Abstract
Hypoxic culture is widely recognized as a method to efficiently expand human mesenchymal stem cells (MSCs) without loss of stem cell properties. However, the molecular basis of how hypoxia priming benefits MSC expansion remains unclear. We report that hypoxic priming markedly extends the cell cycle lifespan rather than augmenting the multipotency of MSC differentiation lineage. Hypoxic priming does not affect to chromosome damage but significantly attenuates the susceptibility of chromosome damage. Our results provide important evidence that multipotency of human MSCs by hypoxic priming is determined by cell cycle lifespan.
Human mesenchymal stem cells (MSCs) are able to self-renew or differentiate to other lineages (1–3), and these cells have been isolated from different tissues such as brain, liver, bone marrow, adipose tissue, foetal tissues, umbilical cord (UC), and placenta (4–7). Various types of human mesenchymal stem cells (MSCs) reside in the hypoic microenvironment, which seems to be conductive to stem cell longevity and the physiological niches (8, 9). Hypoxia has a strong effect on several aspects of cell biology such as metabolism, angiogenesis, innate immunity and stemness induction, and hypoxic is also essential for the self-renewal and the maintenance of multipotency of human MSCs and hematopoietic stem cells (HSCs) (9–11). In recent years, many studies support that the hypoxic culture of human MSCs inhibits cellular senescence, maintains MSCs properties, augments the differentiation capacity, and enhances their tissue regenerative potential, indicating that hypoxia increases the lifespan and the differentiation potential of MSCs (10–13).
MSCs-based cell therapy is a potential therapeutic approach for the treatment of various diseases, including stroke and myocardial infarction (14–17), traumatic brain injuries (18), diabetes mellitus (19), inflammatory bowel disease (20), and acute kidney (21, 22) and liver injuries (23–25). However, the large amount of engrafted MSCs decreased dramatically after transplantation due to immune rejection and toxic microenvironments. Thus, adopting appropriate priming strategies provide an effective way of promoting survival and avoiding immune rejection (14). In addition, recent studies have also shown that hypoxic priming induces the expression of pro-survival markers (26), and growth factors involved in cell proliferation, anti-apoptosis and angiogenesis (27) in MSCs. However, it is not yet clear whether the benefit of hypoxic priming is the expansion, cellular longevity, or multi-potent differentiation capacity of human MSCs. In this study, we found that hypoxic priming extends cell cycle lifespan but reduce genetic damage susceptibility, and thus maintain the multipotency of MSCs during differentiation lineage.
Human umbilical cord blood derived mesenchymal stem cells (hUCB-MSCs; PromoCell) were grown in Dulbecco’s Modified Eagle’s Medium (DMEM; Hyclone) containing 10% fetal bovine serum (FBS; GIBCO) and 1% Penicillin/Streptomycin antibiotics at 37°C in a 5% CO2 incubator with 21% O2 (normoxia) or 1% O2 (hypoxia).
