Introduction
Materials and Methods
Materials
Cell culture and trophoblast differentiation
Receptor inhibition
Growth inhibition
Western blotting
Luciferase reporter transactivation
Statistical analysis
 | Fig. 1TGF-β isoforms induce trophoblast progenitor differentiation and Glut-1 transactivation. SM10 cells were treated for 72 hrs with (A) vehicle control, (B) 5 ng/ml TGF-β1, (C) 5 ng/ml TGF-β2, or (D) 2 ng/ml TGF-β3 to induce differentiation. (E) Glut-1 luciferase reporter activity in SM10s cells treated with vehicle control, TGF-β1, TGF-β2, or TGF-β3. Cells were transiently transfected with Glut1-lux and pRLSV40 using Metafectene. Twenty-four hours post-transfection, cells were treated with vehicle control, TGF-β1 (5 ng/ml), TGF-β2 (5 ng/ml), or TGF-β3 (2 ng/ml) for 24 or 72 hours. Luciferase activity was analyzed using the dual luciferase assay system. The Glut1-lux transactivation values were normalized to the constitutively active reporter, pRLSV40. Error bars represent standard error of the mean. (θ) indicates a significant increase in Glut1 luciferase transactivation compared to vehicle control at 24 hours and (*) indicates a significant increase from control at 72 hours. p<0.05. |
