2. Kawashima N, Mita T, Yoshikawa M. 2013; Inter-individual difference in the effect of mirror reflection-induced visual feedback on phantom limb awareness in forearm amputees. PLoS One. 8:e69324. DOI:
10.1371/journal.pone.0069324. PMID:
23935984. PMCID:
PMC3723857.

3. Nardone R, Langthaler PB, Höller Y, Bathke A, Frey VN, Brigo F, et al. 2015; Modulation of non-painful phantom sensation in subjects with spinal cord injury by means of rTMS. Brain Res Bull. 118:82–6. DOI:
10.1016/j.brainresbull.2015.09.006. PMID:
26405006.

4. Wilkins KL, McGrath PJ, Finley GA, Katz J. 2004; Prospective diary study of nonpainful and painful phantom sensations in a preselected sample of child and adolescent amputees reporting phantom limbs. Clin J Pain. 20:293–301. DOI:
10.1097/00002508-200409000-00003. PMID:
15322435.

5. Sherman RA, Sherman CJ. 1983; Prevalence and characteristics of chronic phantom limb pain among American veterans. Results of a trial survey. Am J Phys Med. 62:227–38. PMID:
6624883.
6. Sherman RA, Sherman CJ, Parker L. 1984; Chronic phantom and stump pain among American veterans: results of a survey. Pain. 18:83–95. DOI:
10.1016/0304-3959(84)90128-3. PMID:
6709380.

7. Davis RW. 1993; Phantom sensation, phantom pain, and stump pain. Arch Phys Med Rehabil. 74:79–91. PMID:
8380543.
8. Dijkstra PU, Geertzen JH, Stewart R, van der Schans CP. 2002; Phantom pain and risk factors: a multivariate analysis. J Pain Symptom Manage. 24:578–85. DOI:
10.1016/S0885-3924(02)00538-9. PMID:
12551807.
9. Montoya P, Larbig W, Grulke N, Flor H, Taub E, Birbaumer N. 1997; The relationship of phantom limb pain to other phantom limb phenomena in upper extremity amputees. Pain. 72:87–93. DOI:
10.1016/S0304-3959(97)00004-3. PMID:
9272791.

10. Kooijman CM, Dijkstra PU, Geertzen JH, Elzinga A, van der Schans CP. 2000; Phantom pain and phantom sensations in upper limb amputees: an epidemiological study. Pain. 87:33–41. DOI:
10.1016/S0304-3959(00)00264-5. PMID:
10863043.

11. Hanley MA, Jensen MP, Smith DG, Ehde DM, Edwards WT, Robinson LR. 2007; Preamputation pain and acute pain predict chronic pain after lower extremity amputation. J Pain. 8:102–9. DOI:
10.1016/j.jpain.2006.06.004. PMID:
16949876.

12. Karanikolas M, Aretha D, Tsolakis I, Monantera G, Kiekkas P, Papadoulas S, et al. 2011; Optimized perioperative analgesia reduces chronic phantom limb pain intensity, prevalence, and frequency: a prospective, randomized, clinical trial. Anesthesiology. 114:1144–54. DOI:
10.1097/ALN.0b013e31820fc7d2. PMID:
21368651.
14. Flor H, Nikolajsen L, Staehelin Jensen T. 2006; Phantom limb pain: a case of maladaptive CNS plasticity? Nat Rev Neurosci. 7:873–81. DOI:
10.1038/nrn1991. PMID:
17053811.

16. Sahin SH, Colak A, Arar C, Tutunculer E, Sut N, Y©¥lmaz B, et al. 2011; A retrospective trial comparing the effects of different anesthetic techniques on phantom pain after lower limb amputation. Curr Ther Res Clin Exp. 72:127–37. DOI:
10.1016/j.curtheres.2011.06.001. PMID:
24648582. PMCID:
PMC3957153.

17. Jang MJ, Bang SM, Oh D. 2011; Incidence of venous thromboembolism in Korea: from the Health Insurance Review and Assessment Service database. J Thromb Haemost. 9:85–91. DOI:
10.1111/j.1538-7836.2010.04108.x. PMID:
20942850.

