This article has been
cited by other articles in ScienceCentral.
Abstract
Background
This study aimed to assess the potential efficacy of purified porcine atelocollagen (PAC) for the management of refractory chronic pain due to suspected connective tissue damage.
Methods
Patients treated with PAC were retrospectively evaluated. Patients with chronic refractory pain, suspected to have originated from musculoskeletal damage or defects with the evidence of imaging studies were included. Pain intensity, using the 11-point numerical rating scale (NRS), was assessed before the procedure, and 1 month after the last procedure.
Results
Eighty-eight patients were finally included for investigation. The mean NRS score was decreased from 5.8 to 4.1 after 1 month of PAC injection (P < 0.001). No independent factor was reported to be directly related to the decrease in NRS score by more than half.
Conclusions
Application of PAC may have potential as a treatment option for refractory chronic musculoskeletal pain. PAC might promote tissue recovery, act as a scaffold for repair, or directly reduce inflammation.
Go to :

Keywords: Chronic Pain, Collagen, Connective Tissue, Injections, Musculoskeletal Pain, Pain Management, Procollagen, Regenerative Medicine
INTRODUCTION
Musculoskeletal disorders are the most common sources of chronic pain worldwide [
1,
2]. These diseases result in functional disability and a heavy economic burden [
3,
4]. Due to its increasing prevalence and economic burden, the need for effective management of chronic musculoskeletal pain has grown.
Although arthritis and joint injuries of the tendons or ligaments can heal completely, chronic cumulative disorders can result in incomplete healing leading to chronic pain [
5]. Several treatments have been introduced to restore the injury site by triggering a local inflammatory response in order to promote a normal healing cascade. However, these treatments can result in problems that take months or even years for complete repair, prolonging the pain associated with local inflammatory reactions [
6,
7]. To compensate for this drawback, there has been increasing interest in application of collagen—which is the final product of the healing cascade—directly onto the injury site [
8,
9].
Type I collagen is found in skin, tendon, vasculature, organs, and bone. Purified porcine atelocollagen (PAC) (Coltrix Tendoregen
TM; Ubiosis, Seongnam, Korea) is a soluble type I collagen with low immunogenicity, due to its lack of an antigenic telopeptide [
10], and good biocompatibility [
11]. PAC injection has been tried in various areas [
12-
14], but there are only a few studies that have investigated its efficacy in the treatment of chronic pain. As such, the aim of this study was to assess the potential of PAC injection for management of refractory chronic pain in patients with suspected connective tissue damage.
Go to :

MATERIALS AND METHODS
This retrospective medical record review study was approved by the Institutional Review Board of Korea University Anam Hospital (approval no. 2020AN0045) and have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. Patient identification data were encoded and scrambled using a restricted computer to protect the privacy of all subjects.
The data were collected on patients (ages 30-90 yr) who visited the pain center for management of chronic pain lasting more than 3 months. Demographic data was collected at the first visit. At every visit, the pain intensity was evaluated using an 11-point numerical rating scale (NRS) (0–10; 0 = no pain/nausea, 10 = worst imaginable pain/nausea). Follow-ups occurred every 1-2 weeks, and any unexpected adverse events were recorded.
Patients who experienced chronic refractory pain, suspected to originate from damage or defects of ligaments, tendons, muscle, or biomembranes, were evaluated for eligibility. The inclusion criteria were confirmation of damage or defects at the site of pain, compared to the painless contralateral side using imaging studies, such as x-ray, computed tomography, magnetic resonance image, or ultrasound. Patients with damage or defects to the bone, muscle, joint, ligament, or tendon were included according to the following criteria. For skeletal defects, patients with chronic pain which might be associated with malunion or deformation were included. Pain originating from the muscle was diagnosed with a significant defect or rupture at the muscle fibers. Ligament- or tendon-associated pain was diagnosed with confirmation of damage to the integrity of the structures connecting the bone and muscle. In addition, patients with significant defects in the joint capsule, including degeneration or injury, were also included. Chronic refractory pain was defined as chronic pain that failed to decrease by more than a half despite one or more pain intervention procedures with conservative management. The exclusion criteria included lack of data for analysis and lack of follow-up before 1 month after treatment.
