INTRODUCTION
MATERIALS AND METHODS
1. Animals, drugs and chemicals
2. CCI-induced neuropathic pain
1) Paw pressure test (mechanical hyperalgesia)
2) Von Frey filament test (mechanical allodynia)
3) Acetone drop test (cold allodynia)
4. Quantification of biochemical parameters
5. Experimental design
Normal: No surgery was performed.
Sham: Surgery was performed without nerve ligation.
CCI: Surgery was performed along with nerve ligation.
Diallyl disulfide (25 mg/kg per os [p.o.]) in CCI: CCI-subjected rats were treated for 14 days with a low dose of diallyl disulfide.
Diallyl disulfide (50 mg/kg p.o.) in CCI: CCI-subjected rats were treated for 14 days with a high dose of diallyl disulfide.
Diallyl trisulfide (20 mg/kg p.o.) in CCI: CCI-subjected rats were treated for 14 days with a low dose of diallyl trisulfide.
Diallyl trisulfide (40 mg/kg p.o.) in CCI: CCI-subjected rats were treated for 14 days with a high dose of diallyl trisulfide.
ANA-12 (0.25 mg/kg) and diallyl disulfide (50 mg/kg) in CCI: To explore the role of BDNF in diallyl disulfide-mediated pain attenuating actions, a low dose of ANA-12 was co-administered with diallyl disulfide (selection of the dose on the basis of the results of groups diallyl disulfide [25 mg/kg p.o.] and diallyl disulfide [50 mg/kg p.o.] in CCI) in CCI-subjected rats.
ANA-12 (0.50 mg/kg) and diallyl disulfide (50 mg/kg) in CCI: A high dose of ANA-12 was co-administered with diallyl disulfide in CCI-subjected rats.
ANA-12 (0.50 mg/kg) and diallyl trisulfide (40 mg/kg) in CCI: To explore the role of BDNF in diallyl trisulfide-mediated beneficial effects, a high dose of ANA-12 (selection of dose on the basis of the results of groups ANA-12 [0.25 mg/kg] and diallyl disulfide [50 mg/kg] and ANA-12 [0.50 mg/kg] and diallyl disulfide [50 mg/kg] in CCI) was co-administered with a high dose of diallyl trisulfide (dose selection on the basis of the results of groups diallyl trisulfide [20 mg/kg p.o.] and diallyl trisulfide [40 mg/kg p.o.] in CCI).
6. Statistical testing
RESULTS
