Discussion
Chronic mild stress model is the most widely applied model of depression. The CMS model of depression involved the presentation of a series of varied and unpredictable environmental stressors. Following such exposure, animals exhibit a persistent reduction in responsiveness to pleasurable stimuli, measured by a decrease in their consumption of 1% sucrose solution. Decrease in sucrose consumption has been advocated as a reliable behavioral measure that is associated with anhedonia (loss of appetite) of depression (
1,
6).
In the present study, SP percent decreased in depressed group confirming occurrence of anhedonia which is a basic sign of depression and so CMS successfully induced depression in the rats.
Chen et al. (
1) induced depression by CMS protocol in normal adult rats and confirmed it through several behavioral and physiological changes including decreased sucrose preference percent.
Improvement of SP in Rhodiola group was in accordance with Chen et al. (
1) who found that introduction of Rhodiola rosea extract for 3 weeks in depressed rats increased sucrose preference to the normal level.
Going parallel with the present findings is the morphometric examination which showed that 4 weeks CMS procedure caused significant decrease in animals’ body weight. Consistent with these findings, a previous study represented an obvious decrease in the body weight of CMS stressed animals (
10). The increase in Bw after Rr supplementation for 3 weeks was in accordance to Chen et al. (
1) who found that depression in rats decreased the rate of Bw increase and Rr supplementation for 3 weeks reversed it to reach the normal level.
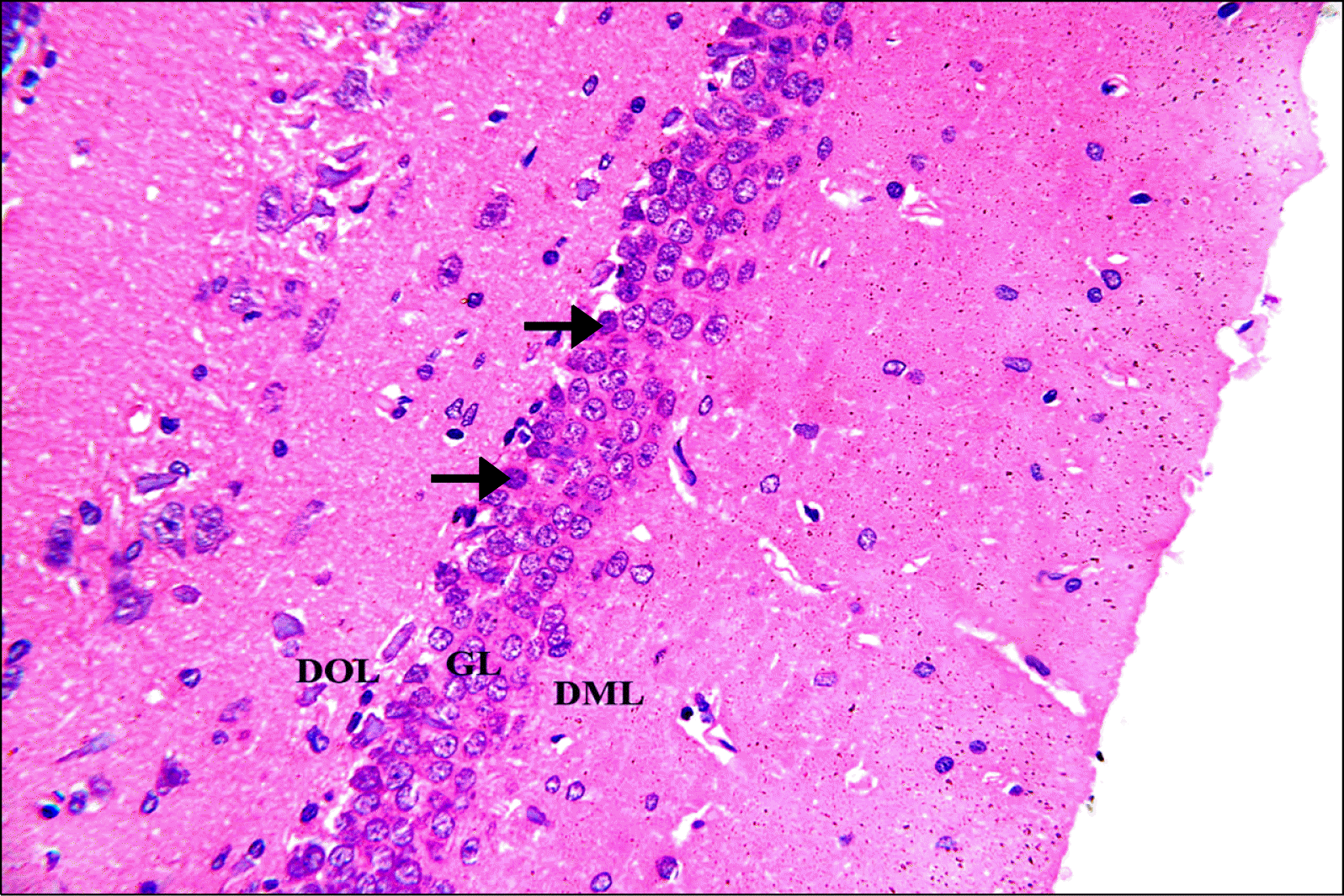
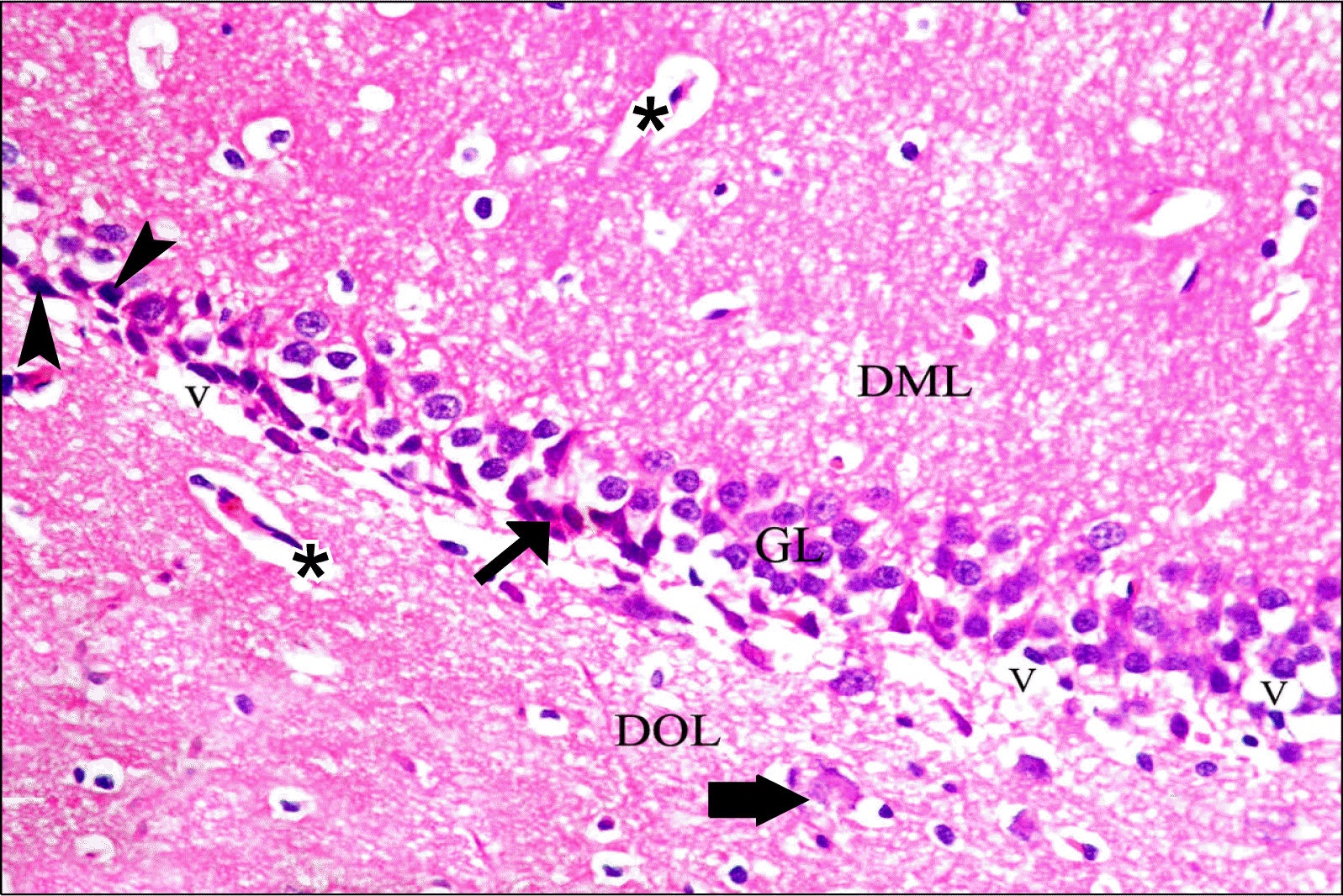
Hippocampal sections of depressed rats revealed degenerating neurons, dark neurons & many vacuolations. The appearance of neuron degeneration in the present study was similar to “eosinophilic neuron degeneration” described by Garman (
11) who mentioned that, the degenerating neurons (sometimes referred to as “red dead neurons” were characterized at the light microscopic level by cell body shrinkage, loss of Nissl substance, intensely stained eosinophilic cytoplasm, and a small/shrunken darkly stained (pyknotic) nucleus that may eventually fragment (undergo karyorrhexis).
Dark neurons might reflect an early cell injury following stress and overexcitation. Early neuronal injury may be due to early damage of cytoskeleton such as micro-tubules or microfilaments. Dark neurons appeared to be in a shrunken or contracted state which may be the result of contraction of cytoskeletal proteins such as actin (
12).
