Abstract
Background:
Engineered blood has the greatest potential to combat a predicted future shortfall in the blood supply for transfusion treatment. The production of red blood cells from hematopoietic stem cells in the laboratory is possible but the mass production of red blood cells to the level present in a blood transfusion unit is currently not possible. The proliferation capacity of the immature red blood cell will need to be increased to enable mass production. This work focused on the hypothesis that exogenous c-Myc can delay the differentiation process of highly proliferative immature erythroblasts, and increase the proliferation capacity of erythroblast cell cultures.
Objectives:
The objective of this research effort was to improve in vitro erythropoiesis from stem cells without gene transfection with the eventual goal of producing blood for transfusion treatment in a manner that could be easily translated into clinical medicine.
Methods:
The hematopoietic stem cell containing mononuclear cell fraction of venous blood samples was cultured in a liquid media containing erythroblasts growth factors with and without exogenous c-Myc combined with a cell -penetrating peptide. The cells were maintained in the liquid culture media for 23 days. Viable cells were counted and analyzed with flow cytometry.
Go to : 
Although the numbers of units of blood collected in the USA in 2009 exceeded the number of units transfused by 13% (1), blood transfusion demand is projected to exceed collections by 2020 (2). Even though significant previous research has long predicted a future shortfall in the US blood supply, an ideal solution has not been found after decades of intense effort (3–7). There are currently seasonal shortages (July–August) of all blood types and there is a continuous shortage of type O negative blood that can serve as an universal donor without risk of blood mismatch, however only 6.6% of the population are type O-(8). Ex vivo engineered blood has the greatest potential to combat the future shortage and assist with seasonal fluctuations in supply. The production of red blood cells from hematopoietic stem cells in the laboratory has been well established in the literature. A critical barrier to the realization of engineered blood is the production scale that would be required to create the 2.5 trillion red blood cells that make up a unit of blood. 2.5 trillion is a massive number of cells, roughly equal to the number of fish in the ocean.
The development of the erythroid cell line from hematopoietic stem cells is a complicated and tightly regulated process. Development begins with erythroid lineage commitment of a hematopoietic stem cell and the subsequent development of highly proliferative erythroid progenitor cells and ending with terminal differentiation of the mature enucleated red blood cell. The hematopoietic stem cell will lose the ability for self-renewal as it matures into erythroid progenitor cells. The first erythroid line committed progenitor cells are the most proliferative and referred to as the erythroid burst-forming unit (BFU-E) (9). The proliferative capacity of the cells decrease as they mature into subsequent erythroid colony forming units (CFU-E). Each cell division leads to serial morphological changes, committing the cell to an increasingly mature state as well as limiting the capacity for further divisions. The cells will typically undergo only 3–4 cell divisions as they mature into morphologically distinct erythroblasts identified as proerythroblasts, basophilic, polychromatophilic, and terminating with the enucleation of the Orthochromic Normoblast which forms the adult red blood cell (10). The maturity of erythroblast cell lines can be identified based on the expression of cell surface proteins. CD117 c-kit expression gradually decreases as cells differentiate from the hematopoietic stem cell to maturing erythroblast stages. The CD71 transferrin receptor is responsible for iron up-take into the erythroblast. CD71 is highly expressed in the early erythroblasts but is down-regulated as the cells pass the basophilic erythroblast stage (11).
Erythropoiesis is regulated by several growth factors, including erythropoietin(EPO), and stem cell factor (SCF). Erythroblasts are initially, highly dependent on erythropoietin for the activation of specific erythroid genes to enable rapid proliferation as well as inhibit apoptosis (12). In the terminal stages of development, the erythroblast cells are less dependent on erythropoietin and become more dependent on α4β1 integrin stimulation (13). The proto-oncogene c-Myc is an erythropoietin early response gene and is rapidly up regulated in response to erythropoietin stimulation. The cellular concentration of the gene product, c-Myc transcription factor protein in normal developing murine erythroblasts increased by fivefold after exposure to erythropoietin (14, 15). It is known that c-Myc plays an important role in the control of proliferation and survival of many types of cells (16, 17). Erythroid progenitor cells require c-Myc for cell cycle progression activity. The protein transcription factor c-Myc can induce cellular proliferation and self-renewal by activating chromatin (18). Supraphysiologic concentrations of c-Myc likely stimulate erythroblast proliferation by up-regulating the G1-S cell cycle checkpoint (15).
The c-Myc transcription factor is known to regulate cell proliferation, differentiation, and apoptosis (19, 20) and c-Myc is vital for many aspects of hematopoietic development and function (21). Studies of c-Myc deletion in mice suggest that both primitive and definitive hematopoiesis is impaired in the absence of Myc (22).