1. Diallyl disulfide and diallyl trisulfide ameliorated CCI-induced hyperalgesia and allodynia
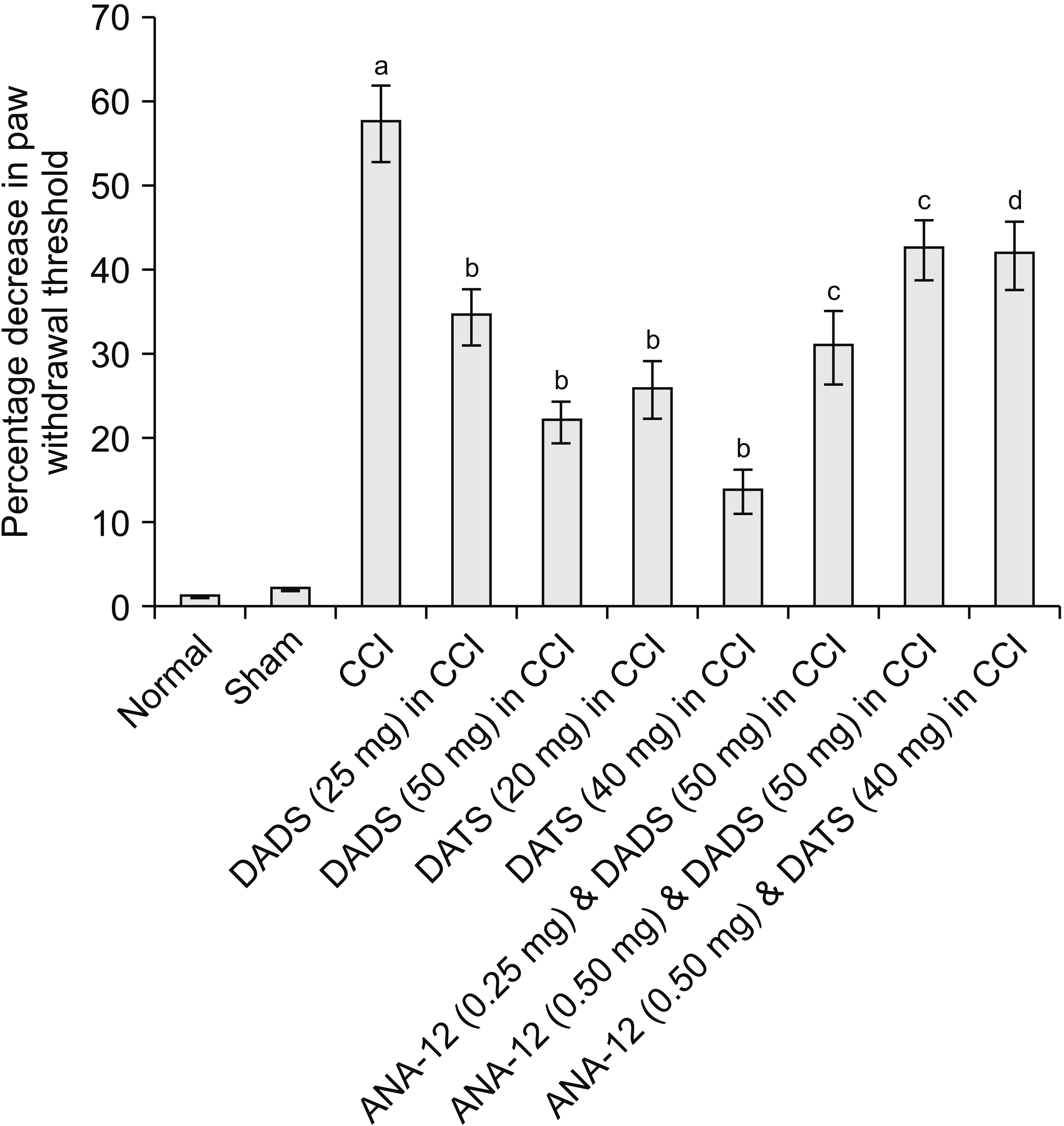 | Fig. 1Effect of different interventions on paw withdrawal threshold (mechanical hyperalgesia) in Randall–Selitto test. The data were represented as percentage decrease in paw withdrawal threshold on 14th day of surgery in comparison to day 1 i.e., before surgery. CCI: chronic constriction injury, DADS: diallyl disulfide, DATS: diallyl trisulfide. aP < 0.05 vs. sham; bP < 0.05 vs. CCI; cP < 0.05 vs. DADS (50 mg/kg) in CCI; dP < 0.05 vs. DATS (40 mg/kg) in CCI. |
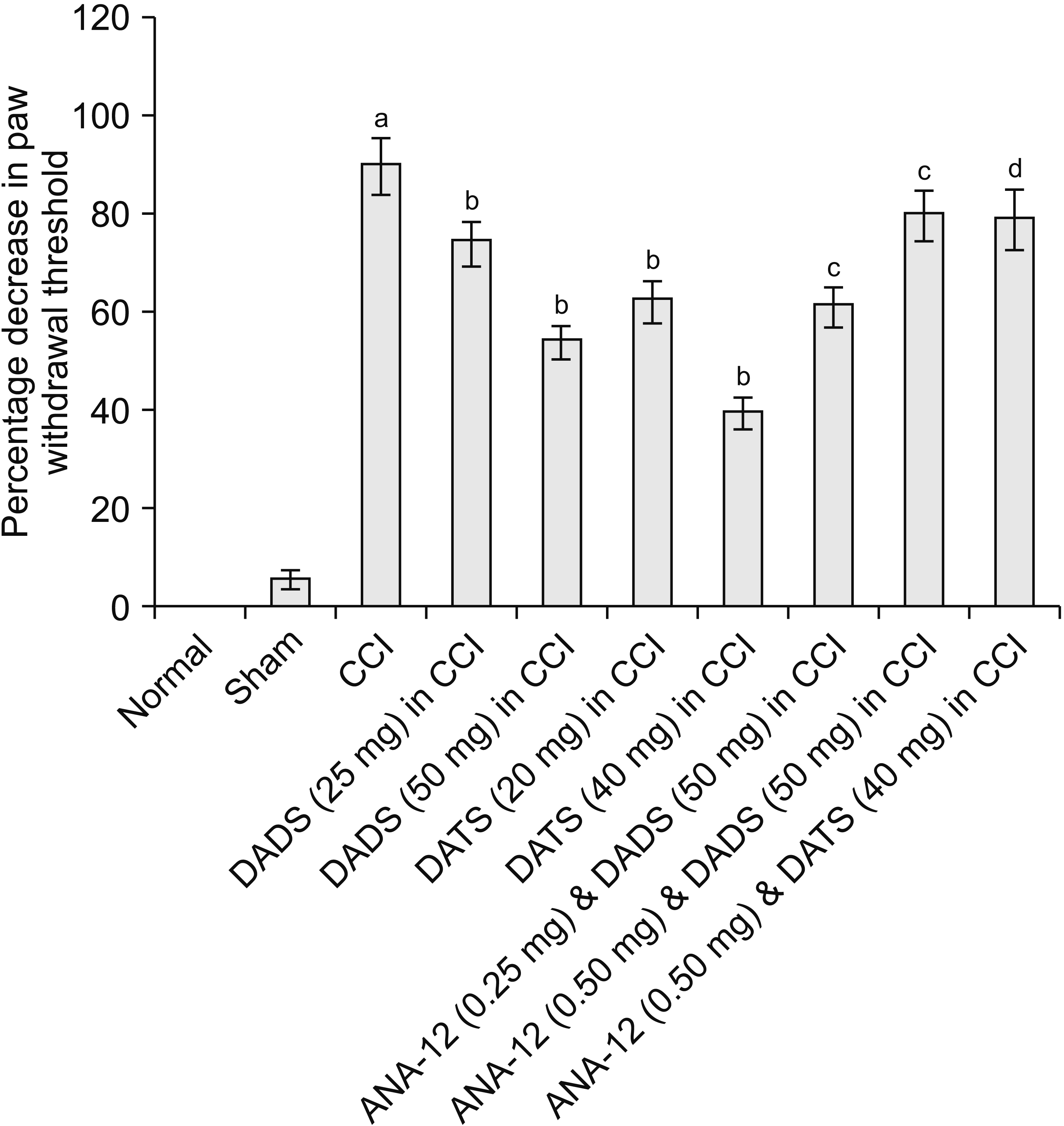 | Fig. 2Effect of different interventions on paw withdrawal threshold (mechanical allodynia) in Von Frey test. The data were represented as percentage decrease in paw withdrawal threshold on 14th day of surgery in comparison to day 1 i.e., before surgery. CCI: chronic constriction injury, DADS: diallyl disulfide, DATS: diallyl trisulfide. aP < 0.05 vs. sham; bP < 0.05 vs. CCI; cP < 0.05 vs. DADS (50 mg/kg) in CCI; dP < 0.05 vs. DATS (40 mg/kg) in CCI. |