In the present study vacuolations could be detected near degenerating neurons which might be attributed to swelling or degeneration of neuronal process. Garman (
11) demonstrated that vacuolations adjacent to the degenerating neurons or those overlying molecular layer are probably swelling/degeneration of neuronal processes, swelling of astrocyte cytoplasmic processes, myelin sheath splitting or intramyelinic edema.
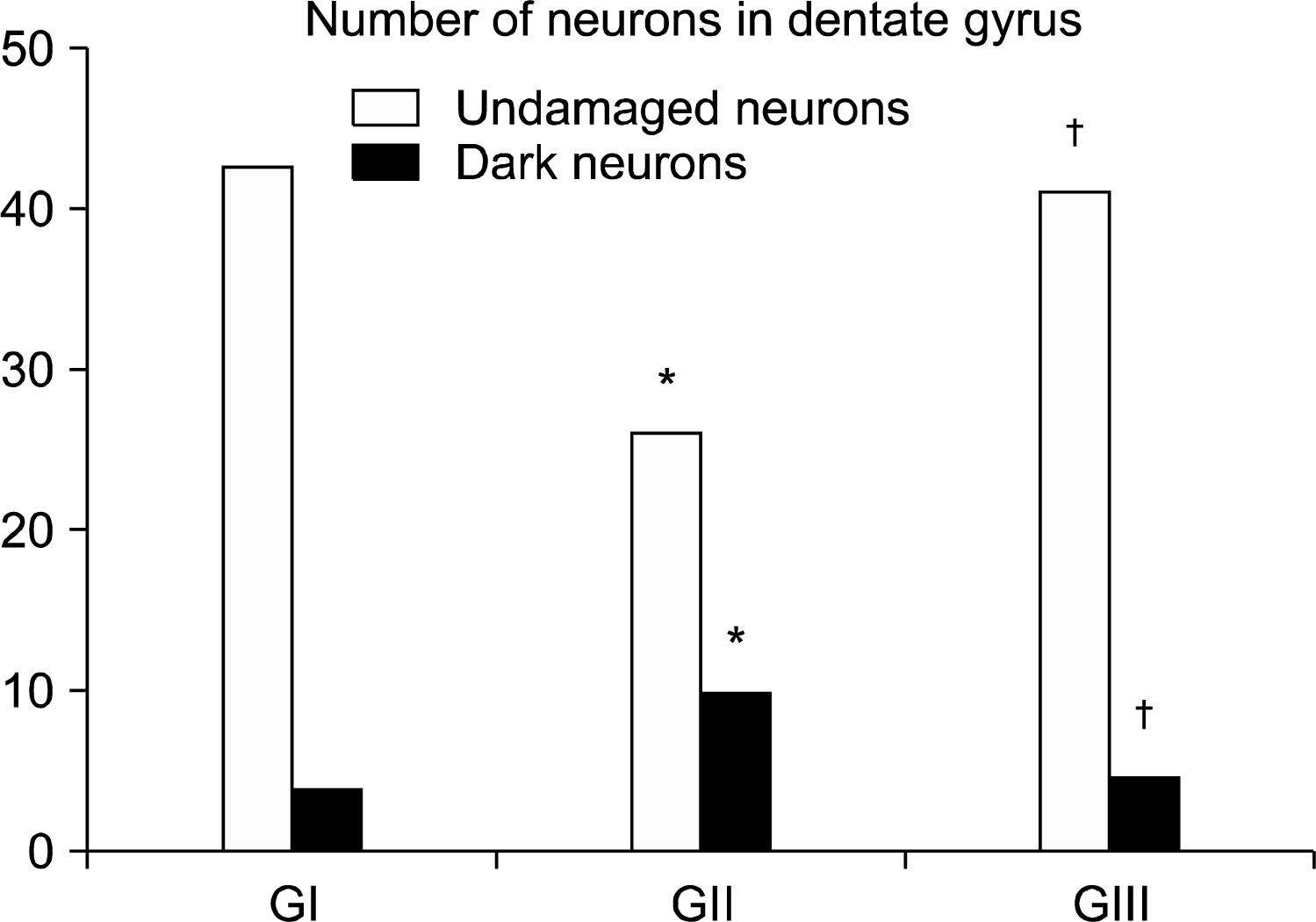
Using morphometric measurment for GII, there was a significant decrease in the number of undamaged neurons and a significant increase in the number of dark neurons in GL as compared to control group. Nowak et al. (
13) detected a significant decrease in the total number of granule cells and in the volume of the GL in the CMS group.
