Abstract
Background and Objectives
The goal of treatment for MS is to reduce the inflammation and induce the regeneration of degenerated axons. Considering the anti-inflammatory and regenerative capacity of mesenchymal stem cell (MSCs), in this study the therapeutic efficacy of allogeneic MSCs and MSCs-derived neural progenitor cells (MSCs-NPs) was investigated in cellular therapy of chronic experimental autoimmune encephalomyelitis (EAE).
Methods and Results
MSCs, MSCs-NPs and MSCs+MSCs-NP were administered intravenously to EAE mice on days 22, 29, and 36 post immunization. The levels of cytokines and PGE2 in sera or supernatant of in vitro cultured splenocytes derived from treated mice were measured by ELISA. The results of this study showed that in comparison to MSCs monotherapy, MSCs-NPs administration had a more profound capability of inhibiting the proliferation of pathogenic MOG35–55-specific T cells, decreasing IFN-γ production and increasing anti-inflammatory IL-10 cytokine production. These findings could be explained by higher ability of in vitro cultured MSCs-NPs in production of PGE2 compared to MSCs. In line with these findings, while the administration of MSCs and MSCs-NPs significantly decreased the clinical scores of EAE in comparison with the untreated EAE group, MSCs-NPs were significantly more efficient in reducing clinical score compared to MSCs. Of interest, combined therapy with MSCs and MSCs-NPs did not provide any benefit over monotherapy with MSCs-NPs.
Multiple sclerosis, or its animal model (EAE), is an immune-mediated inflammatory demyelinating disease of the central nervous system (CNS) which is characterized by extensive mononuclear cell infiltration and destruction of the myelin sheath. The role of interferon-gamma (IFN-γ)-producing T helper cells (Th1 cells), as well as IL-17-producing Th cells (Th17 cells) in the immunopathogenesis of EAE have been well documented in various studies (1–4). Undoubtedly, the ultimate goal in the treatment of MS is the inhibition of inflammatory reactions to prevent the development of new neuronal lesions as well as the induction of remyelination in order to restore damaged neuronal functions. Due to the fact that mesenchymal stem cells (MSCs) have the potential to repopulate myelin producing cells and can also suppress ongoing inflammation through the production of anti-inflammatory factors (5, 6), a great promise has been created for MSCs therapy of patients with MS (5, 6). In addition, these cells have the capability of differentiating into neuroectodermal lineage, such as neural progenitor cells, under suitable stimuli in the cell culture (7, 8) and also in inflamed CNS upon in vivo administration (9). In fact, it has been shown that transplantation of syngeneic MSCs to chronic form of EAE in C57BL/6 mice significantly reduced clinical scores accompanied with an increase in the number of regulatory T cells (10). Moreover, due to the anti-inflammatory effects of MSCs, the treatment of chronic EAE mice either with human or allogenic MSCs have similar therapeutic effects as syngeneic MSCs transplantation through targeting of both inflammation and neuro-regeneration (11–13). Injection of MSCs before disease onset (preventive protocol) and at different time points after disease occurrence (therapeutic protocol) strikingly ameliorated EAE (11, 12, 14), but this amelioration was not significant after one injection in the peak of the disease (14).
An important alternative approach for cell therapy of EAE is the use of neural precursors. Some studies have shown the efficacy of sub ventricular zone-neural progenitor cells (SVZ-NPs) in the treatment of established EAE (15, 16). The capacity of these cells in replacing of damaged tissues as well as local or systemic immune modulation has also been reported (15, 16). Given the fact that isolation of SVZ-NPs is an invasive procedure, access to non-aggressive methods to obtain a sufficient number of NPs is of utmost importance. Accordingly, in vitro production of neural precursors from embryonic stem cells or adult MSCs (MSCs-NPs) has attracted much attention. Of interest, both in vitro generated stem cells-derived NPs and ex vivo produced neural progenitors have shown the ability to reduce the severity of disease in EAE mice and modulation of the immune responses in inflamed CNS after intrathecal or intraventricular administration (17–19). Although the capacity of MSCs and MSCs-NPs in regeneration of myelin producing cells and subsidizing of inflammation in EAE mice is well recognized, no research has been conducted to compare their efficacy in amelioration of EAE. In addition, the effect of concurrent administration of MSCs and MSCs-NPs on inhibition of inflammatory responses and improvement of EAE has not been acknowledged. Therefore, the aim of this study was to compare the capability of MSCs and MSCs-NPs in the treatment of EAE and their immunomodulatory effects. Moreover, the effect of co-administration of MSCs+ MSCs-NPs on production of Th1- and Th17-related cytokines and amelioration of clinical manifestation of EAE were compared to MSCs or MSCs-NPs administration alone.
Mouse MSCs were derived from 4~6 weeks old BALB/c mice. Bone marrow cells were obtained from the femurs and tibias of the mice by flushing with 10% FBS in DMEM media. They were kept at a density of 2×106 cells/cm2 in a humidified 5% CO2 incubator. Non-adherent hematopoietic cells were removed by the complete change of media after 72 hours. Cell passages were done by trypsinization when cells grew up to 70~80% confluence. MSCs were characterized by immunophenotyping and their ability to differentiate into adipocytes and osteoclasts. MSCs at passage 6~8 were used for study.
To determine the expression of CD45, CD44, CD34, Sca-1 and MHCII on the surface of MSCs, cells were resuspended in 1:50 dilution of pooled mice sera in staining buffer (2% FBS/PBS) for half an hour. Subsequent to blocking, MSCs were incubated with the appropriate antibodies (PE-conjugated rat anti-mouse CD45, Sca-1, and CD44 as well as FITC-conjugated rat anti-mouse CD34 and MHCII; eBioscience, UK) and their isotype-matched controls for 30 minutes at room temperature. After washing, flow cytometric analysis was performed with a BD-FACS Calibur Flow cytometer (BD, USA). 10,000 cells were analyzed and data were collected by Cell-Quest software. The experiments were replicated three times and the acquired data was analyzed by the Win MDI (2.8) software.