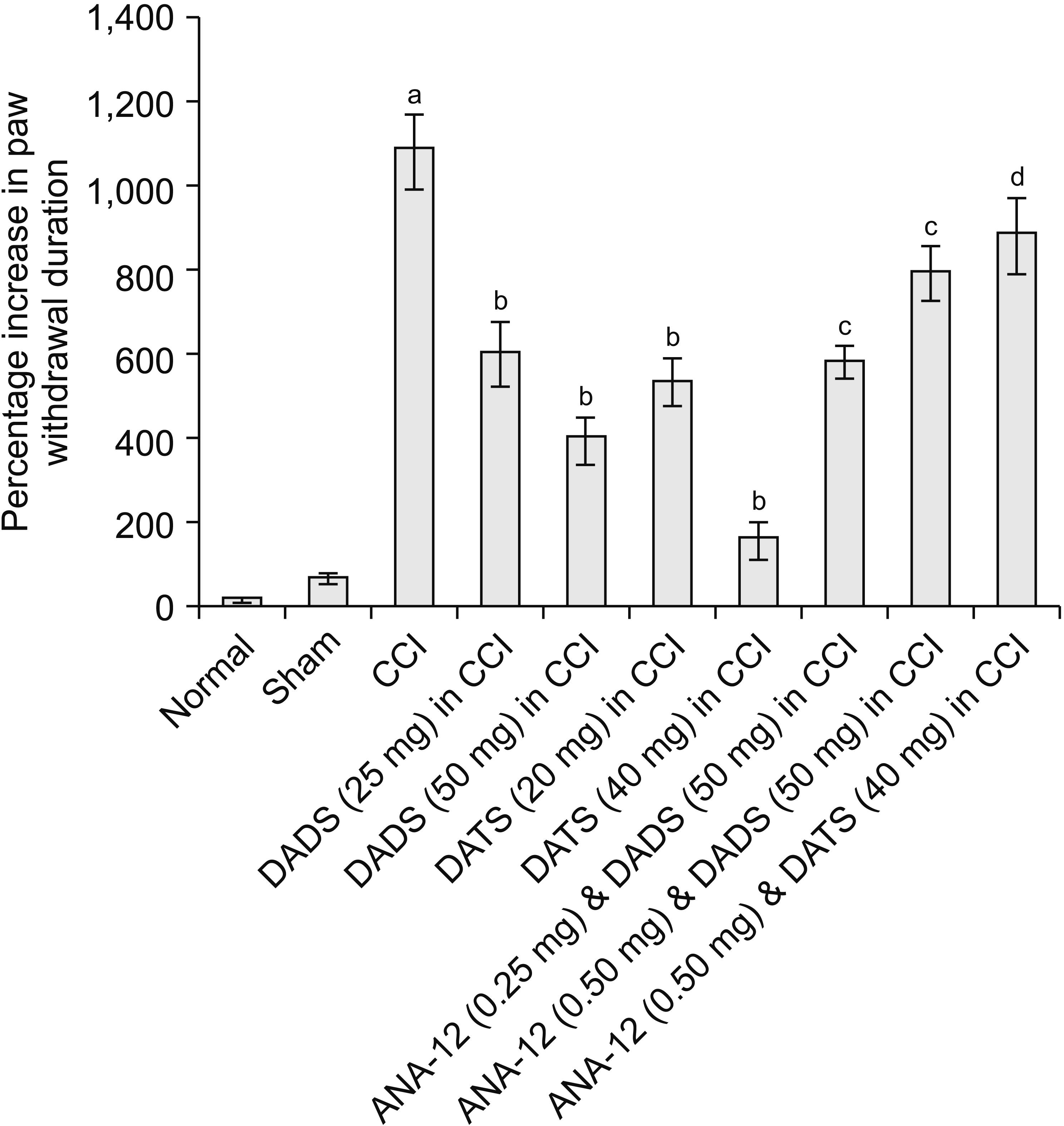 | Fig. 3Effect of different interventions on paw withdrawal duration (cold allodynia) in cold acetone drop test. The data were represented as percentage increase in paw withdrawal threshold on 14th day of surgery in comparison to day 1 i.e., before surgery. CCI: chronic constriction injury, DADS: diallyl disulfide, DATS: diallyl trisulfide. aP < 0.05 vs. sham; bP < 0.05 vs. CCI; cP < 0.05 vs. DADS (50 mg/kg) in CCI; dP < 0.05 vs. DATS (40 mg/kg) in CCI. |
Table 1
| Days | Normal | Sham | CCI | DADS (25 mg/kg) in CCI | DADS (50 mg/kg) in CCI | DATS (20 mg/kg) in CCI | DATS (40 mg/kg) in CCI | ANA-12 (0.25 mg/kg) & DADS (50 mg/kg) in CCI | ANA-12 (0.50 mg/kg) & DADS (50 mg/kg) in CCI | ANA-12 (0.50 mg/kg) & DATS (40 mg/kg) in CCI | F value |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mechanical hyperalgesia (paw withdrawal threshold in grams) | |||||||||||