For assessment of the potential of cell proliferation, MSCs were trypsinized and washed once with phosphate buffered saline (PBS). CFSE (Invitrogen, 10 mM in PBS) was added to the cells and incubated at 37°C in the dark for 15 min. An equal volume of serum containing growth medium was added for quench the CFSE reaction. Cells were again incubated at 37°C in the dark for 5 min. CFSE-labeled MSCs were washed twice with growth medium and seeded in a culture plate.
Cells were fixed with 4% formaldehyde for 10 min and incubated overnight at 37°C with 1 mg/ml X-gal (5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside), 5 mM K3Fe(CN)6, 5 mM K4Fe(CN)6, 150 mM NaCl, and 2 mM MgCl2 in 40 mM citric acid/sodium phosphate pH 6.0.
hUCB-MSCs were grown on coverslips (1×104 cells) for 2 days. Cells were fixed with 4% formaldehyde in PBS for 10 min and rinsed three times with a wash solution of (0.1% Triton X-100 in PBS. Cells were blocked with 3% skim milk/wash solution for 30 min and incubated with primary antibody (1:200) and secondary antibody (1:200) for 1 hr.
Cells were incubated with colcemid (final concentration 100 ng/ml) for cell cycle arrest around metaphase. Mitotic cells were then collected by gentle pipetting, washed with PBS twice, counted, and suspended in a hypotonic solution (0.8% sodium citrate). After 15 min incubation at room temperature, swelled cells were centrifuged (1,000 rpm, 4 min, 4°C). Most of the supernatant was aspirated and the pellet was gently in the remaining approximately 500 μl of supernatant. Carnoy’s fixative solution (75% methanol, 25% acetic acid) was slowly added and incubated at room temperature for 10 min. The fixation was repeated 3~4 more times. The suspended cells were dropped onto cold wet slides and allowed to dry at room temperature. The slides were mounted and examined by fluorescence microscopy (Carl Zeiss).
All data were presented as means±standard errors of means (SEMs). Statistical analyses were performed using the Student t test.
Human UCB-MSCs were evaluated using surface marker detection at passage 5 (P5) to confirm the effect of oxygen concentration (hypoxia and normoxia) on MSC characterization. hUCB-MSCs at 80% confluence were harvested and suspended in FACS buffer (1×107 cells/ml). Antibody was then added to each samples: Anti-CD90 fluorescein isothiocyanate (FITC), Anti-CD44 phycoerythrin (PE), Anti-CD105-PerCP-Cy5.5, Anti-CD73-allophycocyanin (APC), MSC negative antibodies set (Anti-CD34/CD11b/CD19/CD45/HLA-DR-PE), positive isotype cocktail (mIgG-FITC, mIgG-PerCP-Cy5.5, mIgG-APC), negative isotype cocktail (mIgG1-PE, mIgG2a-PE) (562245, BD Biosciences) followed by incubation at 4°C for 30 min. For cell cycle analysis using propidium iodide, cells were fixed with cold 70% ethanol overnight at −20°C. They were then washed once with PBS and incubated in PBS containing 50 μg/ml propidium iodide and 1 mg/ml RNase A for 30 min at room temperature. After staining, cells were washing with PBS and measured by flow cytometry. Antibodies listed above are purchased from BD Biosciences.
To delineate the key mechanistic benefit of hypoxic priming, human MSCs were isolated from human umbilical cord blood (UCB) by adhesion to tissue culture-coated plates in complete culture medium as previously described (14). Culture passaging generated a homogenous population of UCB-MSCs (hereafter MSCs) that were used to measure proliferation rate, multi-potency, and senescence (Fig. 1A~1C). Human MSCs were cultured under normoxic (21% oxygen) or hypoxic (1% oxygen) conditions. When the culture became nearly confluent, the cells were trypsinized and subcultivated. As expected, hypoxia significantly accelerated the proliferation rate and extended the life span of human MSCs compared to normoxic cultures (Fig. 1B and 1C). Similar results were obtained with MSCs from two different donors (data not shown).