MSCs were plated at 5000 cells/cm2 in DMEM with 10% FBS medium for 48 hours and after that differentiation-induced media was added for 14 days with media change every 2~3 days. Adipogenic medium was DMEM supplemented with 50 μg/ml indomethacin (Sigma, Germany), 10−7M dexamethasone (sigma, Germany) and 50 μg/ml ascorbate-2-phosphate (Sigma). For osteogenesis, the cells were incubated in DMED supplemented with 50 μg/ml β-glycerol phosphate (sigma, Germany), 50 μg/ml ascorbate-2-phosphate (Sigma, Germany) and 10−8M dexamethasone (sigma, Germany). To visualize lipid formation, cultures were fixed in 4% formalin for 1 hour and stained with 0.5% Oil red O in 99% isopropanol for 15 minutes and stained cells were observed by light microscopy. To check osteogenesis, differentiated cells were fixed in 99% methanol for 15 minutes and stained with Alizarin Red S for 5 minutes at room temperature and was visualized by light microscopy.
MSCs were differentiated towards MSCs-NPs according to the method developed by Shiri et al. (20). Briefly, 5×105 MSCs/ml were cultured in 1% agarose (Sigma-Aldrich, Germany) coated plate in 10% FBS neurobasal media (Gibco, USA) containing 2% of B27 (Invitrogen-Gibco, USA), 1% insulin-transferring-selenite (ITS, Invitrogen Gibco, USA), L-glutamine (2 mM), 1% penicillin and streptomycin supplemented with 10 ng/ml bFGF (Sigma-Aldrich, Germany) and 1 μM dehydroepiandrosterone (DHEA, Sigma- Aldrich, Germany) in 5% CO2 incubator for 7 days with media changes after every 24~48 hours. Floating neurospheres were visible after two days (Fig. 1A). At the seventh day cells were collected and subsequent to washing in serum free media re-suspended in 500 μl of 0.5% trypsine solution containing 0.01% EDTA (Gibco, USA) to obtain single cell suspension. The viability of single cells was checked by Trypan blue staining. To confirm differentiation of MSCs to neural progenitor cells, total cellular RNA was extracted from MSCs and MSCs-NPs using High Pure RNA Isolation kit and first-strand cDNA was synthesized from equal amounts of RNA (2 μg) for each sample using random hexamer primer and Fermentase Revert Aid H minus kit (Fermentase, UK) for 60 minutes at 42°C. Nestin mRNA expression was measured by SYBR Green quantitative Real-time PCR assay using the following primer sequences: F:5′-TACAGAGTCAGATGGCTCAGATCC3′, R:5′CAGCAGAGTCCTGTATGTAGCCAC-3′. In addition, PCR reactions were normalized using designed forward and reverse primers of β-actin (F: 5′-CATCCATGGCGAACTGGTG-3′, R:5′AGCTTCTTTGCAGCTCCTTCG-3′) as endogenous reference control gene. The Real-time PCR parameters were as follows; 95°C for 10 minutes followed by 40 cycles of denaturation at 95°C for 10s, annealing and extension at 60°C for 60s. At the end of the PCR, the temperature was increased from 60°C to 95°C, and accuracy was measured by melting curve analysis.
All animal experiments were performed on 12 weeks old female C57BL/6 mice. Mice were immunized subcutaneously with 200 μg of MOG35–55 (Pepteron company, Korea) diluted in 200 μl PBS and re-suspended in an equal volume of incomplete Freund’s adjuvant (IFA, Sigma-Aldrich, Steinheim, Germany). Mycobacterium tuberculosis (Difco, Detroit, MI, USA) was added to the final concentration of 1 mg/ml. Mice received intraperitoneal injection of 200 ng pertussis toxin (List Biological laboratories, USA) immediately and 2 days post immunization. Neurological disability was scored daily using a 0~5 scale (0, asymptomatic; 1, partial loss of tail tonicity; 2, tail paralysis and hind limb weakness; 3, hind limb paralysis; 4, hind limb paralysis and forelimb weakness or paralysis; 5, death due to EAE). Mice were observed for 45 days following immunization. Mice that had developed EAE by day 22 were divided into four treatment groups (five mice in each group). The mean of clinical score in each group was two and there were no significant difference in clinical score among groups. Mice received three intravenous injections of 1×106 cells/200 μl of PBS or PBS alone on days 22, 29, and 36 post immunization. Groups were as follows: group 1, PBS; group 2, MSCs; group 3, MSCs-NPs; group 4, MSCs+MSCs-NPs. All animal experiments were approved by the ethics committee for animal experimentation of the Shiraz University of the Medical Sciences.
In vitro proliferation of splenocytes isolated from MSCs-, MSCs-NPs- or MSCs+MSCs-NPs-treated EAE mice as well as EAE control mice (received a sham injection of PBS) were studied in the presence of MOG35–55 peptide. For all proliferation experiments, 2×105 splenocytes/well were cultured in 96-well microtiter plates in the presence of 20 μg/ml MOG35–55 peptide for 72 hours and 1 μCi of [3H]-Thymidine (MPbiomedical, USA) was added to each well 18 hours before the end of cultures. The extent of [3H]-Thymidine incorporation was determined by Wallace liquid scintillation counter (Wallac, Germany) and results were expressed as count per minute (CPM).
Quantitative analysis of PGE2 (Enzo life science, USA), IL-10, IFN-γ and IL-17 (Mabtech, Sweden) was performed by commercially available ELISA kits. To determine the amount of PGE2 and IL-10 produced by MSCs and MSCs-NPs, 5×105 cells/well were cultured in 2 ml DMEM or 2 ml neurobasal media, respectively. After 24 hours and 48 hours supernatants were collected. The concentrations of cytokines and PGE2 were reported after reduction from basal levels. Serums and spleen cells were also isolated from EAE mice transplanted with MSCs, MSCs-NPs, combination of MSCs+MSCs-NPs and control mice with EAE on day 45. Isolated splenocytes were cultured (2×105 cells/well) in triplicate in medium supplemented with 20 μg/ml MOG35–55 and the supernatants were collected after 72 hours.
Homologous cell populations from the bone marrow of BALB/c mice with adherent MSCs morphology, were characterized by flow cytometry. More than 99% of the cells studied showed cell surface CD45−/ CD44+/ Sca-1+/CD34−/ MHCII− phenotypes. These cells were being differentiated towards MSCs-NPs with a “neurosphere morphology” which is characterized by small spheres of floating cells in suspension (Fig. 1A). The neural lineage potential of MSCs-NPs was also confirmed by 2-fold upregulation in nestin (neural stem cell marker) gene expression. In addition, MSCs-NPs showed a markedly lower expression of MSCs cell surface markers such as CD44 and Sca-1 compared to un-manipulated MSCs (geometric mean for CD44: 22.05 and 64.28, respectively; geometric mean for Sca-1: 106.87 and 2057.19, respectively). Expression of CD45, CD34, and MHCII remained absent in the MSCs-NPs (Fig. 1B). Moreover, to determine whether the differentiation capability of MSCs has been decreased after differentiating into MSCs-NPs, neural precursors were also cultured in adipogenic and osteogenic inducting media. After 14 days of culture, MSCs-NPs showed markedly reduced Oil Red O fat vesicles as well as noticeably decreased Alizarin red calcium depositions compared to MSCs (Fig. 1A).