For the treatment of chronic pain, a mixture was prepared using equal amount of PAC and local anesthetics (total 2 mL) to reduce pain during injection. After sterilization, the mixture was injected into the space between the surrounding tissue and the site of the greatest suspected musculoskeletal damage or defect that may have irritated adjacent structures, causing the chronic pain. The procedures were performed under ultrasound guidance. When the application site was large, the treatment was applied up to 3 times at intervals of 1 to 2 weeks, but only when the first procedure was effective.
Patients’ demographic data and pre-procedural pain profiles—including age, sex, comorbidities (i.e., hypertension or diabetes mellitus), duration of pain, and presence of tenderness at the pain site—were evaluated. Patients’ diagnosis was classified into 4 categories (i.e., skeletal, muscular, ligament and tendon, or joint) and recorded. The site of pain was categorized and recorded by region (i.e., spine, upper limb, lower limb, trunk, and other). Pain intensity, using the 11-point NRS, was assessed before the procedure, and 1 month after the last procedure.
1. Statistical analysis
Statistical analysis was performed using SAS software version 9.4 (SAS Institute, Cary, NC). Results were expressed as mean ± standard deviation, or number of patients. We compared pre- and post-procedural NRS with a paired t-test or Wilcoxon signed-rank test for parametric and non-parametric data. Simple logistic regression analysis was used to identify factors associated with a decrease in pain intensity by more than half. Odds ratios (ORs) and 95% confidence intervals were estimated for each factor. P values < 0.05 were considered statistically significant.
Go to :

RESULTS
A total of 135 patients were assessed for enrollment. Among them, 32 were excluded due to incomplete medical records, and 15 were excluded due to lack of follow-up. Of the initial 135, 88 patients were ultimately included in the study (
Fig. 1). Demographic and pre-procedural data of the 88 analyzed patients are presented in
Table 1. The mean NRS score was 5.8 before the procedure. There were 31, 13, 25, and 19 patients who were diagnosed in the skeletal, muscular, ligament and tendon, and joint category, respectively. The most common injection site was the spine, followed by the lower limb, upper limb, and trunk.
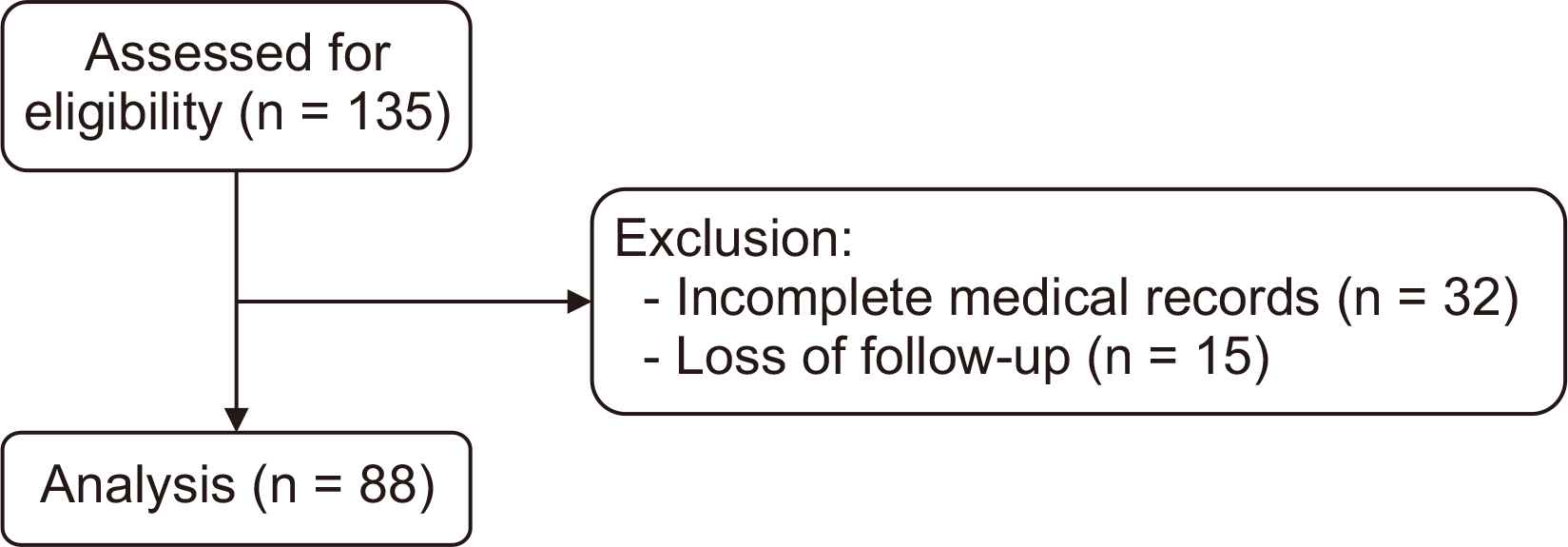 | Fig. 1Flow chart indicating patient selection and exclusion criteria. 