| Day 1 | 294.2 ± 5.6 | 284.3 ± 4.9 | 287.1 ± 6.3 | 275.0 ± 5.8 | 294.5 ± 4.9 | 24.4 ± 5.3 | 295.5 ± 5.0 | 278.0 ± 5.7 | 287.2 ± 6.0 | 292.3 ± 6.4 | For time, F (1,140) = 2,301.3 |
| Day 14 | 290.1 ± 4.8 | 278.1 ± 6.5 | 151.5 ± 5.4a | 180.2 ± 4.9b | 229.3 ± 6.4b | 210.3 ± 5.7b | 254.5 ± 5.8b | 192.2 ± 6.4c | 165.4 ± 4.8c | 170.3 ± 6.1d | For treatment, F (9,140) = 1,301.4 |
| P value | - | - | < 0.001 | < 0.05 | < 0.001 | < 0.01 | < 0.001 | < 0.05 | < 0.001 | < 0.001 | |
| Mechanical allodynia (paw withdrawal threshold in grams) | |||||||||||
| Day 1 | 26 ± 0 | 26 ± 0 | 26 ± 0 | 26 ± 0 | 26 ± 0 | 26 ± 0 | 26 ± 0 | 26 ± 0 | 26 ± 0 | 26 ± 0 | For time, F (1,140) = 2,505.6 |
| Day 14 | 26 ± 0 | 24.6 ± 3.9 | 2.7 ± 1.1a | 6.8 ± 1.8b | 12 ± 3.2b | 9.9 ± 2.5b | 15.8 ± 4.4b | 10.1 ± 2.1c | 5.3 ± 1.5c | 5.5 ± 1.4d | For treatment, F (9,140) = 1,613.9 |
| P value | - | - | < 0.001 | < 0.05 | < 0.001 | < 0.01 | < 0.001 | < 0.05 | < 0.001 | < 0.001 | |
| Cold allodynia (paw withdrawal duration in sec) | |||||||||||
| Day 1 | 1.5 ± 0.2 | 1.6 ± 0.2 | 1.7 ± 0.2 | 2.0 ± 0.1 | 1.7 ± 0.2 | 1.9 ± 0.2 | 1.6 ± 0.1 | 1.8 ± 0.2 | 2.0 ± 0.1 | 1.7 ± 0.2 | For time, F (1,140) = 2,714.6 |