MSCs and MSCs-NPs have an array of immunoregulatory properties which include the suppression of T cell activation, proliferation and production of immunosupresssive factors. Taken this into consideration, the production of PGE2 and IL-10 in the supernatant of cultured MSCs-NPs and MSCs were examined. As demonstrated in Fig. 2A, the results showed higher concentration of PGE2 in the supernatant of cultured MSCs-NPs compared to MSCs after 24 hours (6,313±239 pg/ml and 5,316±328 pg/ml, respectively; p=0.05) or 48 hours (6,519±49 pg/ml and 5,828±229 pg/ml; respectively; p=0.05). However, there were no differences in the levels of IL-10 produced by MSCs and MSCs-NPs cultured after 24- and 48-hours (for 24 hours: 24.4±3.4 pg/ml and 28.8±4 pg/ml, for 48 hours: 21.07±2.3 pg/ml and 23.1±2.3 pg/ml, respectively; p= 0.2; Fig. 2B).
To compare the effect of allogenic MSCs, MSCs-NPs or their combination in the treatment of established EAE, three injections of 1×106 cells were administered intravenously at the onset of the chronic phase of disease (on day 22). Four treatment groups were followed up to 3 weeks post the initiation of cell therapy. As shown in Fig. 3, PBS injected EAE mice (control mice) displayed a chronically stable disease. However, the average of EAE scores in control group (2.4±0.26) was significantly higher compared to those in mice receiving MSCs, MSCs-NPs, and co-injections of MSCs+MSCs-NPs (1.6±0.17, 1.1±0.29 and 1.1±0.36, respectively; p=0.001). In addition, the EAE score in MSCs-NPs and MSCs+MSCs-NPs treated EAE mice have shown significant reduction compared to MSCs-treated EAE mice (1.1±0.29 and 1.1±0.36 versus 1.6±0.17, respectively; p=0.001). However, there was no significant difference in the reduction of post-treatment EAE scores between MSCs-NPs-injected and co-injected groups (1.1±0.29 and 1.1±0.36, respectively; p=0.96).
In order to clarify whether the injection of MSCs and MSCs-NPs possess any immunomodulatory effect in EAE mice, ex vivo proliferation of MOG35–55-specific splenocytes in all four groups were evaluated after 45 days of EAE induction. As shown in Fig. 4, injection of MSCs-NPs has the most significant effect on reduction of splenocytes proliferation after in vitro stimulation by MOG35–55 compared to the control EAE mice (4,905±1426 CPM and 13,162±2,332 CPM, respectively; p=0.001). Although MOG35–55 specific proliferation of splenocytes showed reduction in MSCs-and MSCs+MSCs-NPs-treated groups, those decreases were not significant compared to the control group (p=0.9 and p=0.09, respectively).
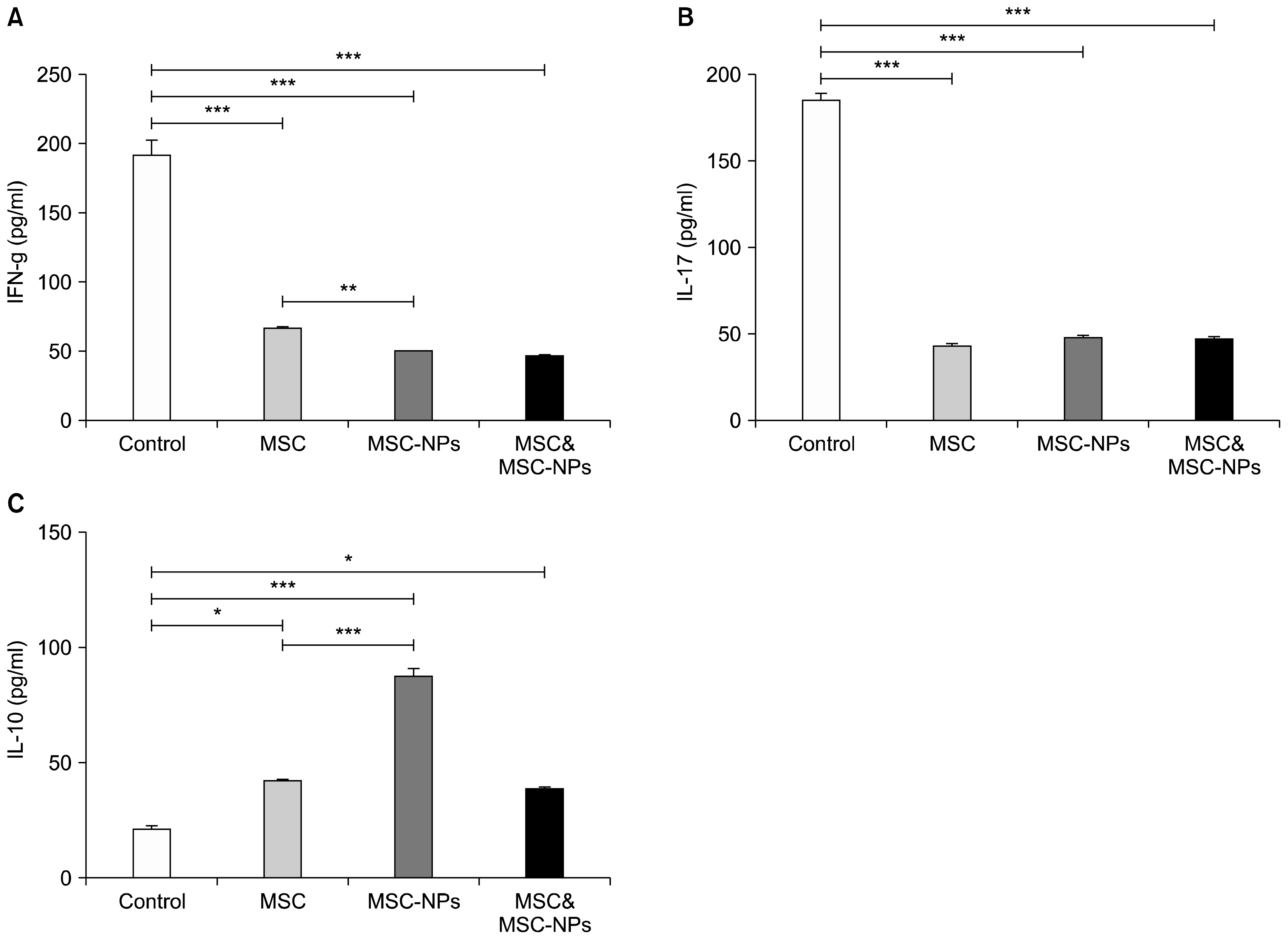
IFN-γ or IL-17 levels in the supernatant of stimulated MOG35–55-specific splenocytes showed a significant decrease in EAE mice treated with MSCs, MSCs-NPs and MSCs+MSCs-NPs compared to the control EAE mice (for IFN-γ: 325±154 pg/ml, 200±15 pg/ml and 252±20 pg/ml versus 1,600±31 pg/ml, respectively; p=0.009; for IL-17: 28±4.2 pg/ml, 27±5.6 pg/ml and 31±4.7 pg/ml versus 108±5.6 pg/ml, respectively; p=0.01; Fig. 5A and B). In addition, compared to the EAE control mice, the levels of IL-10 were also significantly higher in the supernatant of MOG35–55-stimulated splenocytes derived from MSCs, MSCs-NPs and “MSCs+MSCs-NPs”-treated mice (31.5±1.3 pg/ml versus 76±9.8 pg/ml, 75±7.4 pg/ml, and 68±3.4 pg/ml, respectively; p=0.05, p=0.03 and p=0.039, respectively; Fig. 5C), suggesting that MSCs and MSCs-NPs promote the generation of cells with regulatory activity. Moreover, there were no significant differences between IL-10 and IL-17 levels in the supernatants of stimulated MOG35–55-specific splenocytes separated from EAE mice treated with MSCs or MSCs-NPs (p=0.67 and p=0.83, respectively). However, reduction in IFN-γ production by splenocytes removed from MSCs-NPs-treated EAE mice compared to MSCs-treated EAE mice reach a statistical significance (200±15 pg/ml and 325±154 pg/ml, respectively; p=0.015).
As shown in Fig. 6A and B and in accordance with the results obtained from MOG35–55-stimulated splenocytes, significant decreases in the levels of IFN-γ and IL-17 were also observed in the sera of MSCs-, MSCs-NPs- and MSCs+MSCs-NPs-treated mice compared to the untreated EAE mice (for IFN-γ: 67±3.2 pg/ml, 50±1.3 pg/ml and 47±1.3 pg/ml versus 192±17 pg/ml, respectively; p=0.01; for IL-17: 43±2.2 pg/ml, 48±1.9 pg/ml and 47±1.8 pg/ml versus 185±9.1 pg/ml, respectively; p=0.01). Interestingly, a significant increase was found in the sera levels of IL-10 in EAE mice treated with MSCs, MSCs-NPs, and MSCs+MSCs-NPs compared to the untreated EAE mice (42±1.2 pg/ml, 87±8.6 pg/ml, 39±1.5 pg/ml, and 21±3.8 pg/ml respectively; p=0.01 for MSCs and MSCs+MSCs-NPs and p=0.001 for MSCs-NPs; Fig. 6C). Moreover, treatment of EAE mice with MSCs-NPs induced much more increase in the sera levels of IL-10 compared to injection of MSCs (p=0.007, Fig. 6C). Similarly, decrease in the sera levels of IFN-γ in the group treated with MSCs-NPs was much more than in the group treated with MSCs so that their difference was statistically significant (p=0.015, Fig. 6A). However, there was no significant difference in IL-17 levels between MSCs- and MSCs-NPs-treated groups (p=0.6, Fig. 6A).
Experimental Autoimmune Encephalomyelitis (EAE), animal model of multiple sclerosis, is mediated by IFN-γ secreting Th1 and IL-17 producing Th17 cells (1–4). Activation of these cells triggers an inflammatory response that culminates in demyelination, axonal damage and paralysis. Given the fact that MSCs have the capacity of neuroregeneration and suppression of pathogenic T cells (10–13), they have been considered as a promising approach in the treatment of CNS-related demyelinating autoimmune diseases. In this respect, it has been shown that BM-derived MSCs are effective in amelioration of inflammatory onset and severity of EAE (9–11, 14, 17). However, the possibility of uncontrolled mesodermal lineage differentiation or formation of other ectopic tissue after MSCs injection should always be taken into consideration (21). For this reason, MSCs-NPs have been intended as a viable alternative to MSCs. Interestingly, compared to MSCs, the benefit of MSCs-NPs do not only limited to the same immunoregulatory and trophic properties of MSCs, but also show the reduced expression of mesodermal markers and reduced capacity for adipogenic or osteogenic differentiation (22). However, the efficacy of MSCs and MSCs-neural precursors in the treatment of EAE has not been compared. To address this issue, in the present study the MSCs-NPs have been prepared and their differentiation capacity, immunomodulatory functions and their ability in attenuation of EAE were compared with MSCs.