Under the hypoxic condition, late passaged MSCs continuously proliferated and maintained a similar morphology as early passaged cells (Fig. 1B and 1C). However, most of late passaged MSCs (passage 8 and later) cultured in the normoxic condition almost completely lost their proliferation capacity and adopted a flat, enlarged shape and ceased proliferation at subconfluent densities, thus manifesting characteristics of senescence (Fig. 2A), indicating that hypoxia extended the cell proliferation life span of MSCs. In support of this notion, cytochemical senescent phenotype analysis involving staining of the senescence-associated β-galactosidase (SA β-Gal) showed that cells in normoxic cultures of late passaged MSCs mostly displayed SA β-Gal activity, while hypoxic cultures showed significantly less amounts of SA β-Gal staining (Figs. 1D and 2A). In addition, the percentage of γH2AX-positive cells (as a marker for DNA double strand breaks) was markedly decreased compared to normoxic culture (Fig. 2B). Further metaphase chromosome spreading assays also showed significantly less population of MSCs with chromosome damage (Fig. 2B and 2C). Together, these results indicate that hypoxia extends cell cycle lifespan but reduces chromosome damage susceptibility.
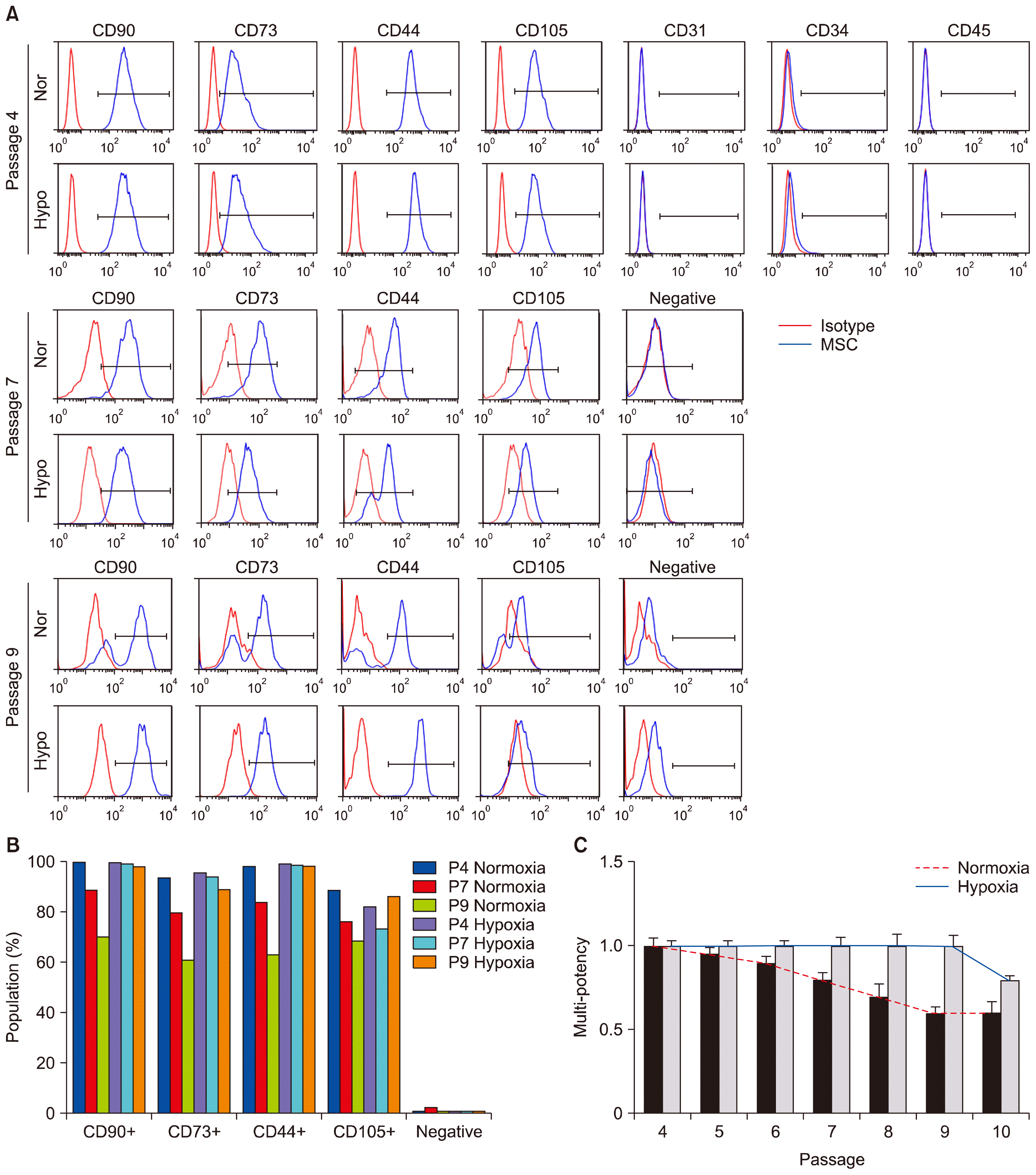
Multi-potency of MSCs by normoxic and hypoxic priming was compared by the examination of the surface expression of multi-potent MSC antigenic markers. Positive markers included CD44, CD73, CD90, and CD105. Negative markers included CD34, CD45, CD11b, and/or CD19 (Fig. 3A and 3B). Unexpectedly, early and middle passage MSCs (passages 4 and 7, respectively) grown under normoxic or hypoxic condition showed very similar levels of surface CD marker profiles; cells were consistently positive for CD44, CD73, CD90, and CD105, and negative for CD34, CD45, CD11b, and/or CD19 (Fig. 3B and 3C), indicating that hypoxic priming does not augment the differentiation capacity of MSCs at early and intermediate passages. However, although hypoxic conditioning drastically extended the cell cycle lifespan and delayed senescence in late passaged MSCs, comparison of the multi-potency by measuring the same number of cells revealed that normoxic primed late passaged MSCs still retained the significant capacity of multi-potency compared to hypoxic conditioning. These results raise the important notion that hypoxic priming is an efficient tool for expanding the actively proliferating pool of MSCs, but not for augmentation of their multi-potency. The multi-potency of MSCs may be able to be determined by measuring their proliferating potential and/or strength of cell cycle lifespan, but not by the expressions of pre-existing MSC markers.
Acknowledgments
This study was supported by a National Research Foundation grant funded by the Korean government (MEST) (2017R1A2B3006776 and 2014R1A2A1A10050775).
References
1. Anderson DJ, Gage FH, Weissman IL. Can stem cells cross lineage boundaries? Nat Med. 2001; 7:393–395. DOI: 10.1038/86439. PMID: 11283651.

2. Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M, Du J, Aldrich S, Lisberg A, Low WC, Largaespada DA, Verfaillie CM. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002; 418:41–49. DOI: 10.1038/nature00870. PMID: 12077603.

3. Mohammadian M, Shamsasenjan K, Lotfi Nezhad P, Talebi M, Jahedi M, Nickkhah H, Minayi N, Movassagh Pour A. Mesenchymal stem cells: new aspect in cell-based regenerative therapy. Adv Pharm Bull. 2013; 3:433–437. PMID: 24312873. PMCID: 3848236.
4. Momin EN, Mohyeldin A, Zaidi HA, Vela G, Quiñones-Hinojosa A. Mesenchymal stem cells: new approaches for the treatment of neurological diseases. Curr Stem Cell Res Ther. 2010; 5:326–344. DOI: 10.2174/157488810793351631. PMID: 20528757.

5. da Silva Meirelles L, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci. 2006; 119:2204–2213. DOI: 10.1242/jcs.02932. PMID: 16684817.

6. Romanov YA, Svintsitskaya VA, Smirnov VN. Searching for alternative sources of postnatal human mesenchymal stem cells: candidate MSC-like cells from umbilical cord. Stem Cells. 2003; 21:105–110. DOI: 10.1634/stemcells.21-1-105. PMID: 12529557.

7. Fukuchi Y, Nakajima H, Sugiyama D, Hirose I, Kitamura T, Tsuji K. Human placenta-derived cells have mesenchymal stem/progenitor cell potential. Stem Cells. 2004; 22:649–658. DOI: 10.1634/stemcells.22-5-649. PMID: 15342929.

8. Davy P, Allsopp R. Hypoxia: are stem cells in it for the long run? Cell Cycle. 2011; 10:206–211. DOI: 10.4161/cc.10.2.14535. PMID: 21239881.

9. Tsai CC, Chen YJ, Yew TL, Chen LL, Wang JY, Chiu CH, Hung SC. Hypoxia inhibits senescence and maintains mesenchymal stem cell properties through down-regulation of E2A-p21 by HIF-TWIST. Blood. 2011; 117:459–469. DOI: 10.1182/blood-2010-05-287508.

10. Rosová I, Dao M, Capoccia B, Link D, Nolta JA. Hypoxic preconditioning results in increased motility and improved therapeutic potential of human mesenchymal stem cells. Stem Cells. 2008; 26:2173–2182. DOI: 10.1634/stemcells.2007-1104. PMID: 18511601. PMCID: 3017477.

11. Suda T, Takubo K, Semenza GL. Metabolic regulation of hematopoietic stem cells in the hypoxic niche. Cell Stem Cell. 2011; 9:298–310. DOI: 10.1016/j.stem.2011.09.010. PMID: 21982230.