18. Hall GC, Morant SV, Carroll D, Gabriel ZL, McQuay HJ. 2013; An observational descriptive study of the epidemiology and treatment of neuropathic pain in a UK general population. BMC Fam Pract. 14:28. DOI:
10.1186/1471-2296-14-28. PMID:
23442783. PMCID:
PMC3599764.

19. Pisansky AJB, Brovman EY, Kuo C, Kaye AD, Urman RD. 2018; Perioperative outcomes after regional versus general anesthesia for above the knee amputations. Ann Vasc Surg. 48:53–66. DOI:
10.1016/j.avsg.2017.10.014. PMID:
29217448.

20. Raichle KA, Osborne TL, Jensen MP, Ehde DM, Smith DG, Robinson LR. 2015; Preoperative state anxiety, acute postoperative pain, and analgesic use in persons undergoing lower limb amputation. Clin J Pain. 31:699–706. DOI:
10.1097/AJP.0000000000000150. PMID:
26153780. PMCID:
PMC6309977.

22. Schley MT, Wilms P, Toepfner S, Schaller HP, Schmelz M, Konrad CJ, et al. 2008; Painful and nonpainful phantom and stump sensations in acute traumatic amputees. J Trauma. 65:858–64. DOI:
10.1097/TA.0b013e31812eed9e. PMID:
18849803.

23. Benzon HT, Rathmell JP, Wu CL, Turk DC, Argoff CE. 2008. Raj’s practical management of pain. 4th ed. Mosby-Elsevier;Philadelphia:
24. Bloomquist T. 2001; Amputation and phantom limb pain: a pain-prevention model. AANA J. 69:211–7. PMID:
11759564.
25. Clark RL, Bowling FL, Jepson F, Rajbhandari S. 2013; Phantom limb pain after amputation in diabetic patients does not differ from that after amputation in nondiabetic patients. Pain. 154:729–32. DOI:
10.1016/j.pain.2013.01.009. PMID:
23433944.

26. Buchheit T, Van de Ven T, Hsia HL, McDuffie M, MacLeod DB, White W, et al. 2016; Pain phenotypes and associated clinical risk factors following traumatic amputation: results from Veterans Integrated Pain Evaluation Research (VIPER). Pain Med. 17:149–61. DOI:
10.1111/pme.12848. PMID:
26177330. PMCID:
PMC6280998.

27. Esfandiari E, Yavari A, Karimi A, Masoumi M, Soroush M, Saeedi H. 2018; Long-term symptoms and function after war-related lower limb amputation: a national cross-sectional study. Acta Orthop Traumatol Turc. 52:348–51. DOI:
10.1016/j.aott.2017.04.004. PMID:
30082112. PMCID:
PMC6205055.

28. Ahmad J, Gupta AK, Sharma VP, Kumar D, Yadav G, Singh S. 2016; Traumatic amputations in children and adolescents: a demographic study from a tertiary care center in Northern India. J Pediatr Rehabil Med. 9:265–9. DOI:
10.3233/PRM-160398. PMID:
27935565.

29. Hinrichs-Rocker A, Schulz K, Järvinen I, Lefering R, Simanski C, Neugebauer EA. 2009; Psychosocial predictors and correlates for chronic post-surgical pain (CPSP) - a systematic review. Eur J Pain. 13:719–30. DOI:
10.1016/j.ejpain.2008.07.015. PMID:
18952472.

30. Montoya P, Ritter K, Huse E, Larbig W, Braun C, Töpfner S, et al. 1998; The cortical somatotopic map and phantom phenomena in subjects with congenital limb atrophy and traumatic amputees with phantom limb pain. Eur J Neurosci. 10:1095–102. DOI:
10.1046/j.1460-9568.1998.00122.x. PMID:
9753177.

33. Smith J, Thompson JM. 1995; Phantom limb pain and chemotherapy in pediatric amputees. Mayo Clin Proc. 70:357–64. DOI:
10.4065/70.4.357. PMID:
7898142.

35. Jensen TS, Krebs B, Nielsen J, Rasmussen P. 1983; Phantom limb, phantom pain and stump pain in amputees during the first 6 months following limb amputation. Pain. 17:243–56. DOI:
10.1016/0304-3959(83)90097-0.