In line with previous investigations (22), the result of this study showed that MSCs-NPs express lower levels of mesenchymal surface markers (CD44 and sca-1), reduced capacity in differentiation to mesodermal lineages, and higher expression of neural stem cell marker (nestin) compared to MSCs (Fig. 1). These results suggest that MSCs have entered early stages of differentiation into neural cells upon culture in neural induction medium. Despite differentiation towards neural cells, MSCs-NPs have retained their capacity in production of immunosuppressive factors (Fig. 2). In fact, alongside the secretion of IL-10, MSCs-NPs produce significantly more PGE2 in the culture supernatant compared to MSCs after 24 hours (6313±239 pg/ml and 5316±328 pg/ml, respectively; p= 0.05) or 48 h (6519±49 pg/ml and 5828±229 pg/ml; respectively; p=0.05) of culture. Of interest, it has been reported that murine MSCs constitutively secret PGE2 and immunosuppressive effects of MSCs is mediated mainly through the secretion of PGE2 (23–26). In addition, MSCs inhibit allogeneic responses in mixed lymphocytic reaction via PGE2 production (23). Consistent with these findings, the results of the present study showed that splenocytes separated from MSCs-NPs-treated EAE mice revealed significantly less proliferation after in vitro stimulation with MOG35–55 compared to MSCs-treated mice (Fig. 4, p<0.05). This might be a result of higher production of PGE2 by MSCs-NPs compared to MSCs. In addition, measurement of Th1 and Th17-dependent cytokines showed that both IFN-γ and IL-17 levels were significantly lower in the supernatant of MOG35–55-stimulated splenocytes derived from MSCs- and MSCs-NPs-treated mice compared to the untreated EAE mice (Fig. 5). Yang et al. has also reported that compared to control mice, splenocytes from EAE mice treated with BM-derived neural precursors displayed decreased levels of IFN-γ and IL-17 after in vitro activation by MOG35–55 (17). However, the production of IFN-γ by MOG35–55-stimulated splenocytes of mice treated with MSCs-NPs is significantly less than MSCs-treated mice (Fig. 5A). Therefore, it can be concluded that MSCs-NPs are more potent than MSCs in controlling of pathogenic Th1 cells in EAE mice. Interestingly, the quantification of serum levels of cytokines duplicates the results of cytokine assay in the culture supernatants of MOG35–55-stimulated splenocytes, except that the mice treated with MSCs-NPs have significantly higher levels of IL-10 compared to those in the sera of MSCs-treated mice. Again these results indicate MSCs-NPs might be more efficient than MSCs in suppression of pathogenic responses by induction of IL-10 producing regulatory cells. It is also worth to mention that the absence of IL-10 in the supernatant of SVZ-derived adult neural stem cells and the significance of engineered IL-10 production by these cells in immunomodulation and remyelination have already been reported (27).
Given the fact that compared with those from MSCs, MSCs-NPs produce significantly more PGE2 in the culture supernatant and induce higher levels of IL-10 in the sera of treated mice, in our current study the in vivo efficacy of these cells in amelioration of EAE severity were also investigated. The results of the present study showed that administration of both MSCs- or MSCs-NPs significantly reduced the clinical score of EAE compared to control EAE mice, though amelioration of clinical score was more prominent in MSCs-NPs- or MSCs+ MSCs-NPs-treated EAE mice compared to those of MSCs-treated EAE mice (Fig. 3). Due to the fact that homing of intravenous injected neural precursors into the injured CNS of EAE mice and their ability in remyelination have been reported by other investigators (17, 18), significant advantage of MSCs-NPs over MSCs in amelioration of EAE might be a result of their neural replacement and remyelination property, in addition to their higher immunoregulatory capacity. It worth mentioning that combination therapy with MSCs and MSCs-NPs did not show any advantages for amelioration of clinical score over the monotherapy with MSCs-NPs. This result also indicates that MSCs-NPs confer all regenerative and immunomodulatory functions of MSCs and accordingly, combined therapy does not provide any benefits over MSCs-NPs therapy.
In conclusion, our result showed that both MSCs and MSCs-NPs can attenuate the EAE severity by suppression of pathogenic Th1 and Th17 cells through the production of PGE2 and IL-10 or may be involved in the disease amelioration by differentiation to neural cells. In addition, MSCs-NPs can be considered as a promising candidate for the treatment of animal model of MS since on one hand in vitro production of MSCs-NPs is not accompanied with difficulties arising from separation of SVZ-derived neural stem cells and on the other hand, unlike MSCs, MSCs-NPs have no risk of unwanted tissue differentiation. Moreover, our results showed that MSCs-NPs not only modulate the pathogenic Th1- and Th17-mediated immune responses stronger, but also after intravenous administration attenuate the EAE more effectively than MSCs.
Acknowledgements
The authors acknowledge staff in the Autoimmune Diseases Research Centre in particular Mrs. Taki for their help to accomplish the goals of the present study. The authors are also grateful to Ms. Yasamin Kamali Sarvestani for thorough English editing and correction of grammatical mistakes. This study was financially supported by the Shiraz University of Medical Sciences and Iranian Molecular Medicine Network (Grant Numbers: 5147, 6022, and 6152). This paper was extracted from M.Sc. theses of F. Nasri for fulfilling her M.Sc. degrees.
References
1. Park TY, Park SD, Cho JY, Moon JS, Kim NY, Park K, Seong RH, Lee SW, Morio T, Bothwell AL, Lee SK. ROR γt-specific transcriptional interactomic inhibition suppresses autoimmunity associated with TH17 cells. Proc Natl Acad Sci U S A. 2014; 111:18673–18678. DOI: 10.1073/pnas.1413687112. PMID: 25527718. PMCID: 4284575.