12. Mathieu J, Zhou W, Xing Y, Sperber H, Ferreccio A, Agoston Z, Kuppusamy KT, Moon RT, Ruohola-Baker H. Hypoxia-inducible factors have distinct and stage-specific roles during reprogramming of human cells to pluripotency. Cell Stem Cell. 2014; 14:592–605. DOI: 10.1016/j.stem.2014.02.012. PMID: 24656769. PMCID: 4028142.

13. Zhang J, Nuebel E, Daley GQ, Koehler CM, Teitell MA. Metabolic regulation in pluripotent stem cells during reprogramming and self-renewal. Cell Stem Cell. 2012; 11:589–595. DOI: 10.1016/j.stem.2012.10.005. PMID: 23122286. PMCID: 3492890.

14. Theus MH, Wei L, Cui L, Francis K, Hu X, Keogh C, Yu SP. In vitro hypoxic preconditioning of embryonic stem cells as a strategy of promoting cell survival and functional benefits after transplantation into the ischemic rat brain. Exp Neurol. 2008; 210:656–670. DOI: 10.1016/j.expneurol.2007.12.020. PMID: 18279854.

15. Francis KR, Wei L. Human embryonic stem cell neural differentiation and enhanced cell survival promoted by hypoxic preconditioning. Cell Death Dis. 2010; 1:e22. DOI: 10.1038/cddis.2009.22.

16. Jaussaud J, Biais M, Calderon J, Chevaleyre J, Duchez P, Ivanovic Z, Couffinhal T, Barandon L. Hypoxia-preconditioned mesenchymal stromal cells improve cardiac function in a swine model of chronic myocardial ischaemia. Eur J Cardiothorac Surg. 2013; 43:1050–1057. DOI: 10.1093/ejcts/ezs549.

17. Muscari C, Giordano E, Bonafè F, Govoni M, Pasini A, Guarnieri C. Priming adult stem cells by hypoxic pretreatments for applications in regenerative medicine. J Biomed Sci. 2013; 20:63. DOI: 10.1186/1423-0127-20-63. PMID: 23985033. PMCID: 3765890.

18. Chang CP, Chio CC, Cheong CU, Chao CM, Cheng BC, Lin MT. Hypoxic preconditioning enhances the therapeutic potential of the secretome from cultured human mesenchymal stem cells in experimental traumatic brain injury. Clin Sci (Lond). 2013; 124:165–176. DOI: 10.1042/CS20120226.

19. Mottaghi S, Larijani B, Sharifi AM. Apelin 13: a novel approach to enhance efficacy of hypoxic preconditioned mesenchymal stem cells for cell therapy of diabetes. Med Hypotheses. 2012; 79:717–718. DOI: 10.1016/j.mehy.2012.08.007. PMID: 22981008.

20. Watanabe S, Arimura Y, Nagaishi K, Isshiki H, Onodera K, Nasuno M, Yamashita K, Idogawa M, Naishiro Y, Murata M, Adachi Y, Fujimiya M, Imai K, Shinomura Y. Conditioned mesenchymal stem cells produce pleiotropic gut trophic factors. J Gastroenterol. 2014; 49:270–282. DOI: 10.1007/s00535-013-0901-3.

21. Liu H, Liu S, Li Y, Wang X, Xue W, Ge G, Luo X. The role of SDF-1-CXCR4/CXCR7 axis in the therapeutic effects of hypoxia-preconditioned mesenchymal stem cells for renal ischemia/reperfusion injury. PLoS One. 2012; 7:e34608. DOI: 10.1371/journal.pone.0034608. PMID: 22511954. PMCID: 3325280.

22. Yu X, Lu C, Liu H, Rao S, Cai J, Liu S, Kriegel AJ, Greene AS, Liang M, Ding X. Hypoxic preconditioning with cobalt of bone marrow mesenchymal stem cells improves cell migration and enhances therapy for treatment of ischemic acute kidney injury. PLoS One. 2013; 8:e62703. DOI: 10.1371/journal.pone.0062703. PMID: 23671625. PMCID: 3650042.