| Day 14 | 1.8 ± 0.7 | 2.7 ± 0.9 | 20.1 ± 1.5a | 14.1 ± 1.1b | 8.5 ± 0.5b | 12.1 ± 1.1b | 4.2 ± 0.4b | 12.3 ± 0.8c | 17.9 ± 1.1c | 16.7 ± 1.2d | For treatment, F (9,140) = 1,749.1 |
| P value | - | - | < 0.001 | < 0.05 | < 0.001 | < 0.01 | < 0.001 | < 0.05 | < 0.001 | < 0.001 | |
| BDNF levels (pg/mg of protein) | |||||||||||
| DRG | 15.2 ± 0.8 | 14.0 ± 0.7 | 3.0 ± 0.1a | 7.4 ± 0.2b | 11.4 ± 0.21b | 9.3 ± 0.4b | 13.4 ± 0.5b | 8.3 ± 0.5c | 4.3 ± 0.2c | 5.3 ± 0.2d | F (9,70) = 256.5 |
| Sciatic nerve | 18.4 ± 0.6 | 17.2 ± 0.3 | 3.8 ± 0.1a | 8.9 ± 0.2b | 13.2 ± 0.2b | 10.2 ± 0.3b | 16.3 ± 0.4b | 7.4 ± 0.23 | 4.8 ± 0.1c | 5.9 ± 0.3c | F (9,70) = 279.7 |
| P value | - | - | < 0.001 | < 0.05 | < 0.001 | < 0.01 | < 0.001 | < 0.05 | < 0.001 | < 0.001 | |
| Hydrogen sulfide (pg/mg of protein) | |||||||||||
| DRG | 25.0 ± 1.3 | 23.2 ± 1.2 | 5.8 ± 0.8 | 12.5 ± 0.7 | 1.9 ± 1.1 | 14.6 ± 1.6 | 22.1 ± 1.7 | 18.3 ± 1.1 | 17.8 ± 1.3 | 21.3 ± 2.1 | F (9,70) = 295.1 |