2. Yang C, He D, Yin C, Tan J. Inhibition of interferon regulatory factor 4 suppresses Th1 and Th17 cell differentiation and ameliorates experimental autoimmune encephalomyelitis. Scand J Immunol. 2015; 82:345–351. DOI: 10.1111/sji.12334. PMID: 26110284.

3. Grifka-Walk HM, Giles DA, Segal BM. IL-12-polarized Th1 cells produce GM-CSF and induce EAE independent of IL-23. Eur J Immunol. 2015; 45:2780–2786. DOI: 10.1002/eji.201545800. PMID: 26220255. PMCID: 5352159.

4. Yeh WI, McWilliams IL, Harrington LE. Autoreactive Tbet-positive CD4 T cells develop independent of classic Th1 cytokine signaling during experimental autoimmune encephalomyelitis. J Immunol. 2011; 187:4998–5006. DOI: 10.4049/jimmunol.1100031. PMID: 21984703. PMCID: 3709433.

5. Liu SP, Fu RH, Huang SJ, Huang YC, Chen SY, Chang CH, Liu CH, Tsai CH, Shyu WC, Lin SZ. Stem cell applications in regenerative medicine for neurological disorders. Cell Transplant. 2013; 22:631–637. DOI: 10.3727/096368912X655145.

6. Payne NL, Sun G, McDonald C, Moussa L, Emerson-Webber A, Loisel-Meyer S, Medin JA, Siatskas C, Bernard CC. Human adipose-derived mesenchymal stem cells engineered to secrete IL-10 inhibit APC function and limit CNS autoimmunity. Brain Behav Immun. 2013; 30:103–114. DOI: 10.1016/j.bbi.2013.01.079. PMID: 23369732.

7. Hermann A, Gastl R, Liebau S, Popa MO, Fiedler J, Boehm BO, Maisel M, Lerche H, Schwarz J, Brenner R, Storch A. Efficient generation of neural stem cell-like cells from adult human bone marrow stromal cells. J Cell Sci. 2004; 117:4411–4422. DOI: 10.1242/jcs.01307. PMID: 15304527.

8. Kabos P, Ehtesham M, Kabosova A, Black KL, Yu JS. Generation of neural progenitor cells from whole adult bone marrow. Exp Neurol. 2002; 178:288–293. DOI: 10.1006/exnr.2002.8039. PMID: 12504887.

9. Constantin G, Marconi S, Rossi B, Angiari S, Calderan L, Anghileri E, Gini B, Bach SD, Martinello M, Bifari F, Galiè M, Turano E, Budui S, Sbarbati A, Krampera M, Bonetti B. Adipose-derived mesenchymal stem cells ameliorate chronic experimental autoimmune encephalomyelitis. Stem Cells. 2009; 27:2624–2635. DOI: 10.1002/stem.194. PMID: 19676124.

10. Zhu J, Zhang J, Li Q, Du Y, Qiao B, Hu X. Transplanting of mesenchymal stem cells may affect proliferation and function of CD4(+)T cells in experimental autoimmune encephalomyelitis. Exp Clin Transplant. 2012; 10:492–500. DOI: 10.6002/ect.2011.0197. PMID: 22817386.

11. Bai L, Lennon DP, Eaton V, Maier K, Caplan AI, Miller SD, Miller RH. Human bone marrow-derived mesenchymal stem cells induce Th2-polarized immune response and promote endogenous repair in animal models of multiple sclerosis. Glia. 2009; 57:1192–1203. DOI: 10.1002/glia.20841. PMID: 19191336. PMCID: 2706928.

12. Rafei M, Birman E, Forner K, Galipeau J. Allogeneic mesenchymal stem cells for treatment of experimental autoimmune encephalomyelitis. Mol Ther. 2009; 17:1799–1803. DOI: 10.1038/mt.2009.157. PMID: 19602999. PMCID: 2835011.

13. Kassis I, Petrou P, Halimi M, Karussis D. Mesenchymal stem cells (MSC) derived from mice with experimental autoimmune encephalomyelitis (EAE) suppress EAE and have similar biological properties with MSC from healthy donors. Immunol Lett. 2013; 154:70–76. DOI: 10.1016/j.imlet.2013.06.002. PMID: 23994102.

14. Zappia E, Casazza S, Pedemonte E, Benvenuto F, Bonanni I, Gerdoni E, Giunti D, Ceravolo A, Cazzanti F, Frassoni F, Mancardi G, Uccelli A. Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood. 2005; 106:1755–1761. DOI: 10.1182/blood-2005-04-1496. PMID: 15905186.

15. Pluchino S, Quattrini A, Brambilla E, Gritti A, Salani G, Dina G, Galli R, Del Carro U, Amadio S, Bergami A, Furlan R, Comi G, Vescovi AL, Martino G. Injection of adult neurospheres induces recovery in a chronic model of multiple sclerosis. Nature. 2003; 422:688–694. DOI: 10.1038/nature01552.

16. Pluchino S, Zanotti L, Rossi B, Brambilla E, Ottoboni L, Salani G, Martinello M, Cattalini A, Bergami A, Furlan R, Comi G, Constantin G, Martino G. Neurosphere-derived multipotent precursors promote neuroprotection by an immunomodulatory mechanism. Nature. 2005; 436:266–271. DOI: 10.1038/nature03889. PMID: 16015332.

