pain control have been reported: Varrassi et al. [
24] reported that 2 g of propacetamol showed a similar analgesic effect to that of 30 mg of ketorolac in a gynecological operation. Zhou et al. [
10] reported that 2 g of propacetamol and 30 mg of ketorolac showed an almost identical effect in acute pain control after a total hip replacement or total knee replacement. Van Aken et al. [
25] claimed that propacetamol 2 g and morphine 10 mg showed a similar analgesic effect after a dental operation, just as paracetamol 1 g, ketorolac 30 mg, and paracetamol 700 mg + morphine 3 mg showed a similar analgesic effect in this study.
According to Moller et al. [
26], the onset time of propacetamol for intravenous injection was 3 to 5 minutes, and the maximum analgesic effect was found 15 to 45 minutes after the injection. They also reported that, since the drug action duration was 4 to 6 hours, the analgesic effect could be maintained up to 6 hours after the operation, showing a similar pharmacodynamic property with that of ketorolac. In this study, considering time of the maximum analgesic effect of the drug, the analgesics were injected 30 minutes before the end of the operation to maximize the analgesic effect. Moderate analgesic effect was shown similarly for 6 hours after the operation in the K Group, P Group, and PM Group.
The postoperative pain evaluation, VAS, in this study was higher in C Group than in K Group, P Group, and PM Group at 30 minutes and 1 hour after the operation, but there was no significant difference in VAS at 2 hours, 4 hours, and 6 hours. This is assumed to be the results of an additional administration of the analgesic carried out to significantly more patients, 6 (30%) and 8 (40%) in C Group, than those in K Group, P Group, and PM Group, within 30 minutes and 1 hour after the operation, respectively. Thus, no significant difference was found among the groups at the times after 1 hour.
For an evaluation of the patients' overall degree of satisfaction regarding the pain control, the 4-point scale created by Zhou et al. [
10] was employed. A measure of dissatisfaction was found in C Group at the times 30 minutes and 1 hour after the operation, as the significantly higher VAS scores showed, but the patients' overall degree of satisfaction regarding the pain control was not significantly different, even in C Group, because the pain was considerably controlled in the times of 2 hours, 4 hours, and 6 hours after the operation by an additional administration of the analgesic to the most of the patients within 1 hour after the operation.
Since paracetamol, different from NSAIDs, does not cause side effects such as platelet function declination, gastrointestinal bleeding, and the reduction of the renal function, it can be used on children for postoperative pain control. It is also a good substituent of NSAIDs for patients who are hypersensitive to NSAIDs, those who have declined renal function, and those have potential risk of a gastric ulcer or gastrointestinal bleeding. When it is used together with an opioid, it can reduce the dose of the opioid, as well as reduce the side effects of opioids, including respiratory depression, sedation, and postoperative nausea and vomiting.
In addition, Bergenstock et al. [
27] reported that, in contrast to the selective cyclooxygenase-II inhibitors, such as celecoxib, that delayed the fracture treatment, acetaminophen showed an analgesic effect without delaying the fracture treatment in the mouse experiment, implying that it can be also used for postoperative pain control of orthopaedic fracture patients.
In this study, the occurrences of postoperative nausea and vomiting were 1 (5%), 1 (5%), 2 (10%), and 2 (10%) in C Group, K Group, P Group and PM Group, respectively. The occurrence was very rare, considering that thyroidectomy normally shows a relatively high rate of postoperative nausea and vomiting occurrence as compared to other operations. The low occurrence of postoperative nausea and vomiting is probably because, just before the end of the operation, an intravenous injection of ramosetron 0.3 mg was administered; this drug has a long action time and is a 5-HT
3 receptor antagonist that has a high affinity to the 5-HT
3 receptor. Fujii and Tanaka [
28] reported that ramosetron was effective in preventing postoperative nausea and vomiting for the patients undergoing thyroidectomy. Other side effects, such as dizziness and sedation, were nearly nonexistent and not even 1 case of respiratory depression was found in C Group, K Group, P Group, and PM Group.
The occurrence of headache as a postoperative side effect was 3 (15%), 4 (20%), 3 (15%), and 4 (20%) in C Group, K Group, P Group, and PM Group, respectively, and it was higher than other side effects. It is thought that such a frequent occurrence might be related with the thyroidectomy operation position, extending the neck excessively to expose the operative part, and further study is required with respect to this.
In conclusion, since a better analgesic effect was found in the 3 groups where the patients were injected with ketorolac 30 mg, paracetamol 1 g, and paracetamol 700 mg and morphine 3 mg, respectively, at 30 minutes before the end of the thyroidectomy, in comparison with the control group, it is thought that paracetamol can be used for postoperative acute pain control, especially for the patients who cannot be medicated with NSAIDs, replacing ketorolac without any specific side effect.
Go to :






 PDF
PDF Citation
Citation Print
Print







 XML Download
XML Download