23. Yu J, Yin S, Zhang W, Gao F, Liu Y, Chen Z, Zhang M, He J, Zheng S. Hypoxia preconditioned bone marrow mesenchymal stem cells promote liver regeneration in a rat massive hepatectomy model. Stem Cell Res Ther. 2013; 4:83. DOI: 10.1186/scrt234. PMID: 23856418. PMCID: 3854783.

24. Bonfield TL, Caplan AI. Adult mesenchymal stem cells: an innovative therapeutic for lung diseases. Discov Med. 2010; 9:337–345. PMID: 20423678.
25. Inamdar AC, Inamdar AA. Mesenchymal stem cell therapy in lung disorders: pathogenesis of lung diseases and mechanism of action of mesenchymal stem cell. Exp Lung Res. 2013; 39:315–327. DOI: 10.3109/01902148.2013.816803. PMID: 23992090.

26. Chacko SM, Ahmed S, Selvendiran K, Kuppusamy ML, Khan M, Kuppusamy P. Hypoxic preconditioning induces the expression of prosurvival and proangiogenic markers in mesenchymal stem cells. Am J Physiol Cell Physiol. 2010; 299:C1562–C1570. DOI: 10.1152/ajpcell.00221.2010. PMID: 20861473. PMCID: 3006322.

27. Ohnishi S, Yasuda T, Kitamura S, Nagaya N. Effect of hypoxia on gene expression of bone marrow-derived mesenchymal stem cells and mononuclear cells. Stem Cells. 2007; 25:1166–1177. DOI: 10.1634/stemcells.2006-0347. PMID: 17289933.