17. Yang J, Yan Y, Ciric B, Yu S, Guan Y, Xu H, Rostami A, Zhang GX. Evaluation of bone marrow- and brain-derived neural stem cells in therapy of central nervous system autoimmunity. Am J Pathol. 2010; 177:1989–2001. DOI: 10.2353/ajpath.2010.091203. PMID: 20724590. PMCID: 2947293.

18. Einstein O, Grigoriadis N, Mizrachi-Kol R, Reinhartz E, Polyzoidou E, Lavon I, Milonas I, Karussis D, Abramsky O, Ben-Hur T. Transplanted neural precursor cells reduce brain inflammation to attenuate chronic experimental autoimmune encephalomyelitis. Exp Neurol. 2006; 198:275–284. DOI: 10.1016/j.expneurol.2005.11.007. PMID: 16472805.

19. Harris VK, Yan QJ, Vyshkina T, Sahabi S, Liu X, Sadiq SA. Clinical and pathological effects of intrathecal injection of mesenchymal stem cell-derived neural progenitors in an experimental model of multiple sclerosis. J Neurol Sci. 2012; 313:167–177. DOI: 10.1016/j.jns.2011.08.036.

20. Shiri EH, Mehrjardi NZ, Tavallaei M, Ashtiani SK, Baharvand H. Neurogenic and mitotic effects of dehydroepiandrosterone on neuronal-competent marrow mesenchymal stem cells. Int J Dev Biol. 2009; 53:579–584. DOI: 10.1387/ijdb.082623eh. PMID: 19378256.

21. Grigoriadis N, Lourbopoulos A, Lagoudaki R, Frischer JM, Polyzoidou E, Touloumi O, Simeonidou C, Deretzi G, Kountouras J, Spandou E, Kotta K, Karkavelas G, Tascos N, Lassmann H. Variable behavior and complications of autologous bone marrow mesenchymal stem cells transplanted in experimental autoimmune encephalomyelitis. Exp Neurol. 2011; 230:78–89. DOI: 10.1016/j.expneurol.2011.02.021. PMID: 21440544.

22. Harris VK, Faroqui R, Vyshkina T, Sadiq SA. Characterization of autologous mesenchymal stem cell-derived neural progenitors as a feasible source of stem cells for central nervous system applications in multiple sclerosis. Stem Cells Transl Med. 2012; 1:536–547. DOI: 10.5966/sctm.2012-0015. PMID: 23197858. PMCID: 3659719.

23. Hsu WT, Lin CH, Chiang BL, Jui HY, Wu KK, Lee CM. Prostaglandin E2 potentiates mesenchymal stem cell-induced IL-10+IFN-γ+CD4+ regulatory T cells to control transplant arteriosclerosis. J Immunol. 2013; 190:2372–2380. DOI: 10.4049/jimmunol.1202996. PMID: 23359497.

24. Duffy MM, Pindjakova J, Hanley SA, McCarthy C, Weidhofer GA, Sweeney EM, English K, Shaw G, Murphy JM, Barry FP, Mahon BP, Belton O, Ceredig R, Griffin MD. Mesenchymal stem cell inhibition of T-helper 17 cell-differentiation is triggered by cell-cell contact and mediated by prostaglandin E2 via the EP4 receptor. Eur J Immunol. 2011; 41:2840–2851. DOI: 10.1002/eji.201141499. PMID: 21710489.

25. Wang L, Shi J, van Ginkel FW, Lan L, Niemeyer G, Martin DR, Snyder EY, Cox NR. Neural stem/progenitor cells modulate immune responses by suppressing T lymphocytes with nitric oxide and prostaglandin E2. Exp Neurol. 2009; 216:177–183. DOI: 10.1016/j.expneurol.2008.11.017.

26. Matysiak M, Orlowski W, Fortak-Michalska M, Jurewicz A, Selmaj K. Immunoregulatory function of bone marrow mesenchymal stem cells in EAE depends on their differentiation state and secretion of PGE2. J Neuroimmunol. 2011; 233:106–111. DOI: 10.1016/j.jneuroim.2010.12.004. PMID: 21354631.

27. Yang J, Jiang Z, Fitzgerald DC, Ma C, Yu S, Li H, Zhao Z, Li Y, Ciric B, Curtis M, Rostami A, Zhang GX. Adult neural stem cells expressing IL-10 confer potent immunomodulation and remyelination in experimental autoimmune encephalitis. J Clin Invest. 2009; 119:3678–3691. DOI: 10.1172/JCI37914. PMID: 19884657. PMCID: 2786785.