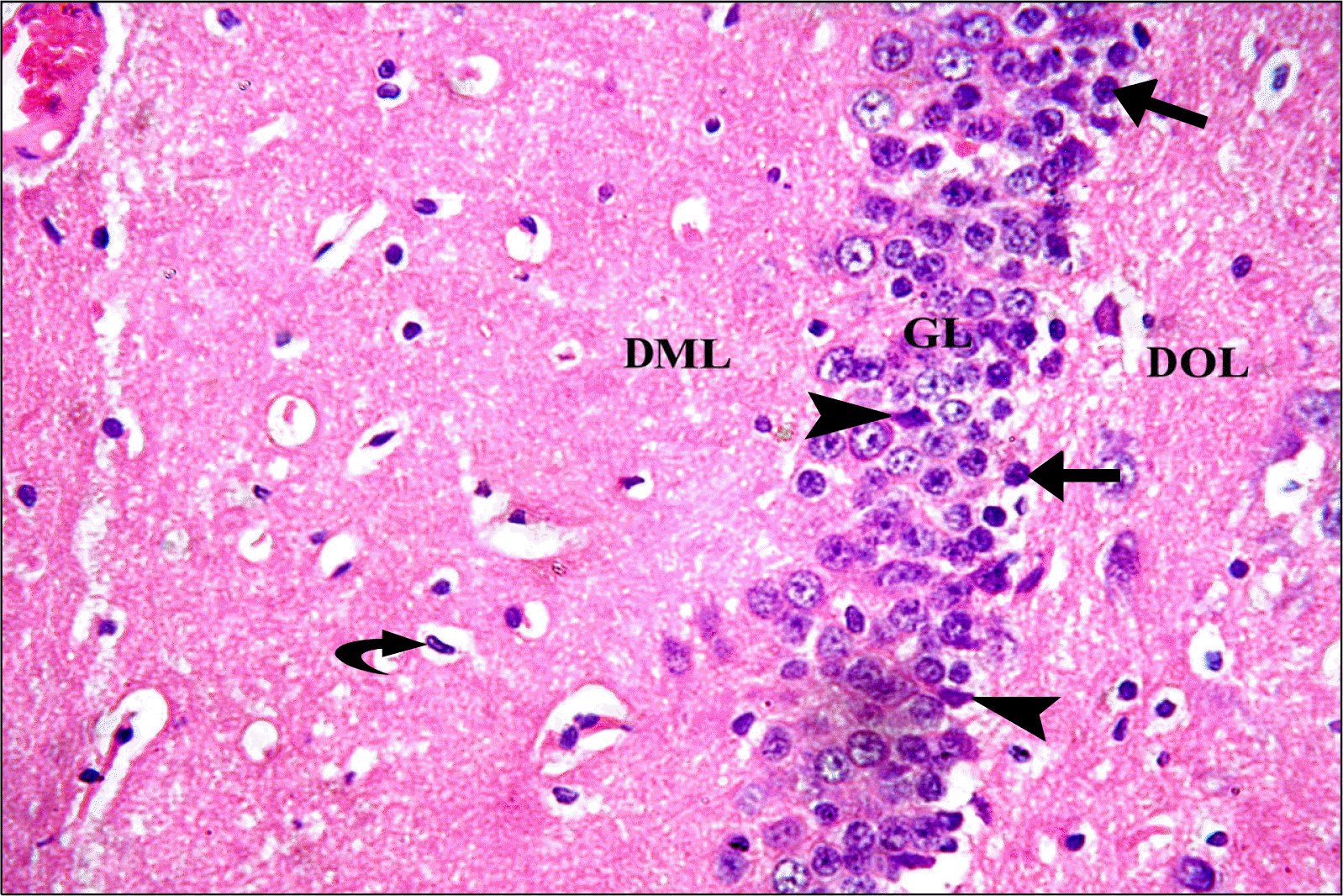
Hippocampal sections of rats which exposed to CMS followed by Rr (GIII) showed signs of recovery in the form of improved neuronal cellularity with the presence of proliferating cells at the basal layer of GL, and consequently no eosinophilic cells, as compared to GII.
Darbinyan et al. (
14) demonestrated that Rr extract alleviates pathological changes in affected hippocampal neurons and promotes survival of the injured hippocampal neurons.
Combining the findings in the present study (GIII) with the previous data reported by others, signals the role of Rr in the improvement of depressed hippocampus through increasing healthy neurons. This may be due to recovery of injured dark neurons or by proliferation of NSCs in DG containing NSCs niche.