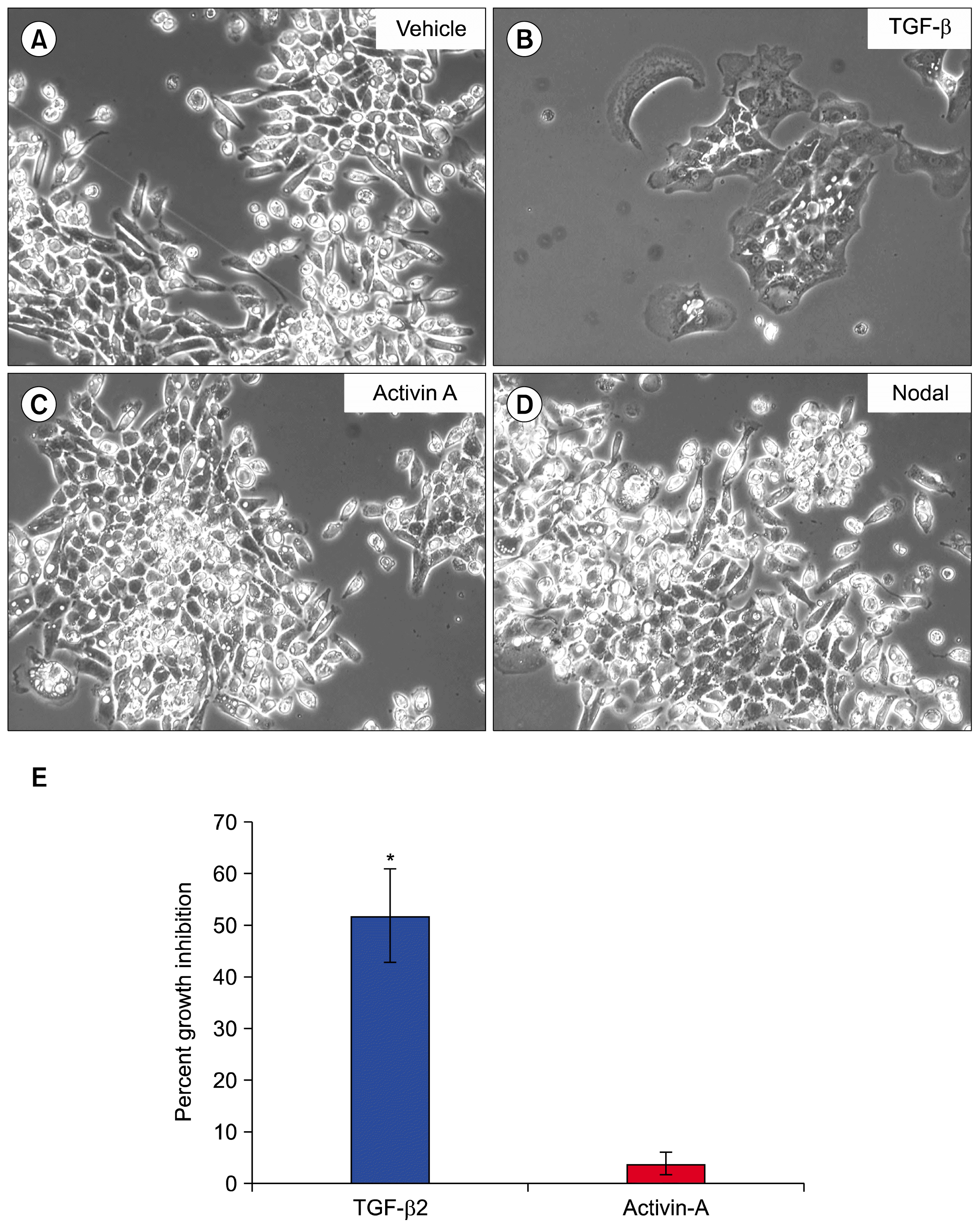 | Fig. 2TGF-β induces SM10 cell differentiation and growth inhibition. SM10 cells were treated with (A) vehicle control, (B) TGF-β2 (5 ng/ml), (C) Activin A (5 ng/ml), or (D) Nodal (250 μg/ml) for 72 hrs to induce differentiation. (E) After 72 hours of treatment, cells were incubated with 1 μCi/ml 3H-Thymidine per well for 4 hours. Cells were then lysed and counted in scintillation fluid. Percent growth inhibition was determined as the amount of 3H-Thymidine incorporation compared to control. Percent growth inhibition is normalized to vehicle treated cells. Error bars represent standard error of the mean. *p<0.001. |
 | Fig. 3TGF-β induced differentiation can be mediated through the Alk-5 receptor. SM10 cells were treated without (A, B) or with (C, D) the Alk inhibitor SB431542 (10 μM) for 1 hour, followed by treatment without (A, C) or with (B, D) TGF-β 2 (5 ng/ml) for 48 hours. |
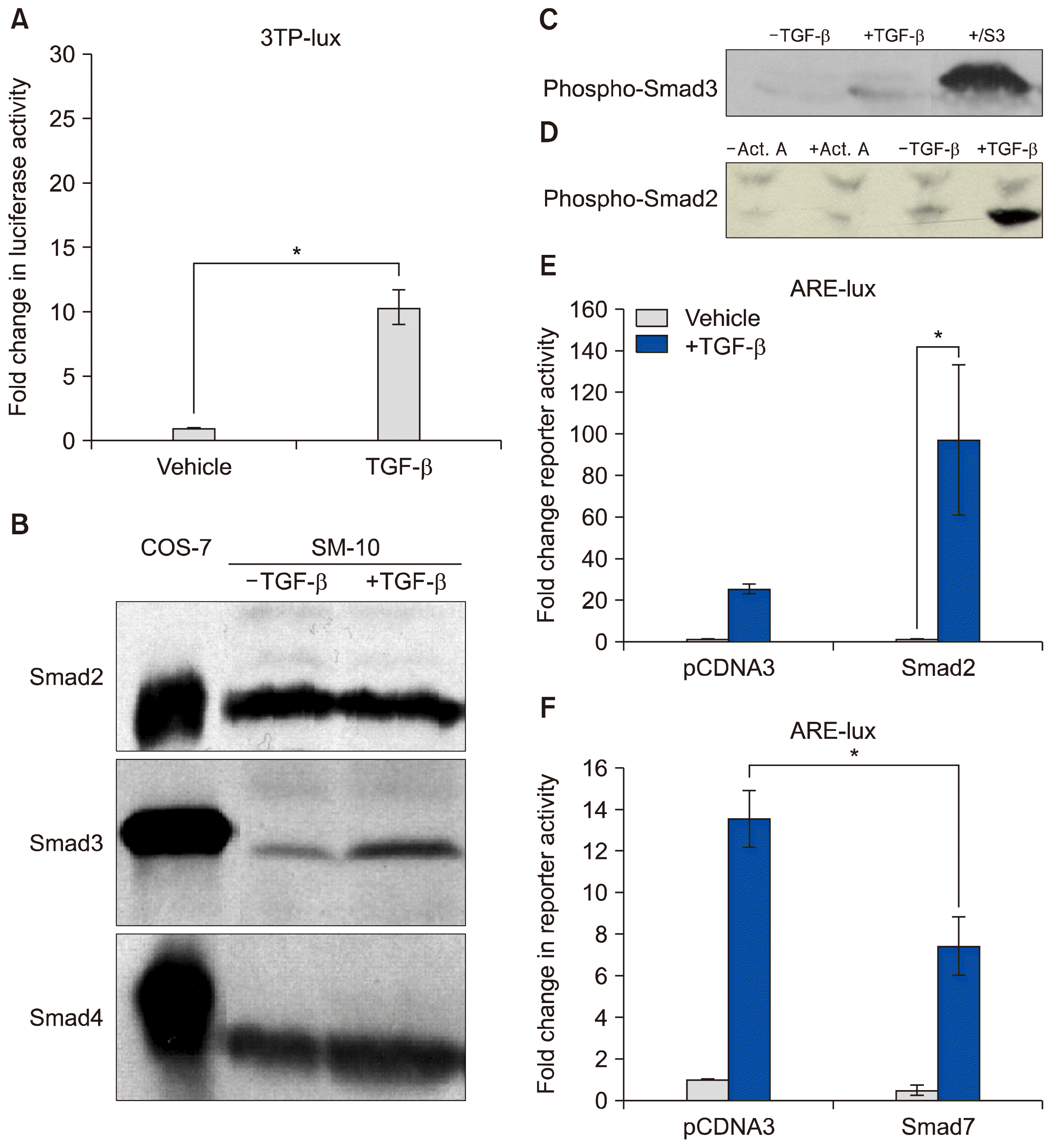 | Fig. 4TGF-β triggers Smad2 phosphorylation and transactivation. (A) 3TP luciferase reporter activity in SM10s cells treated with vehicle control or TGF-β2. Cells were transiently transfected with 3TP-lux and pRLSV40 using Metafectene. Twenty-four hours post-transfection, cells were treated with vehicle or TGF-β2 (5 ng/ml) for 72 hours. Luciferase activity was analyzed using the dual luciferase assay system. The 3TP-lux transactivation values were normalized to the constitutively active reporter (pRLSV40). (B) Western blotting