|
Table 1
Demographic and Pre-procedural Data
|
Variable |
Data (n = 88) |
|
Age (yr) |
68.2 ± 12.6 |
|
Sex (M/F) |
26/62 |
|
Hypertension |
38 |
|
Diabetes mellitus |
18 |
|
Duration of pain (mo) |
28.9 ± 35.2 |
|
Pre-procedural NRS (0–10) |
5.8 ± 1.7 |
|
Tenderness at pain site |
37 |
Categorical diagnosis
Skeletal
Muscular
Ligament or tendon
Joint |
31
13
25
19 |
Pain region
Spine
Upper limb
Lower limb
Trunk
Other |
45
13
21
4
5 |

One month after PAC injection, the mean NRS score had reduced to 4.1 (
P < 0.001,
Fig. 2).
Table 2 lists the various associated factors, and their respective number of patients whose NRS score decreased by more than half. There was no independent factor, including the diagnosis category and the site of pain, which was identified as being associated with a decrease in pain intensity by more than half. No adverse effects that might have been caused by treatment were recorded during the follow-up period.
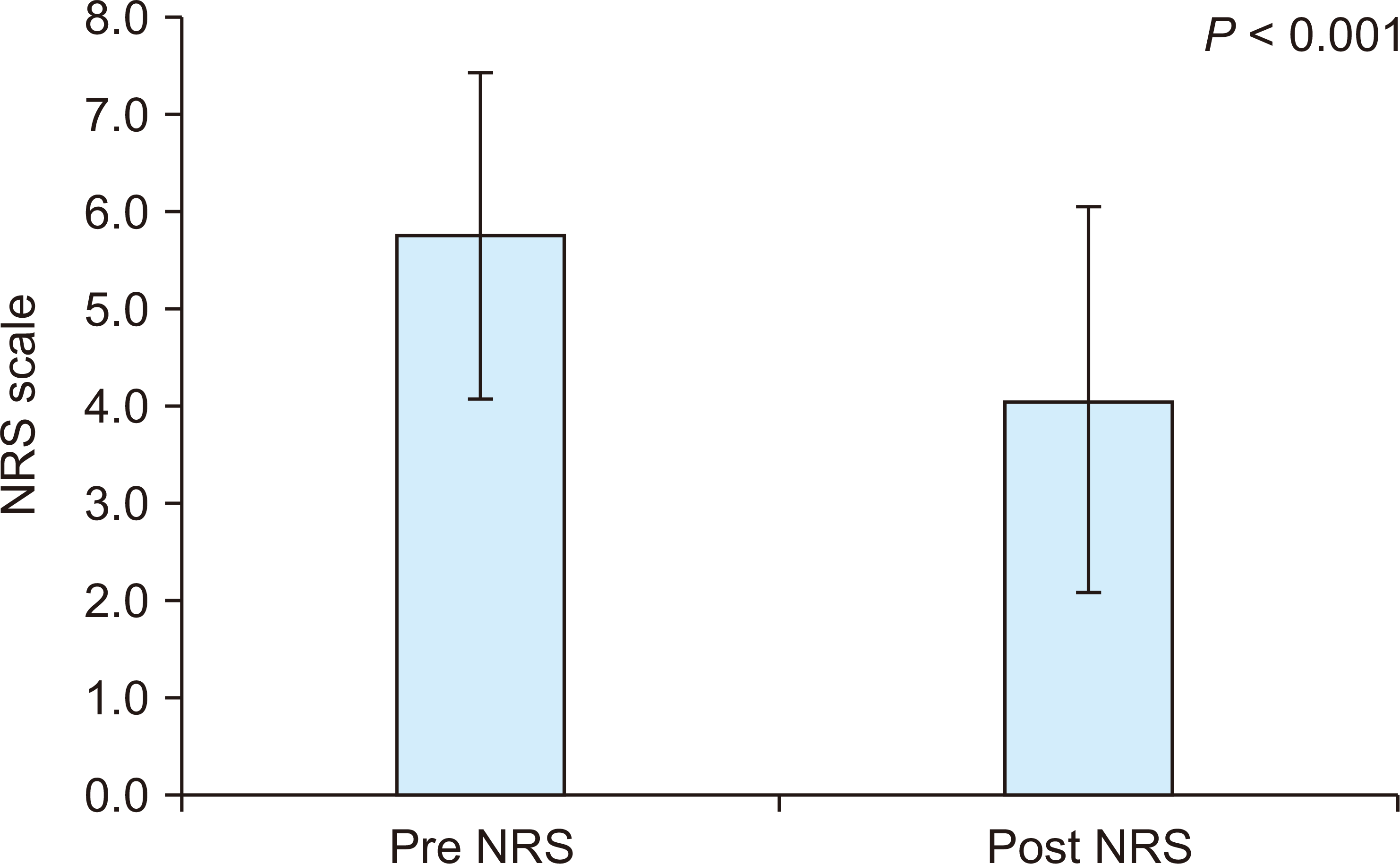 | Fig. 2Change in the numerical rating scale (NRS) score 1 month after porcine atelocollagen injection. 
|
Table 2
Factors Associated with Decrease in Pain Intensity by More Than a Half
|
Predictor |
Effect |
Odds ratio (95% confidence interval) |
P value |
|
Total |
37 (42.0) |
|
|
|
Age (yr) |
|
|
|
|
< 65 |
16 (47.1) |
Ref |
|
|
≥ 65 |
21 (48.9) |
0.72 (0.30–1.71) |
0.450 |
|
Sex |
|
|
|
|
Male |
9 (34.6) |
Ref |
|
|
Female |
28 (45.2) |
1.56 (0.60–4.02) |
0.362 |
|
Hypertension |
14 (36.8) |
0.69 (0.29–1.62) |
0.390 |
|
Diabetes mellitus |
8 (44.4) |
1.13 (0.40–3.21) |
0.817 |
|
No. of interventions |
|
|
|
|
1 |
3 (30.0) |
Ref |
|
|
2 |