Fig. 1
Comparison of potentialities of proliferation, multipotency, and senescence between normoxic and hypoxic conditioning. (A) Phase contrast images of human umbilical cord blood-derived MSCs (MSCs) were cultured in normoxic (21% O2) or hypoxic (1% O2) conditions through multiple passages (passages 3, 5 and 7, respectively). (B) Several different passages of MSCs derived from the same source of MSCs were further cultured in normoxic (21% O2) and hypoxic (1% O2) conditions, and cell numbers were counted at designated times. (C) The graph shows the relative comparison of proliferating cell number following normoxic or hypoxic priming at several passages of MSCs culture. (D) The graph shows the relative incidence of senescence associated β-galactosidase positive MSCs. Data (mean±SEM) are representative of 3 independent experiments.
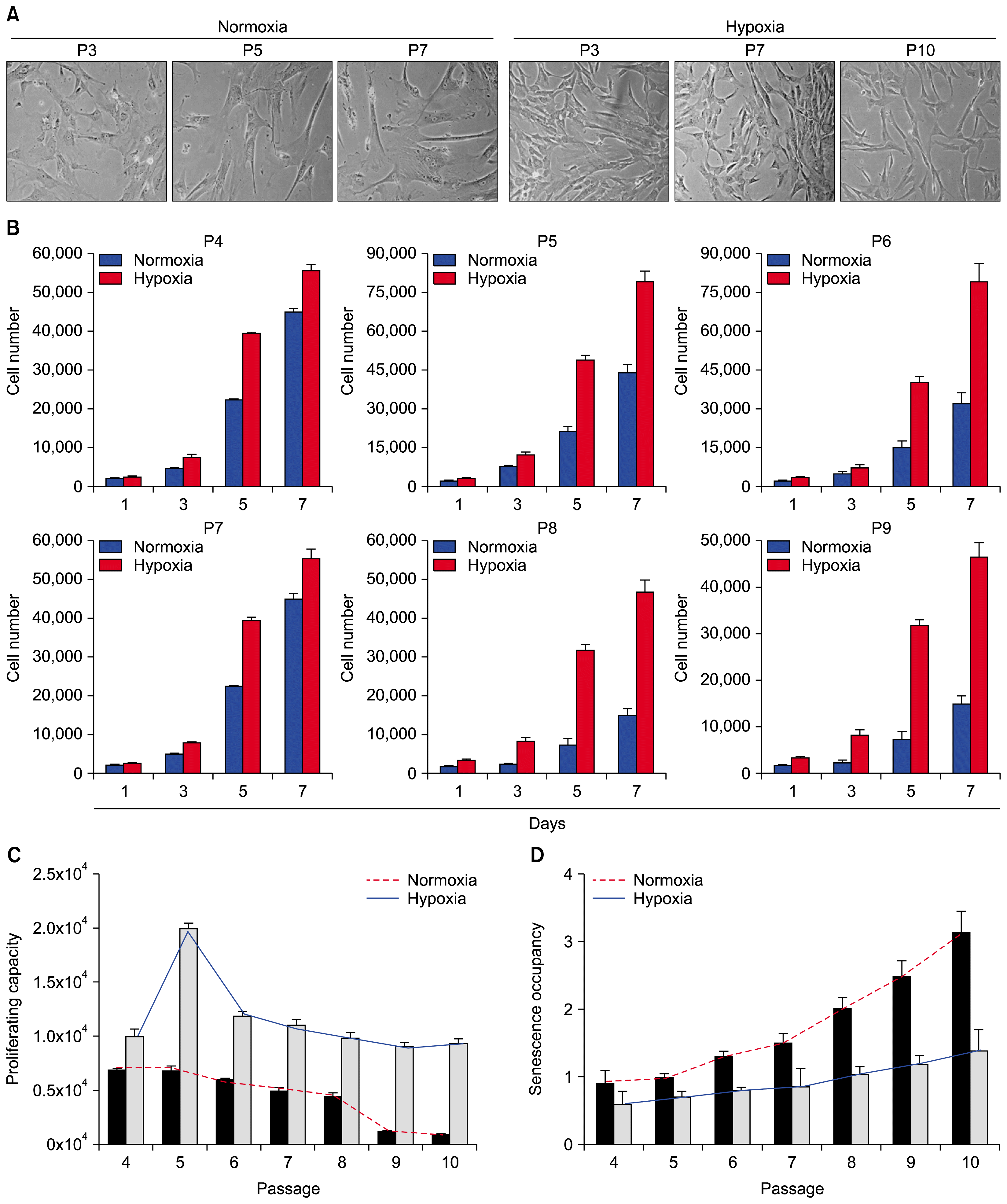
Fig. 2
Effect of hypoxic priming on the DNA and chromosome damage and senescence of MSCs. (A) Three different passages of MSCs grown in normoxic or hypoxic condition were analyzed by senescence associated β-galactosidase (SA-β-gal) staining. The graph shows the relative percentage of SA-β-gal positive cells. (B) MSCs were cultured in normoxic or hypoxic condition, or in normoxic condition in combination with 0.5 mM doxorubicin (Doxo) treatment, and stained with anti-γH2AX (a marker for DNA double-strand breaks) and DAPI for DNA. Graph shows the percentage of γH2AX-positive cells at three different passages. (C) Three different passages of