analysis of Smad2, Smad3, and Smad4 in TGF-β2 or vehicle-treated SM10 cells. COS-7 cells were used as a positive control. (C) Western blotting analysis of Phospho-Smad3 in TGF-β2 or vehicle-treated SM10 cells. COS-7 cells transiently transfected with the plasmid, pXIX-Smad3 and treated with TGF-β were used as a positive control. (D) Western blotting analysis of Phospho-Smad2 in the TGF-β2, Activin A, or vehicle-treated SM10 cells. (E) ARE luciferase reporter activity in SM10 cells transiently transfected with pCDNA3 control or pRK5-Smad2 and treated with vehicle control or TGF-β2. Cells were transiently transfected with ARE-lux, pLv-CMV-hFAST-1, and pRLSV40 using Metafectene. Twenty-four hours post-transfection, cells were treated with vehicle or TGF-β2 (5 ng/ml) for 72 hours. Luciferase activity was analyzed using the dual luciferase assay system. The ARE-lux transactivation values were normalized to the constitutively active reporter (pRLSV40). (F) ARE luciferase reporter activity in SM10 cells transiently transfected with pCDNA3 control or pRK5-Smad7 and treated with vehicle control or TGF-β2. Cells were transiently transfected with ARE-lux, pLv-CMV-hFAST-1, and pRLSV40 using Metafectene. Twenty-four hours post-transfection, cells were treated with vehicle or TGF-β2 (5 ng/ml) for 72 hours. Luciferase activity was analyzed using the dual luciferase assay system. The ARE-lux transactivation values were normalized to the constitutively active reporter (pRLSV40). Error bars represent standard error of the mean. *p<0.05. |