7 (43.8) |
1.81 (0.34–9.68) |
0.486 |
|
≥ 3 |
27 (43.5) |
1.80 (0.43–7.62) |
0.425 |
Categorical diagnosis
Skeletal
Muscular
Ligament or tendon
Joint |
12 (48.0)
6 (33.3)
11 (47.8)
8 (36.4) |
Ref
0.54 (0.15–1.90)
0.99 (0.32–3.09)
0.62 (0.19–2.00) |
0.339
0.990
0.422 |
|
Tenderness at pain site |
|
|
|
|
No |
16 (40.0) |
Ref |
|
|
Yes |
21 (43.8) |
1.17 (0.50–2.73) |
0.723 |
Pain region
Spine
Upper limb
Lower limb
Trunk
Other |
17 (37.8)
3 (23.1)
11 (52.4)
3 (75.0)
3 (60.0) |
Ref
0.49 (0.12–2.05)
1.81 (0.64–5.16)
4.94 (0.48–51.40)
2.47 (0.37–16.32) |
0.332
0.266
0.181
0.181 |

Go to :

DISCUSSION
This retrospective study evaluated the potential of PAC injection treatment in the management of refractory chronic pain due to suspected connective tissue damage. In the present study, we studied patients who were not responding to other conservative treatments. In these patients, normal recovery or inflammatory reactions are usually attenuated due to repeated damage [
15,
16]. For the treatment of patients with this condition, regenerative therapies such as prolotherapy have been introduced and developed. Despite application of various agents such as dextrose, polydeoxyribonucleotide or platelet rich plasma [
6,
7,
17,
18], all these treatments aim to repair the damaged tissue by inducing an endogenous healing reaction
via injection of a specific drug around the damaged structure, although the endogenous healing reaction may not occur or may be incomplete, especially owing to age [
19]. Therefore, attempts have been made to directly inject collagen, the final product of the healing process, to treat chronic pain [
8,
9].