Fig. 1
MSCs and MSCs-NPs characterization. (A) Mice bone marrow derived MSCs at passage 6 (10× or 20×magnification) and MSCs-NPs at the fourth day post-induction (10×magnification) are compared for their morphology and capability in mesodermal differentiation (osteogenesis and adipogenesis). Neurosphere morphology and reduced ability in osteogenesis and adipogenesis was found in MSCs-NPs (lower row). (B) Cell surface phenotyping of MSCs and MSCs-NPs by flow cytometry. Isotype controls are represented by gray filled histograms.
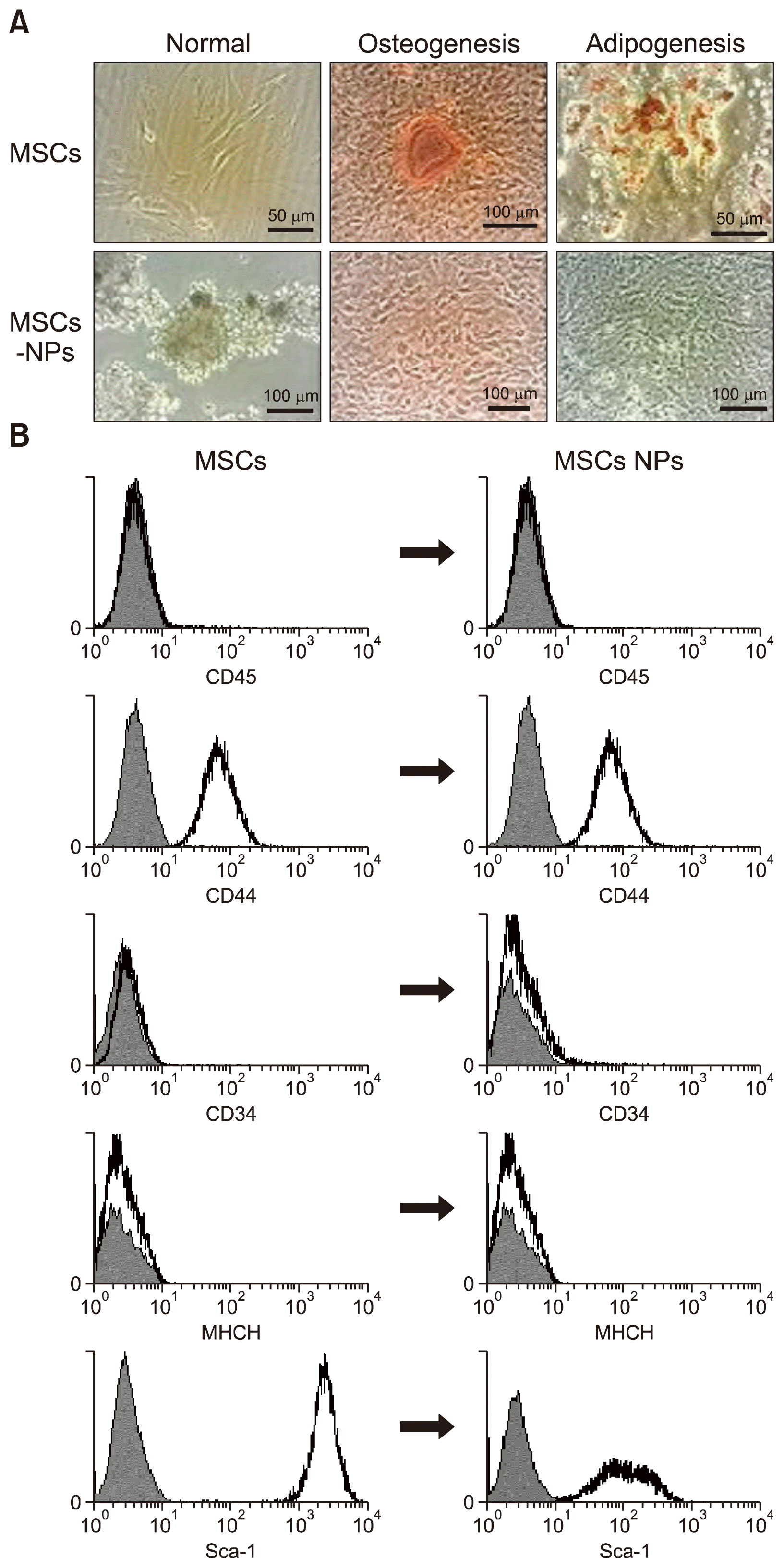
Fig. 2
Comparison of MSCs and MSCs-NPs for secretion of PGE2 (A) or IL-10 (B) after 24 hours or 48 hours of culture. Bars represent mean±SD from three experiments. *p<0.05, **p<0.01.
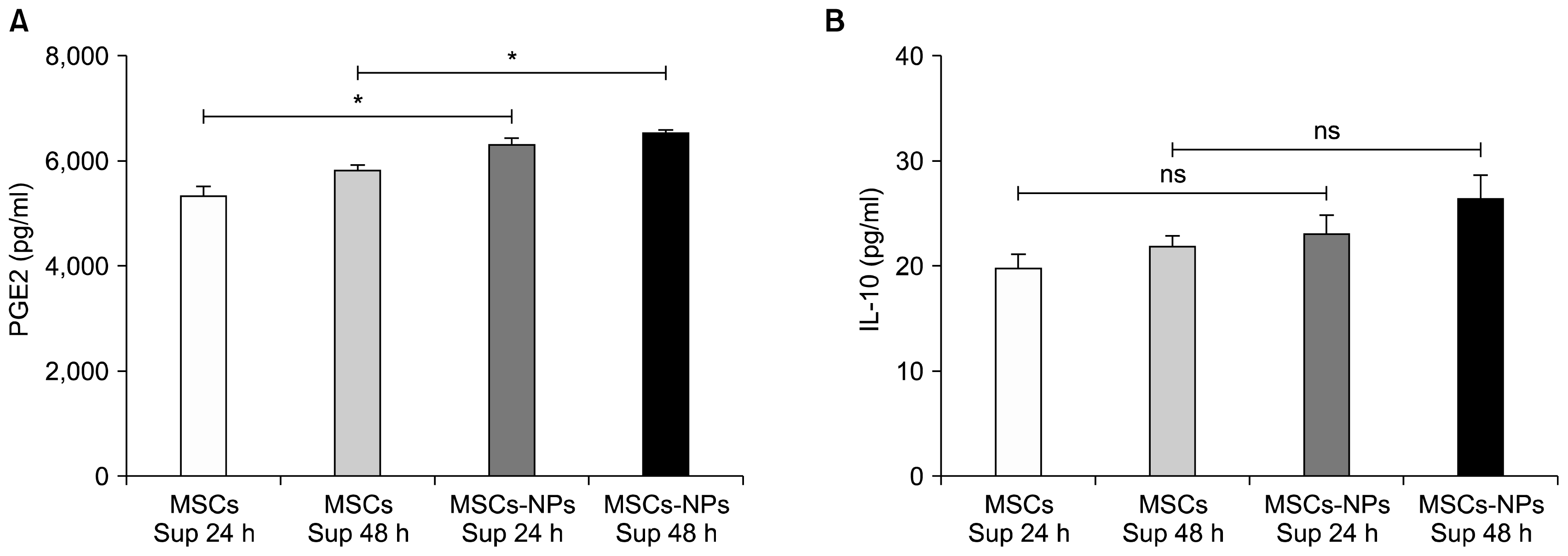
Fig. 3
Three times intravenous injection of MSCs, MSCs-NPs and co-injection of MSCs+MSCs-derived NPs significantly decreased EAE clinical scores in treated mice compared to control mice. Error bars represent SEM from day 12 onwards. Arrows indicate times of injection. ***p<0.001.