| Sciatic nerve | 29.1 ± 1.7 | 27.2 ± 1.7 | 6.8 ± 0.5a | 15.3 ± 0.4b | 23.4 ± 0.9b | 18.3 ± 0.9b | 26.4 ± 1.4b | 22.1 ± 1.3c | 22.7 ± 1.5c | 25.3 ± 1.2d | F (9,70) = 310.7 |
| P value | - | - | < 0.001 | < 0.05 | < 0.001 | < 0.01 | < 0.001 | < 0.05 | < 0.001 | < 0.001 | |
| Nrf2 (relative expression in percentage) | |||||||||||
| DRG | 100.0 ± 5.1 | 95.2 ± 5.7 | 41.3 ± 4.3 | 58.3 ± 4.1 | 83.2 ± 3.7 | 65.1 ± 5.4 | 91.3 ± 6.4 | 61.3 ± 6.7 | 49.0 ± 3.7 | 52.1 ± 5.2 | F (9,70) = 210.1 |
| Sciatic nerve | 100.0 ± 6.2 | 92.1 ± 6.1 | 39.2 ± 3.2a | 55.1 ± 4.7b | 80.1 ± 6.4b | 63.0 ± 3.5b | 87.2 ± 7.8b | 57.4 ± 4.5c | 43.4 ± 3.6c | 48.4 ± 5.3d | F (9,70) = 231.9 |
| P value | - | - | < 0.001 | < 0.05 | < 0.001 | < 0.01 | < 0.001 | < 0.05 | < 0.001 | < 0.001 | |
2. Treatment with diallyl disulfide and diallyl trisulfide induced biochemical changes in the sciatic nerve and DRG of CCI-subjected rats