Results
TGF-β isoforms differentiate SM10 progenitor cells
TGF-β, but not Activin A or Nodal, promote SM10 progenitor differentiation
TGF-β induced differentiation is dependent upon TGFBR1 signaling
TGF-β induces phosphorylation of Smad2 but not Smad3 in SM10 cells
TGF-β induces transactivation of Activin Responsive Element via Smad2
Smad7 reduces activation of the activin responsive element
Discussion
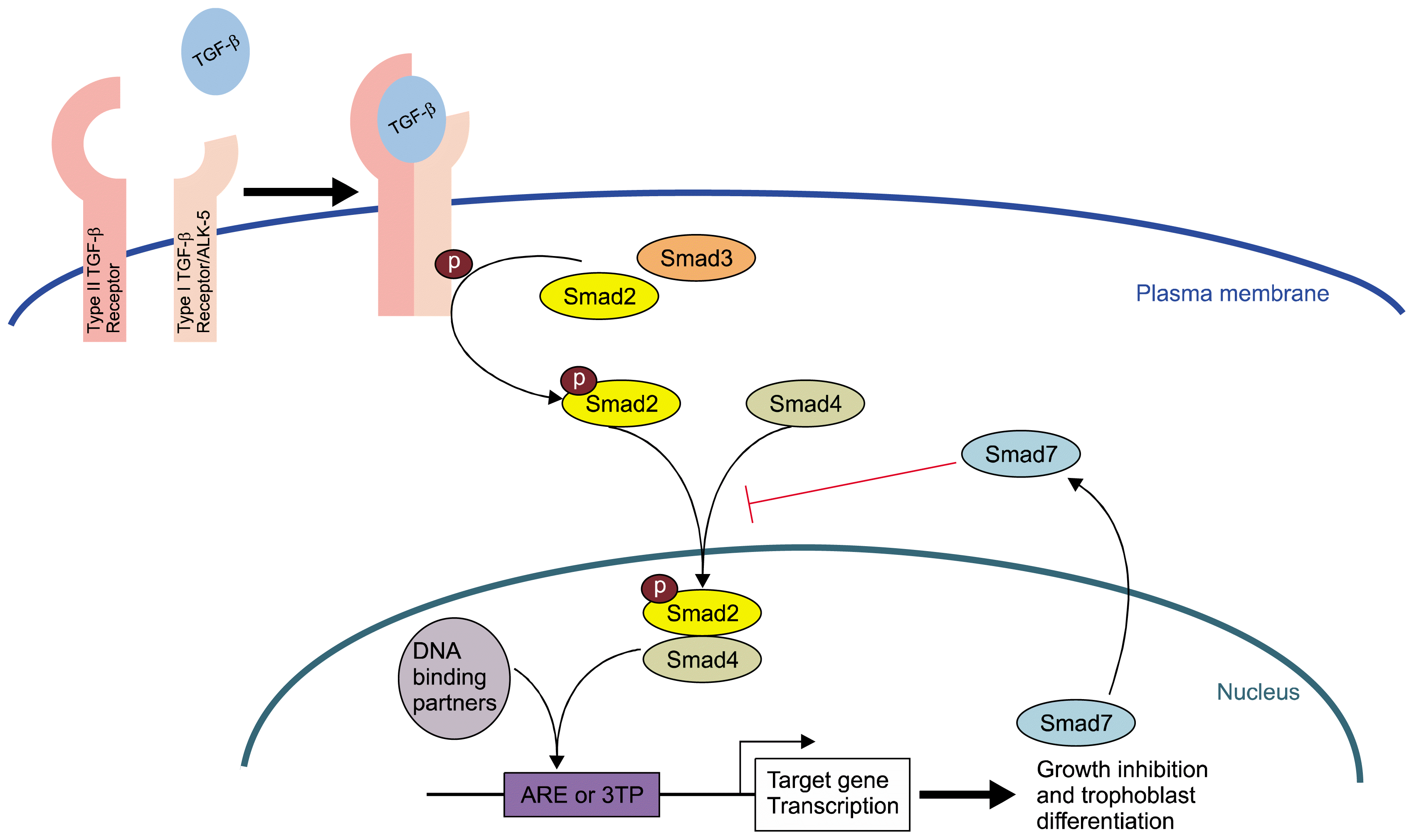 | Fig. 5TGF-β induces Smad2 Phosphorylation, ARE Induction, and Trophoblast Differentiation Diagrammatic representation of the TGF-β signaling pathway in SM10 growth inhibition and trophoblast differentiation. The ligand, TGF-β, binds to cognate receptors and triggers activation via the TGF-β Type I receptor (ALK-5) to induce the phosphorylation of Smad2. Phospho-Smad2, via classically presumptive Smad 4 binding, triggers the transactivation of ARE response elements in target genes that inhibit cell growth and promote labyrinthine trophoblast differentiation. Smad 7 acts as a classical negative feedback inhibitor for the TGF-β signaling pathway. |




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download