PAC treatment has been used for regeneration of various organs, including bones [
20], articular cartilages [
14,
21], and ligaments [
8,
9]. However, there are limited studies on the clinical application of PAC for chronic pain. To our best knowledge, this is the first study to evaluate the use of PAC for refractory chronic pain with suspected musculoskeletal damage. The significant reduction in the NRS score of patients treated in this study suggests the applicability of PAC injection for the treatment of intractable chronic pain caused by extensive tissue damage. In this study, we injected PAC into the space between the suspected damage or defect and its surrounding tissues. However, the exact mode of action or mechanism by which this application contributed to the improvement of the chronic pain is not fully known.
There are possible mechanisms which might explain the therapeutic effect of PAC. First, PAC may have directly promoted tissue recovery. Since collagen is the final product of tissue damage repair, it may have helped directly repair damaged tissue. Stopak et al. [
22] reported that type I collagen injected into developing tissue was rearranged at the injection site. In addition, Suh et al. [
8] reported histological and biomechanical repair at 12 weeks after fixation of PAC between damaged bone and tendon junctions. These studies may suggest the possibility that the healing cascade was promoted by direct engraftment of the end product. In the present study, the PAC that we administered around the damaged tissue may have been engrafted directly and was possibly involved in regeneration. However, such successful collagen engraftment may not be easily achieved through a simple injection into the degenerative tissue of elderly patients. Secondly, PAC might have acted as a scaffold for repair. This mechanism has been studied for bones, ligaments, tendons, and cartilage through laboratory studies [
8,
12,
14,
20,
21]. PAC has a long half-life because of its resistance to degradation by enzymatic breakdown, due to its triple helix structure and non-immunity [
14,
23]. Therefore, it is estimated that PAC might have acted as a scaffold to facilitate the tissue regeneration reaction around damaged tissue in the present study. In this respect, it is possible that using progenitor cells or proliferation-related factors may have a synergistic effect [
19,
24,
25]. Finally, the effect might have been caused by the reduction of inflammation. Because PAC, which does not easily degrade, may simply remain around the injection site, and thus reduce chronic inflammation through a protective role from repetitive stimulation by degeneration or damaged tissue.
In studies involving prolotherapy, the prognoses were different depending on the region of pain [
26]. However, according to the results of the present study, the region of pain was not a significant factor impacting the therapeutic effect of the PAC. Differences between the mechanisms of prolotherapy and PAC application may explain this result, but a lack of statistical power, due to insufficient sample size, may also be the cause. A well-designed study with a larger sample size is necessary for more clarification.
There are some limitations to this study. Since there was no control group, it was not possible to present a relative comparative analysis of the effects from other treatments. However, because this study targeted patients with refractory chronic pain—for whom there was no relief after other various treatments, including anti-inflammatory injection, prolotherapy, medication or physical therapy, et al.—it was difficult to designate specific treatments as a control. In addition, the area of pain targeted for this study is diverse, which has limitations in specifying the indications for the procedure. The short follow-up period of 1 month and a lack of control over the use of other treatments, due to the retrospective study design, were other limitations of this study. Nevertheless, this study is significant in that it is the first study to evaluate the possibility of PAC as a treatment option for chronic intractable musculoskeletal disorders. It is anticipated that further well-designed studies with histological investigation in the future will give a better understanding of the potential of PAC as a treatment option for chronic refractory pain.
In conclusion, NRS score reduction was obtained by administering PAC to patients with refractory chronic pain. Application of PAC may have potential as a treatment option for refractory chronic musculoskeletal pain.
Go to :