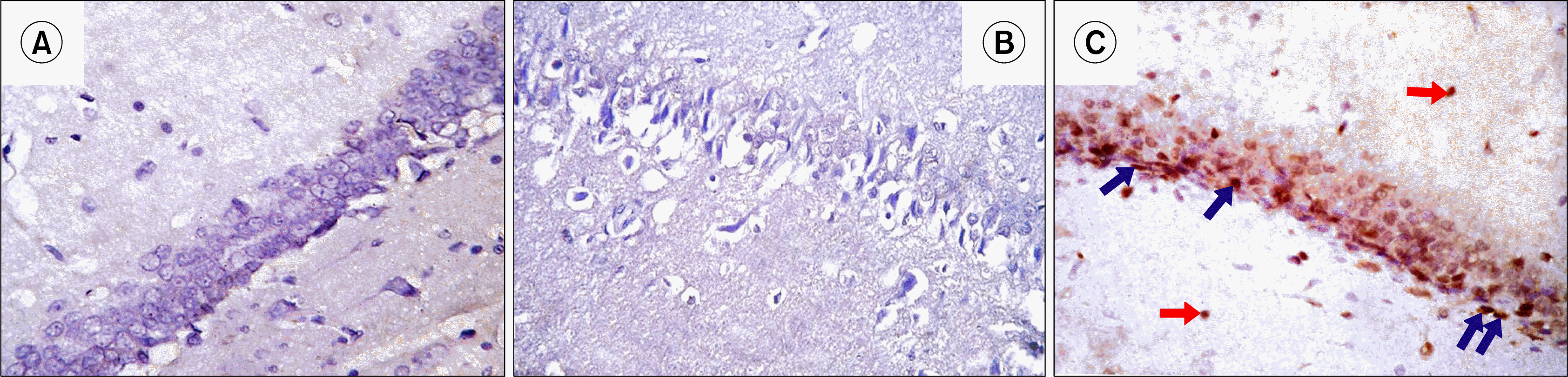
Presence of apoptotic cells in GII was confirmed by using anti-CASP 3 immunostaining. The immunoreactivity was detected in the majority of granule cells of DG mainly in SGZ. On the contrary, GI had negative immunoreactivity detected in all layers of DG. This was in accordance with Sima and Li (
15) who did not found apoptotic cells in all layers of hippocampus of the control rats using TUNEL immunostaining.
Bachis et al. (
16) demonstrated that CMS induced neuronal death through apoptosis as it increased the number of caspase-3 positive neurons in the cerebral cortex. They suggested that increased glucocorticoids secondary to the hyperactivity of the hypothalamic-pituitary-adrenal (HPA) axis in CMS might be responsible for the apoptosis seen in some cortical neurons.
Moreover, Sze et al. (