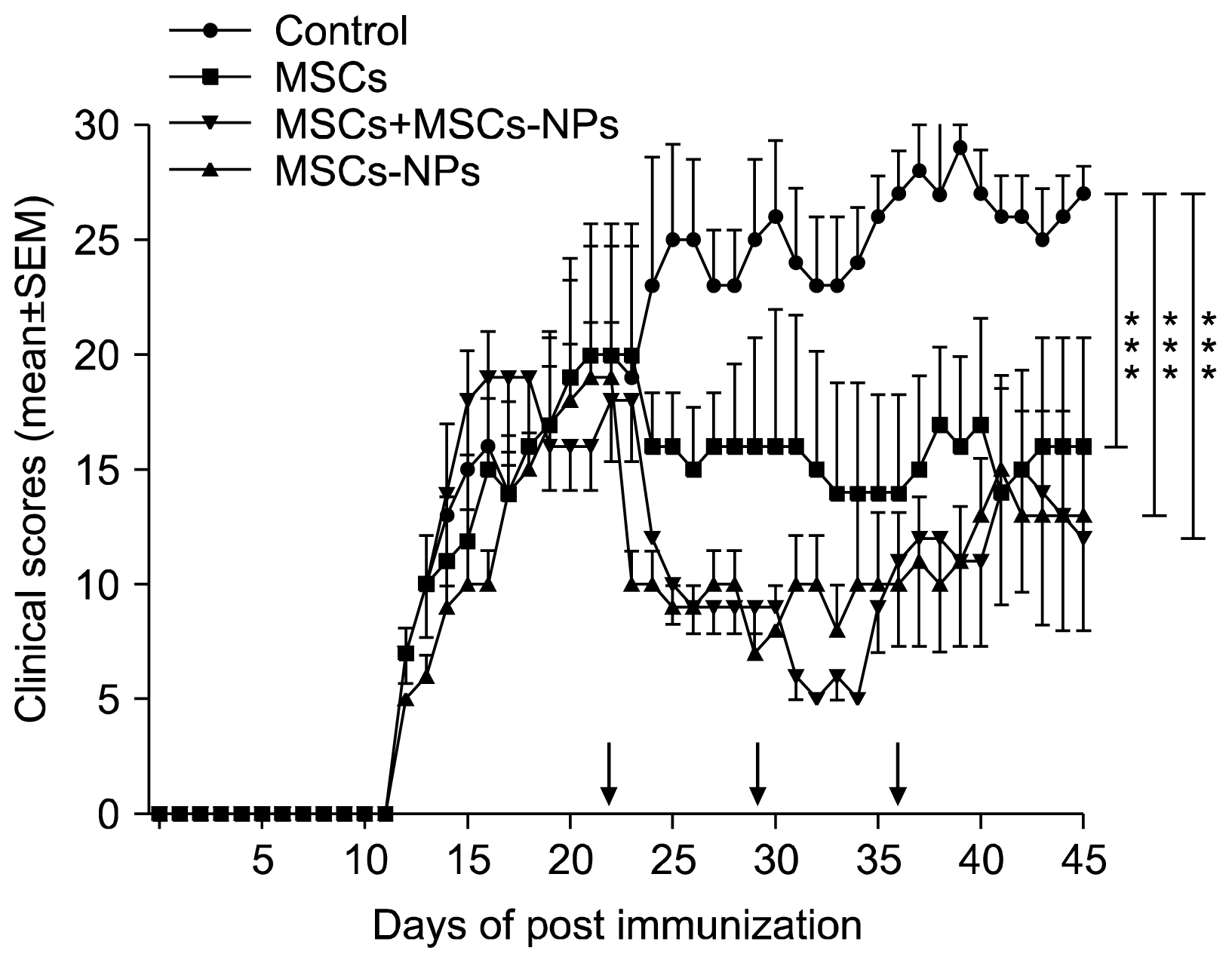
Fig. 4
Ex vivo effect of MSCs and MSCs-derived NPs on proliferation of MOG35–55-specific splenocytes in [3H]-Thymidine incorporation. *≤0.05, **≤0.01 and NS: nonsignificant.
Fig. 5
Cytokine levels in the supernatant of MOG35–55-stimulated splenocytes. (A, B) IFN-γ and IL-17 levels were significantly decreased in the supernatant of activated splenecytes derived from treated mice compared to control group. (C) IL-10 showed significant increases in the supernatant of activated splenecytes derived from treated animals. Bars represent mean±SD from five mice/condition. *p<0.05, **p<0.02, ***p<0.001.
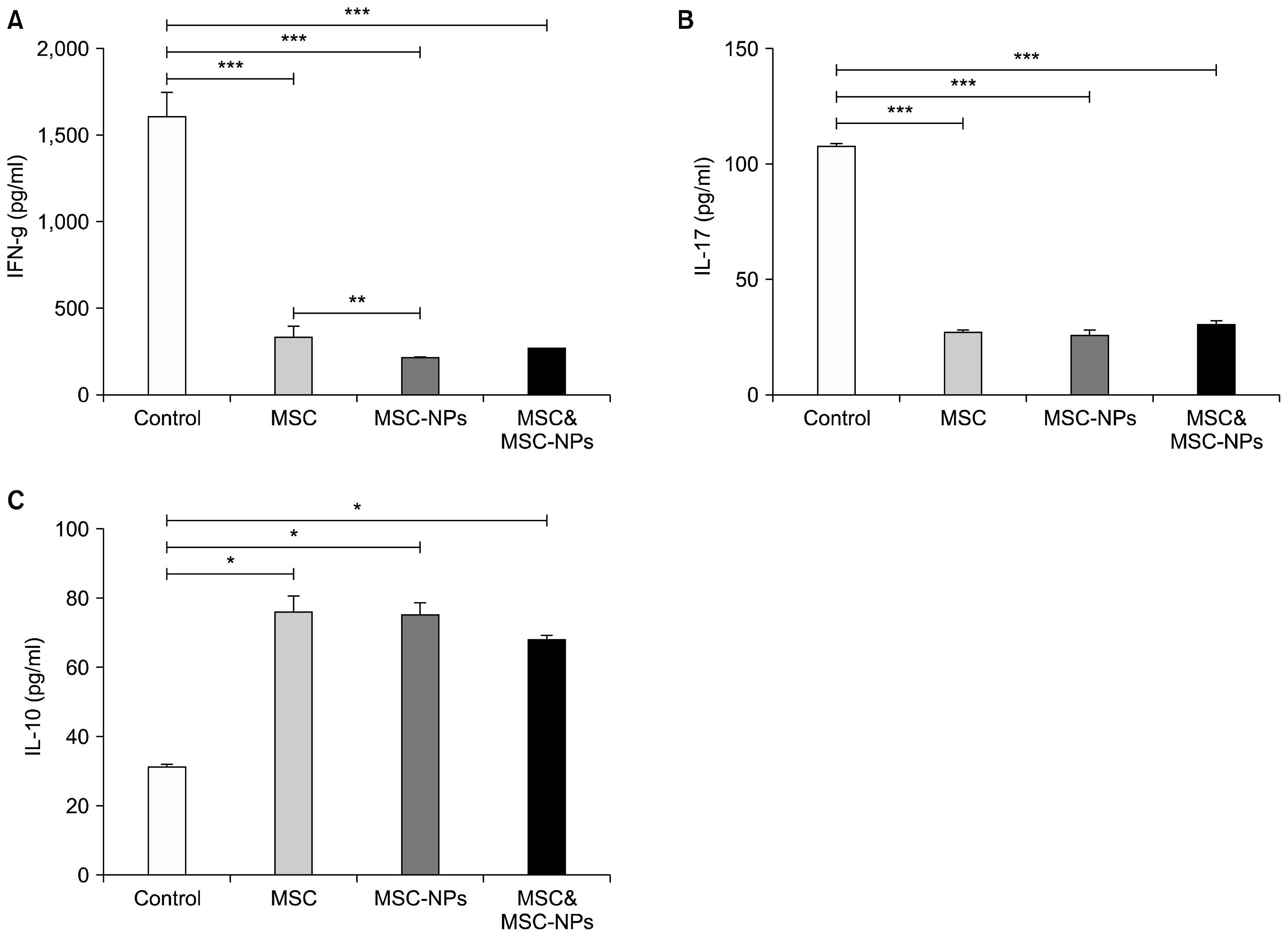
Fig. 6
Cytokine levels in the sera of treated and untreated mice. (A) IFN-γ and (B) IL-17 levels were significantly decreased in the sera derived from treated mice while (C) IL-10 levels showed significant increase in sera of treated animals in comparison to untreated ones. Bars represent mean±SD from five mice/condition. *p<0.05, **p=0.015, ***p=0.01.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download