36. Jensen TS, Krebs B, Nielsen J, Rasmussen P. 1985; Immediate and long-term phantom limb pain in amputees: incidence, clinical characteristics and relationship to pre-amputation limb pain. Pain. 21:267–78. DOI:
10.1016/0304-3959(85)90090-9. PMID:
3991231.

37. Nikolajsen L, Ilkjaer S, Kr©ªner K, Christensen JH, Jensen TS. 1997; The influence of preamputation pain on postamputation stump and phantom pain. Pain. 72:393–405. DOI:
10.1016/S0304-3959(97)00061-4. PMID:
9313280.

38. Bach S, Noreng MF, Tjéllden NU. 1988; Phantom limb pain in amputees during the first 12 months following limb amputation, after preoperative lumbar epidural blockade. Pain. 33:297–301. DOI:
10.1016/0304-3959(88)90288-6.

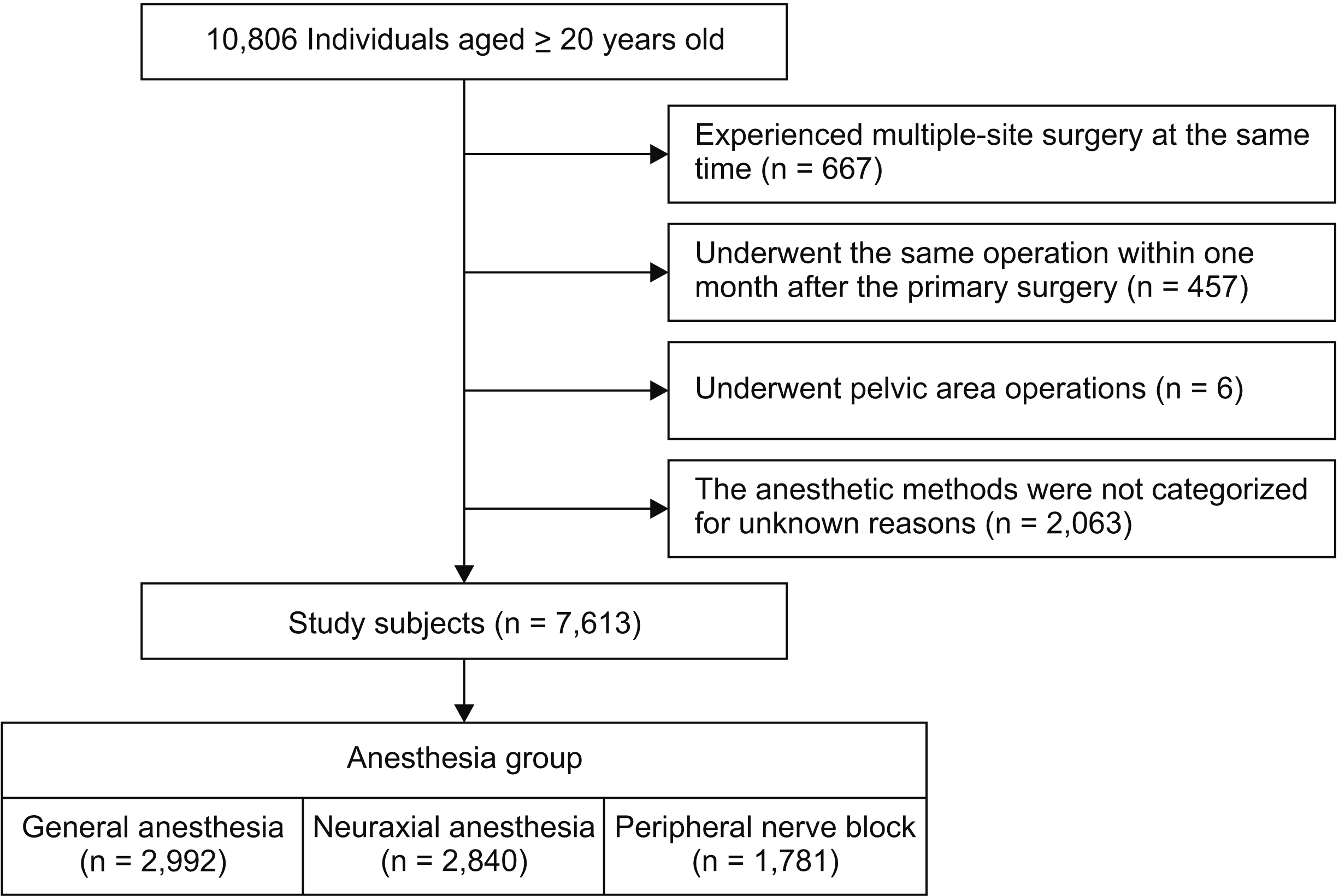
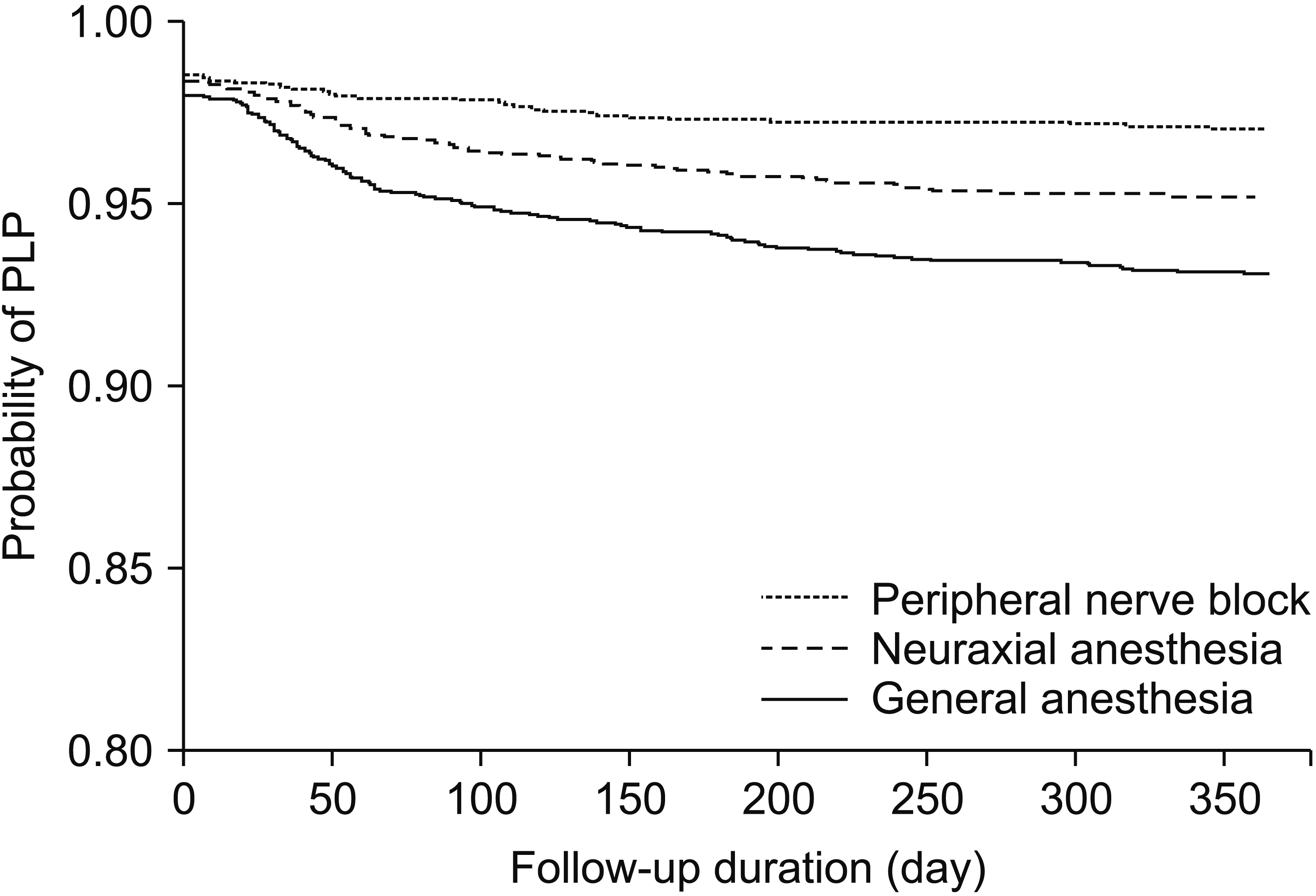




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download