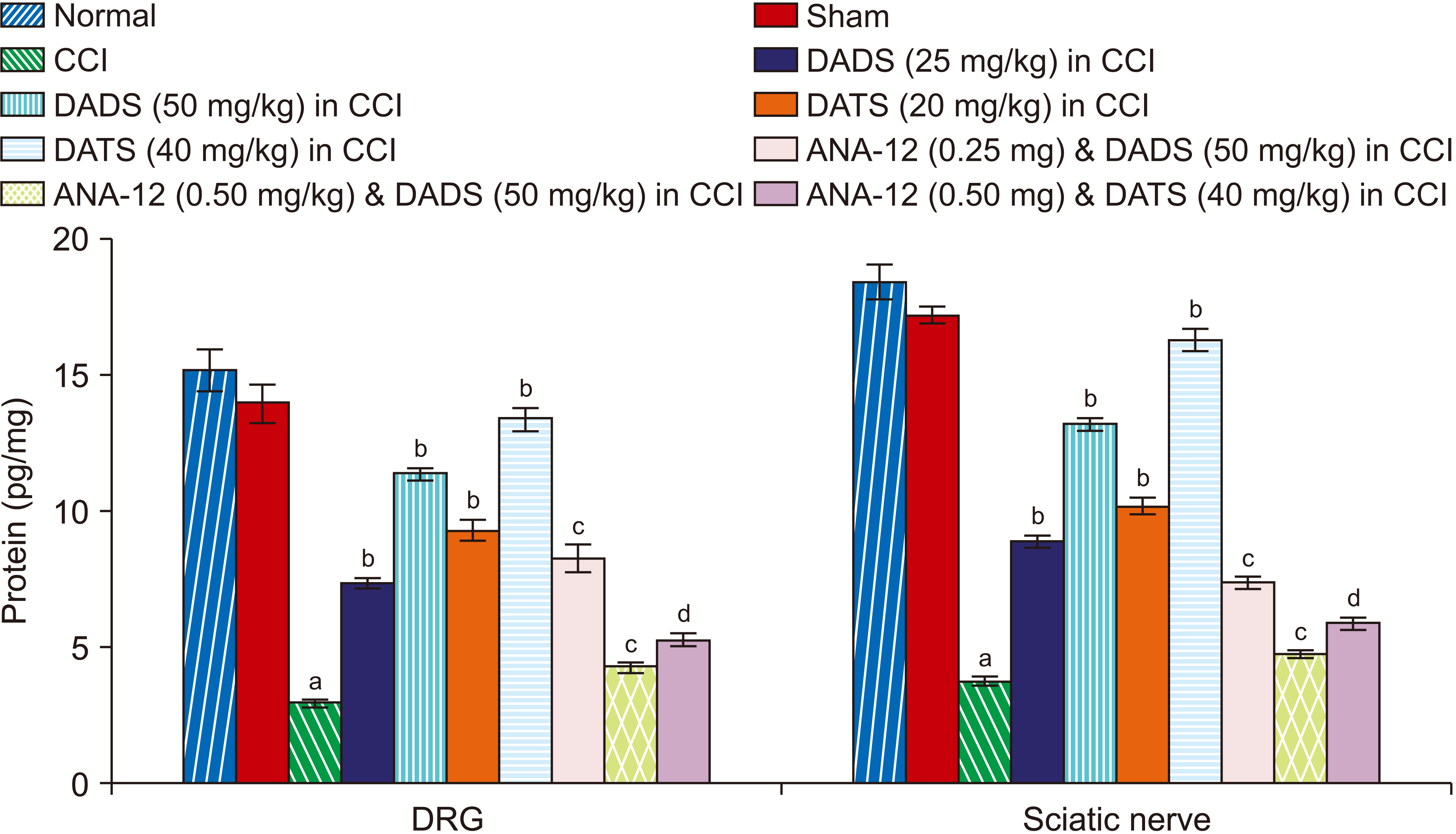 | Fig. 4Effect of different interventions on the BDNF levels in the DRG and sciatic nerve. BDNF: brain-derived neurotrophin factor, DRG: dorsal root ganglia, CCI: chronic constriction injury, DADS: diallyl disulfide, DATS: diallyl trisulfide. aP < 0.05 vs. sham; bP < 0.05 vs. CCI; cP < 0.05 vs. DADS (50 mg/kg) in CCI; dP < 0.05 vs. DATS (40 mg/kg) in CCI. |
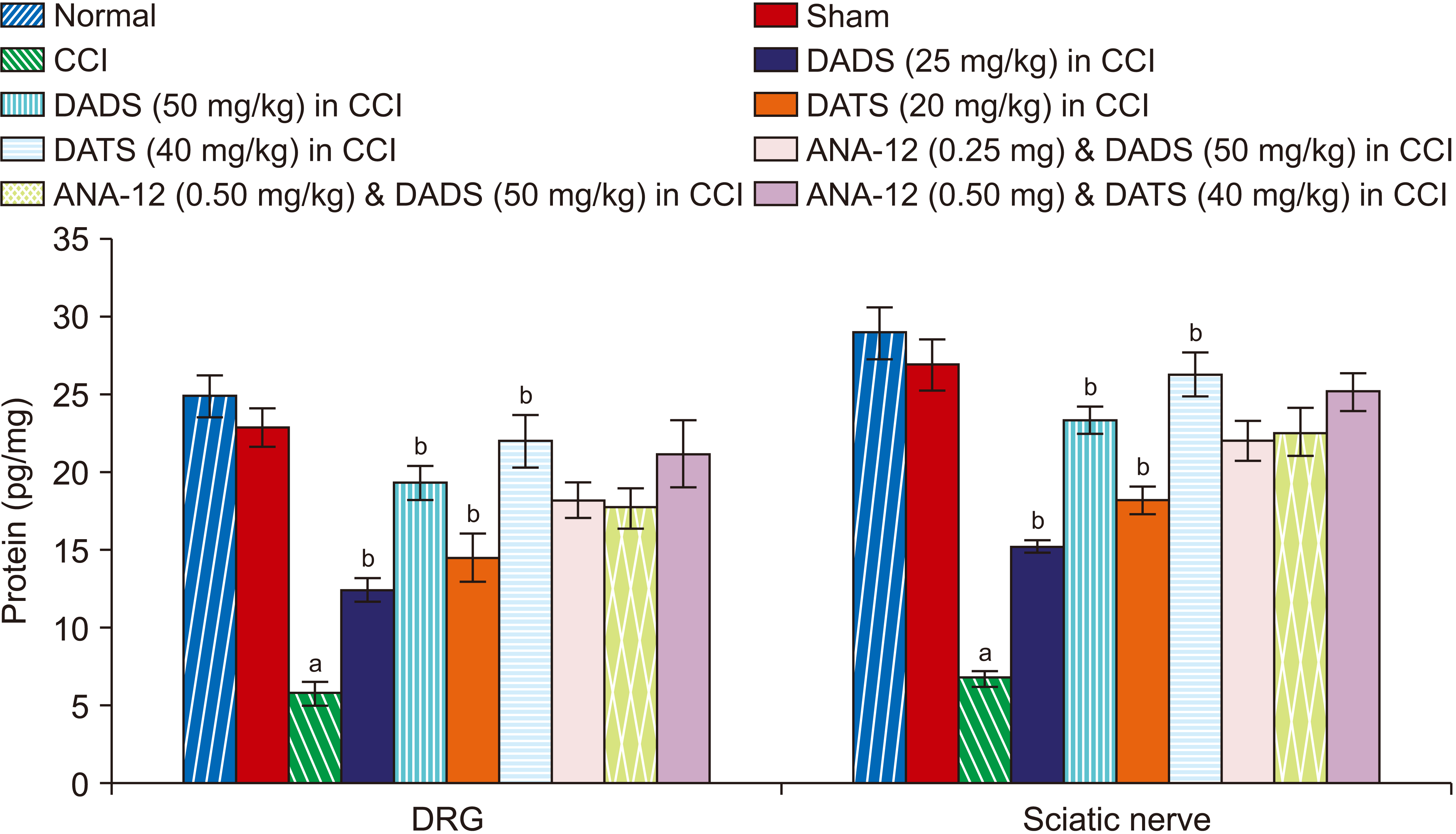 | Fig. 5Effect of different interventions on the H2S levels in the DRG and sciatic nerve. DRG: dorsal root ganglia, CCI: chronic constriction injury, DADS: diallyl disulfide, DATS: diallyl trisulfide. aP < 0.05 vs. sham; bP < 0.05 vs. CCI. |
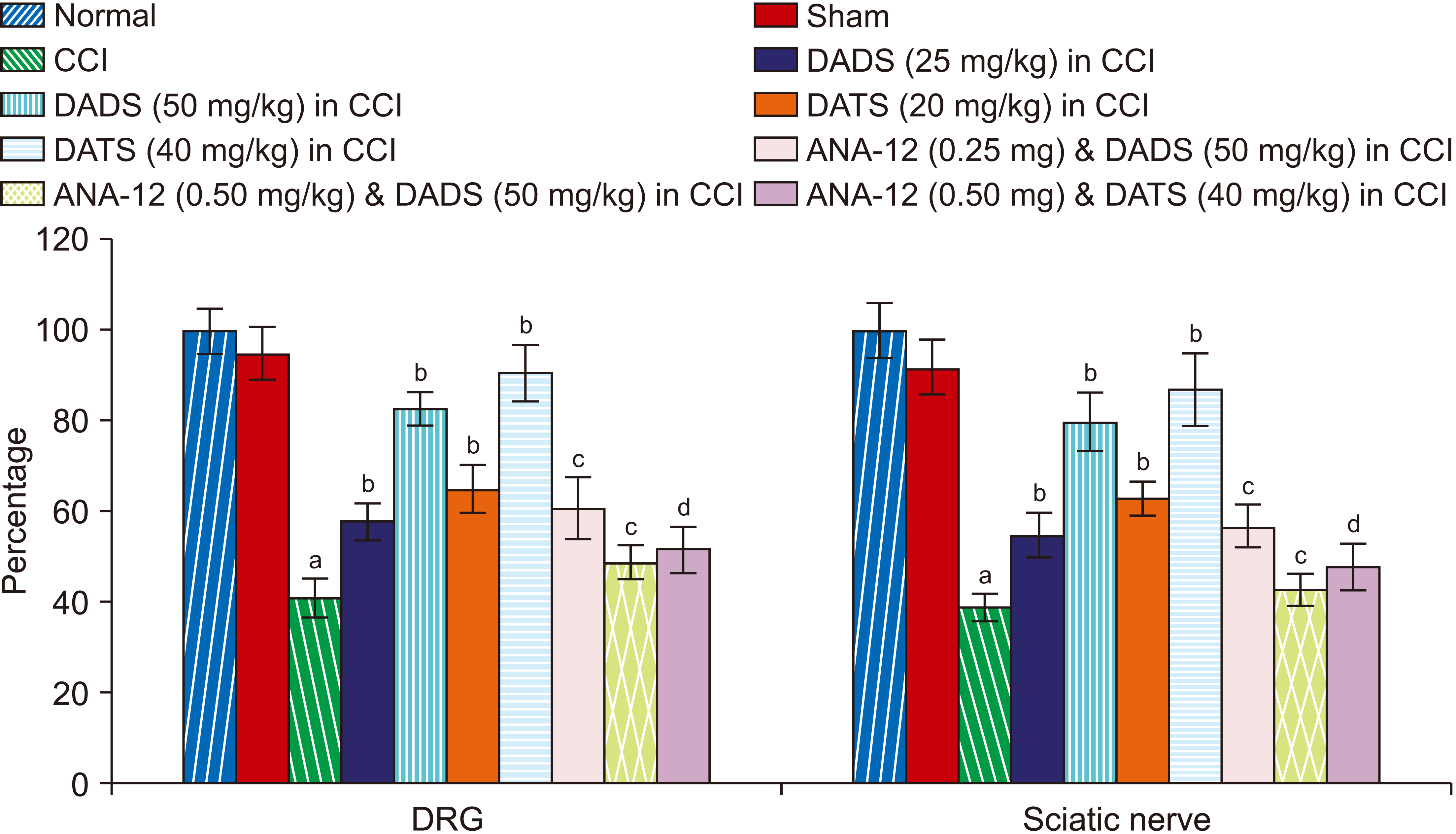 | Fig. 6Effect of different interventions on the Nrf2 levels in the DRG and sciatic nerve. Nrf2: nuclear factor erythroid 2-related factor 2, DRG: dorsal root ganglia, CCI: chronic constriction injury, DADS: diallyl disulfide, DATS: diallyl trisulfide. aP < 0.05 vs. sham; bP < 0.05 vs. CCI; cP < 0.05 vs. DADS (50 mg/kg) in CCI; dP < 0.05 vs. DATS (40 mg/kg) in CCI. |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download