17) found that dexamethasone increased the number of caspase-3 positive cells in DG. It stimulated glucocorticoid receptors which are present in a high density on hippocampal neurons leading to apoptosis. They proved that, the apoptotic cells in DG were mainly neuroprogenitor cells. In the present study, the existence of anti-CASP 3 immunoreactivity mainly in SGZ of DG (NSCs niche) indicates that depression is associated with apoptosis of these cells.
On the contrary to the present study, Nowak et al. (
13) could not find differences in the number of Caspase 3 immunopositive cells between the control and CMS rat groups. They explained that their results may be due to exposure to less stressors that failed to decrease the number of hippocampal neurons.
Hippocampal sections of Rr group (GIII) stained with anti-CASP 3 antibody revealed no immunoreactivity in all layers of DG. This was in accordance with Palumbo et al. (
18) who showed the neuroprotective effect of Rr in cortical neurons. It significantly increased cell survival, decreased apoptosis and significantly prevented the plasma membrane damage and the morphological disruption caused by oxidative stress.
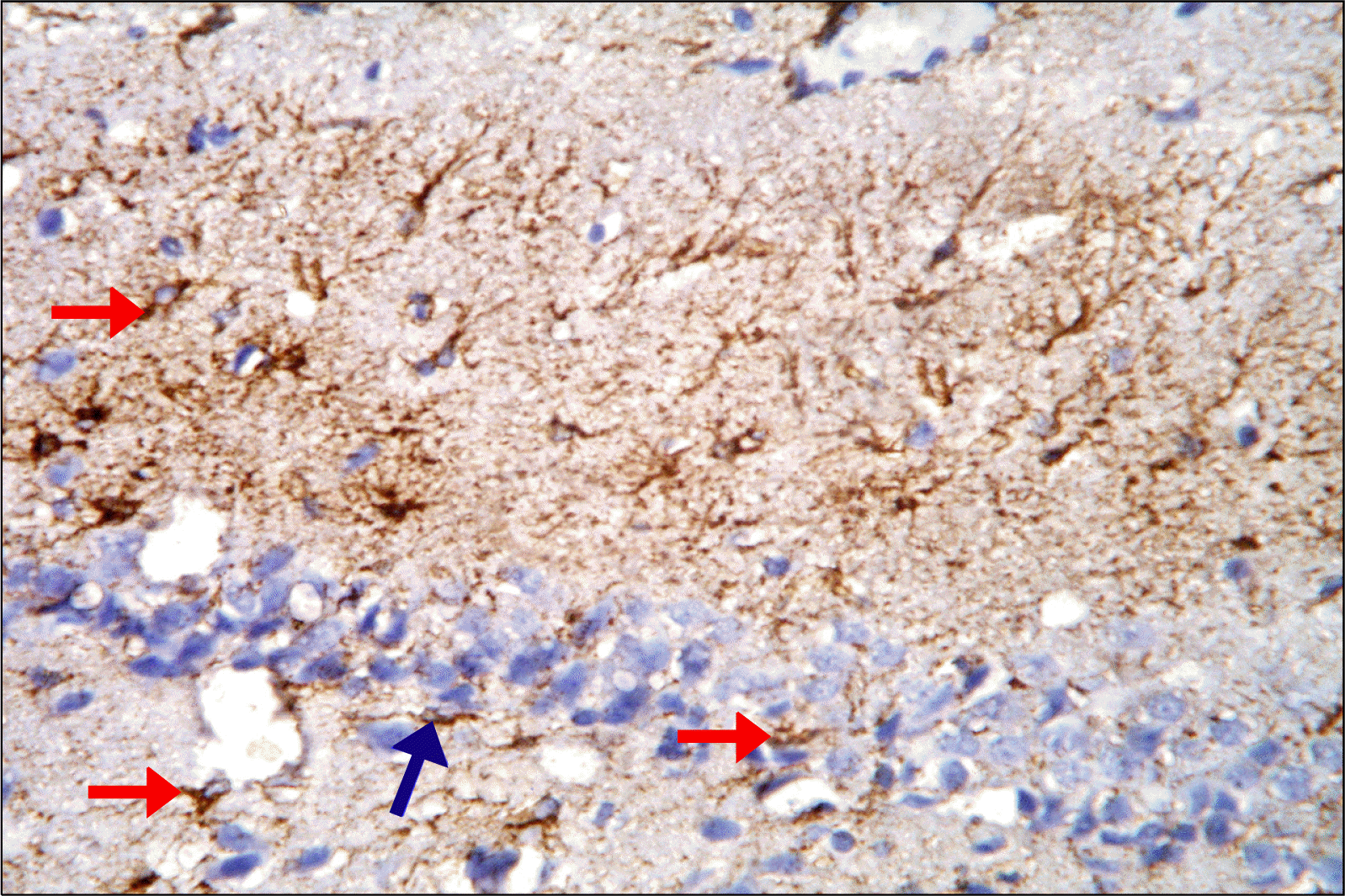
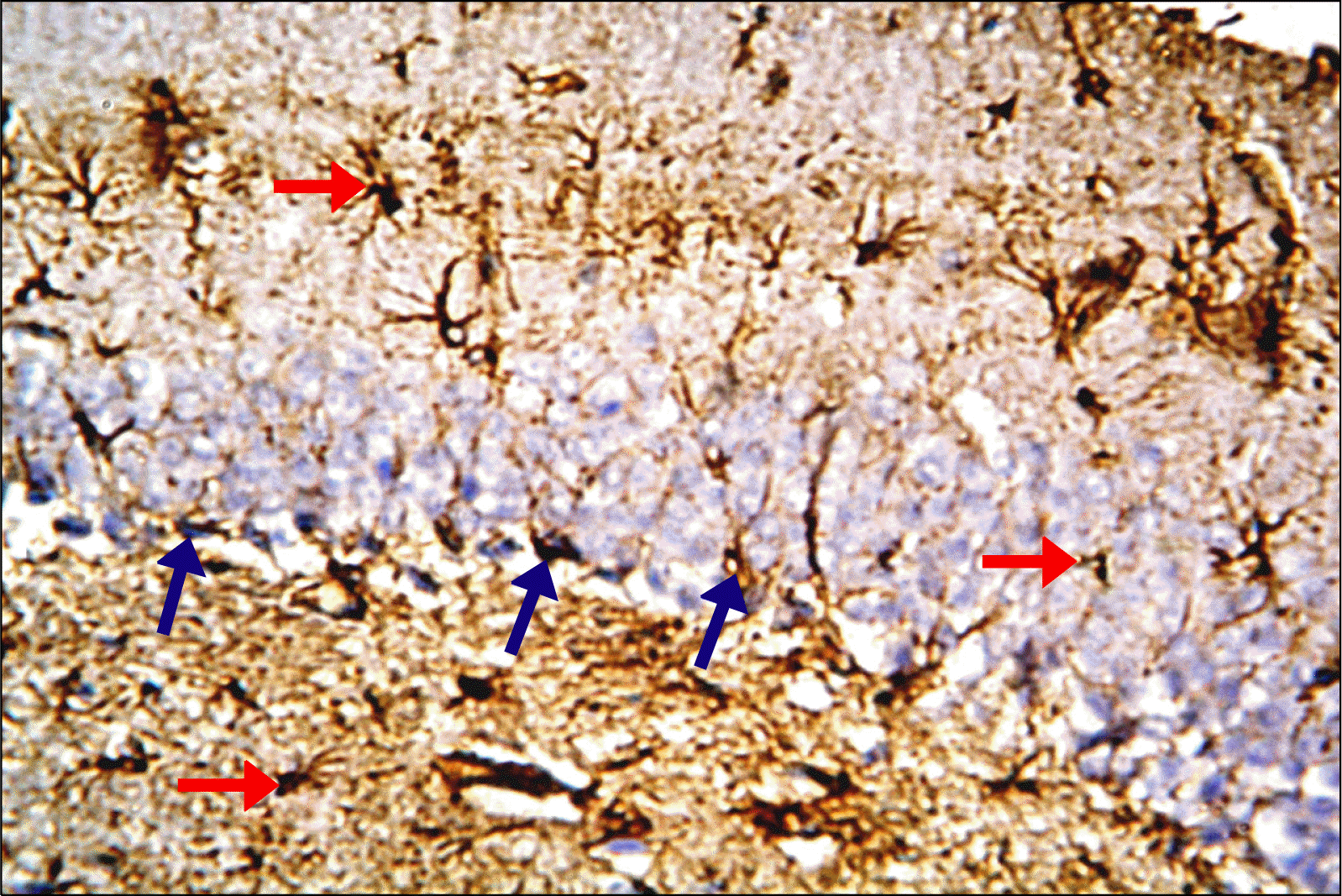
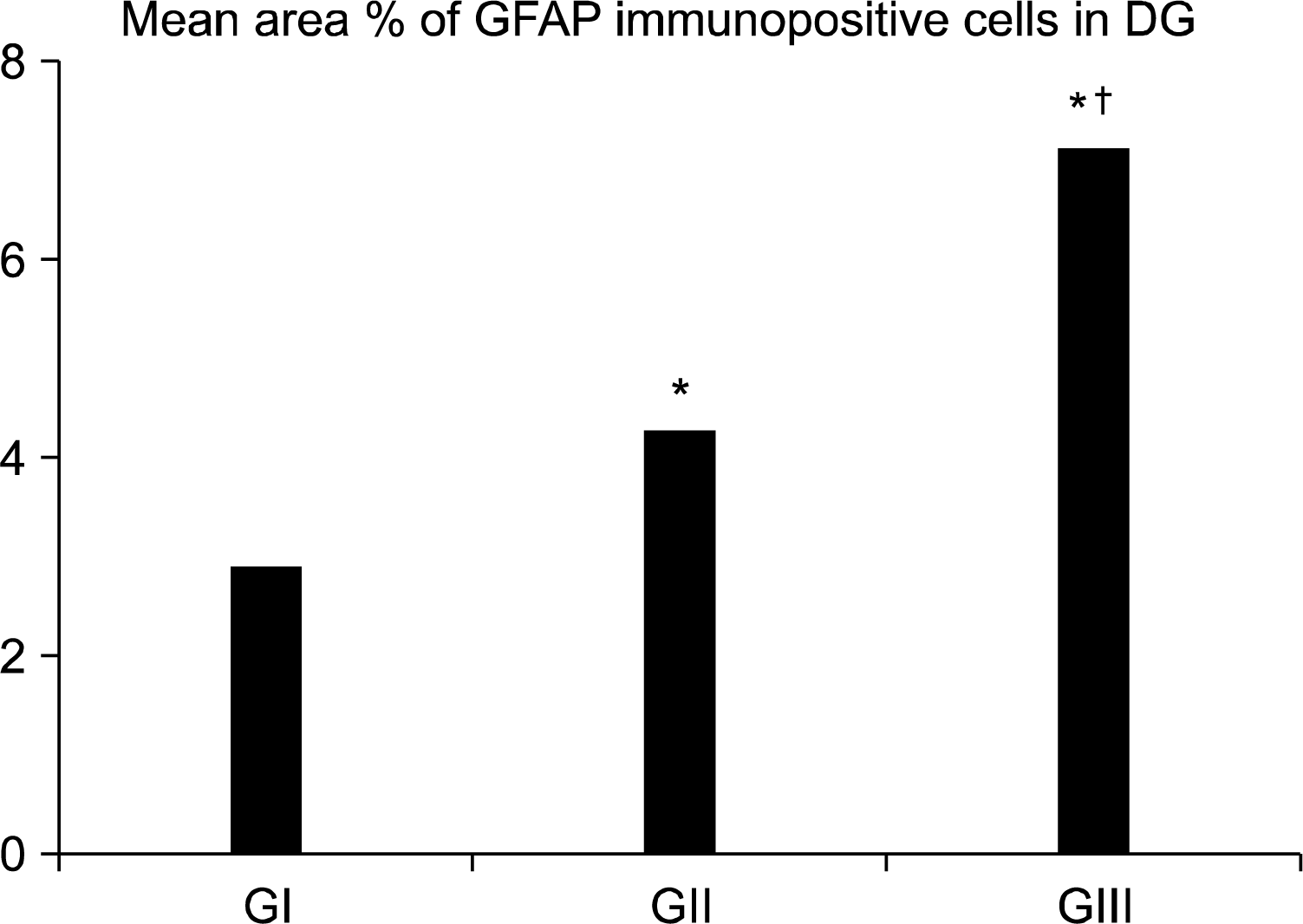
Sections of depressed rats (GII) stained with anti-GFAP antibody revealed increased immunostaining that were detected in all layers of DG. The astrocytes were extending between granule cells mainly in SGZ of GL. They were closely related to dark cells. Using morphometric measurements, the mean area percent of GFAP immunopositive cells of DG were significantly increased as compared to the controls (GI).