MSCs were grown in normoxic or hypoxic condition, and harvested for metaphase chromosome spreading analysis. Arrows indicate the break chromosome. The graphs show the percentage of metaphase MSCs containing the break chromosome. (D) Total number of chromosome in each MSC was examined at passages 4, 8, and 12, respectively. Data (mean±SEM) are representative of 3 independent experiments.
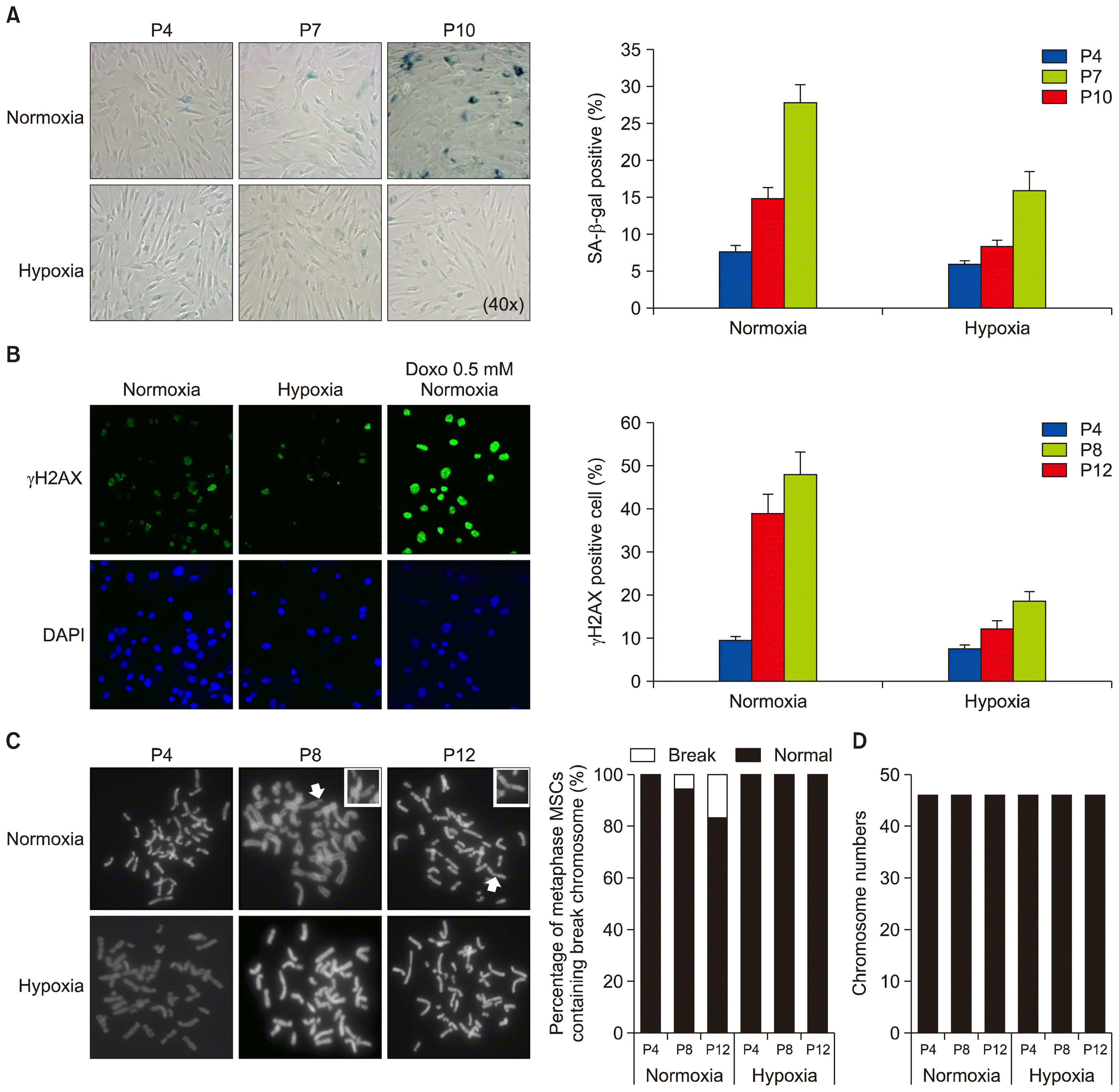
Fig. 3
Application of hypoxia to MSCs stimulates their proliferating potential and the potential of multi-lineage differentiation. (A) Multi-passaged MSCs were cultured in normoxic or hypoxic condition, labeled with antibodies against specific surface antigens, CD44, CD73, CD90, and CD105 for MSC positive markers and CD31, CD34, and CD45 for MSC negative markers, and analyzed by counting 10,000 cells at passages 4, 7, and 9, respectively. The surface antigen phenotype was characterized by FACS. Immunoglobulin isotype was used as a negative control for FACS analysis. Negative indicates the labelling of cells with CD34, CD45, and CD11b antibodies. Red-colored histograms illustrate the control immunoglobulin, and blue-colored histograms represent the staining against each specified antibodies as indicated. (B) The graph shows the positive percentages of each MSC marker expression. (C) The graph shows the potency of adipogenic differentiation rate at indicated passages. Data (mean±SEM) are representative of 3 independent experiments.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download