Injured astrocytes become hypertrophic, change their morphology and start to proliferate. They also increase the expression of GFAP, vimentin and nestin. These cells are called reactive astrocytes (
19). Contrary to the present results, Li et al. (
20) suggested that astrocyte atrophy contributes to the pathogenesis of depression as GFAP mRNA and protein levels in depressed rats were significantly decreased than control rats.
In the present study, presence of astrocytes closely related to dark cells and mainly in SGZ of GL may be for support or phagocytosis of dark neurons. Local astrocytes can offer structural support, secrete regulatory factors and regulate synapse formation and synaptic transmission. Astrocytes are component of SGZ neural stem cell niche and control neurogenesis by stimulation of proliferation and differentiation of NSCs (
21). Astrocytes and microglia express certain membrane receptors. These receptors recognize molecules released by altered and degenerating neurons leading to the phagocytosis of these cells and neuronal debris (
22).
Sections of Rr treated rats (GIII) revealed prominent immunoreactivity for GFAB as compared to GI & GII. This increase was confirmed by morphometric measurment of GFAP immunopositive cells area percent. This may be for more neuronal protection, support and stimulation of NSCs proliferation & differentiation by astrocytes (
21).
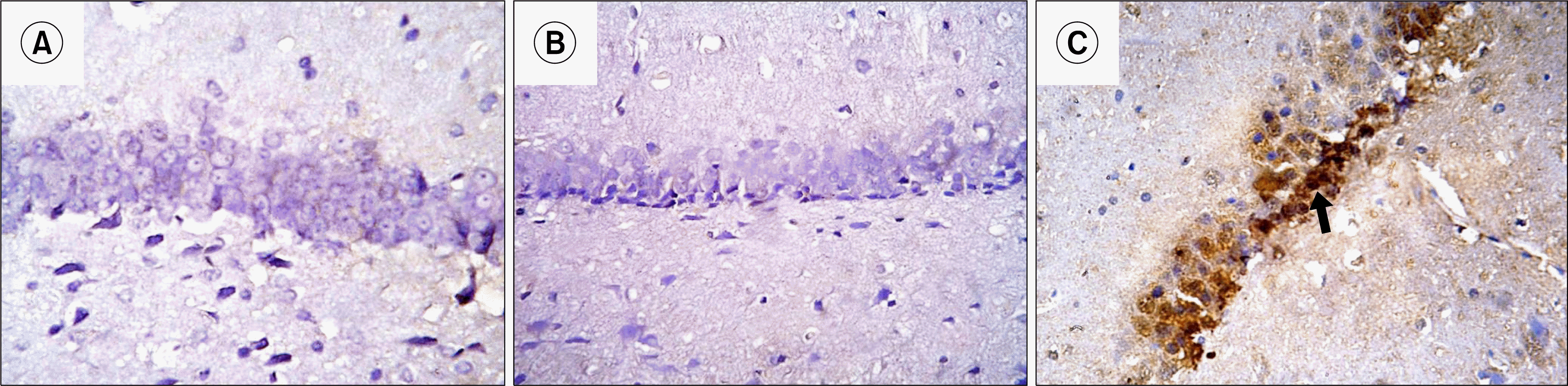
In the present study, anti-PCNA antibody was used to detect proliferating cells. Nowak et al. (
13) mentioned that proliferating cells in SGZ of the DG represent heterogeneous populations of cells, including progenitor of granule cells, radial and horizontal astrocytes in addition to endothelial cells.
Depressed rats (GII) hippocampal sections stained with anti-PCNA antibody revealed negative immunoreactivity in all layers of DG. This was in accordance to Chen et al. (
1) who reported that depression was associated with suppressed proliferation in the rat DG after chronic stress.
Moreover, proliferating cells were not present in control rats. This does not exclude presence of NSCs as they may be in quiescent state and need a marker for quiescent NSCs detection. This also does not exclude presence of glial cells as they do not proliferate in the healthy brain or self-renew when cultured in vitro (
19).
In Rr treated rats, nuclear immunoreactivity for PCNA was detected in many granule cells of DG mainly in SGZ. Few immunoreactive nuclei were detected in other layers of DG.
The current results were in accordance to Chen et al. (
1) who detected an increase in hippocampus proliferating cells after 3 weeks of Rr extract introduction in rats depressed by CMS. The proliferating cells were present in SGZ (NSCs niche). They concluded that Rr extract could improve 5-HT level, induce neural stem cell proliferation and repair the injured hippocampal neurons of depressed rats.
Salidroside content of Rr is a potent antioxidant. It promotes neurogenesis in the hippocampus. It reduces ROS levels and NSCs death and increases NSCs proliferation and differentiation (
23).
In conclusion, Rr extract has a role in affecting the cerebral hippocampal neurons and improving depression in rats. Further studies are required to evaluate the safety/toxicity of Rr and to evaluate their role in preventing human depression.
Go to :






 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download