Supraphysiologic expression of c-Myc has been shown to block p27 induced erythroid differentiation of human leukemia K562 (23). We, therefore, theorize that the addition of supraphysiologic doses of exogenous c-Myc to the growth medium could temporarily block human erythro-blast differentiation in culture. A block in cellular differentiation at an early and highly proliferative stage of development could potentially increase the proliferation capacity of erythroblasts in culture. Temporarily delaying differentiation of early more immature and highly proliferative pronormo erythroblast may increase culture expansion capacity. Toward this end, we investigated the effects of supraphysiologic doses of exogenous c-Myc protein on the differentiation process of human erythroblast in culture.
Go to : 
Non-mobilized adult peripheral blood samples were obtained from healthy volunteers who gave informed consent in a protocol approved by the Santa Barbara Cottage Hospital Institutional Review Board. Mononuclear Cells (MNCs) were isolated from 10 mL samples of whole blood by centrifugation through density purification using Percoll (Sigma Aldridge, St Louis, MO) with density ρ1.077. The whole blood samples were centrifuged at 400g at room temperature for 30 minutes. The serum was removed from the specimen and the mononuclear cells were washed in sterile 1 x phosphate-buffered saline (PBS) at 300 g at room temperature for 15 minutes. After two washes, the cells were suspended in the liquid culture media.
Purified mononuclear cells from the same adult volunteer were resuspended in liquid culture media at 10^6 cells/mL in 6 well plates, suspended in the serum-free media Stemspan SFEMTM (Stem Cell Technologies, Vancouver CA) supplemented with 3 ng/mL recombinant human interleukin-3 (IL-3) and 100 ng/mL recombinant human Stem Cell Factor (SCF), 40 ng/mL Insulin like Growth Factor1 (IGF-1), 6 U/mL erythropoietin (EPO). The media also contained 1uM Dexamethasone (DXM) and 1uM Estradiol (E2). In the c-Myc protocol, recombinant human c-Myc-11R protein was added to media for a final concentration of 400 nM. Partial growth media changes were preformed daily for up to 23 days.
Recombinant human IL-3, SCF, IGF1 and EPO were all from PeproTech (Rocky Hill, NJ). DXM and E2 were obtained from Sigma (St Louis, MO). The cMyc-11R Protein was obtained from LD Biopharma, Inc. (San Diego, CA) with a Recombinant Protein Sequence of MDFFRVVENQQPPATMPLNVSFTNRNYDLDYDSVQ PYFYCDEEENFYQQQQQSELQPPAPSEDIWKKFELLP TPPLSPSRRSGLCSPSYVAVTPFSLRGDNDGGGGSFST ADQLEMVTELLGGDMVNQSFICDPDDETFIKNIIIQD CMWSGFSAAAKLVSEKLASYQAARKDSGSPNPARGH SVCSTSSLYLQDLSAAASECIDPSVVFPYPLNDSSSPKS CASQDSSAFSPSSDSLLSSTESSPQGSPEPLVLHEETPP TTSSDSEEEQEDEEEIDVVSVEKRQAPGKRSESGSPSA GGHSKPPHSPLVLKRCHVSTHQHNYAAPPSTRKDYP AAKRVKLDSVRVLRQISNNRKCTSPRSSDTEENVKRR THNVLERQRRNELKRSFFALRDQIPELENNEKAPKV VILKKATAYILSVQAEEQKLISEEDLLRKRREQLKHK LEQLRNSCAESGGGGSPGRRRRRRRRRRR
Cells were maintained in the liquid culture media at 37°C, in high humidity, pCO2 5%, and pO2 5%. Cells were analyzed in vitro under an Olympus Phase contrast microscope. Cell contrast was achieved using May-Grunwald. The analysis of the immunophenotyping of the cells was performed at the Santa Barbara Cottage Hospital Flow Cytometry laboratory. Flow Cytometric analysis was performed in which cells were incubated for 15 min with fluorescein isothiocyanate (FITC) conjugated CD71 and phyocerythrin (PE)-conjugated CD117 (C-Kit)
Go to : 
Each culture protocol started with the mononuclear fraction of a 10 mL sample of peripheral blood. Circulating adult hematopoietic stem (CD34+) cells are found within the mononuclear cell fraction. Hematopoietic stem cells occur in non mobilized peripheral blood at frequencies of 0.001−0.007×10^6 cell/mL of blood (24). Since we started each experimental protocol with a 10 mL of blood, there were 0.01−0.07×10^6 hematopoietic stem cells present at the start of the culture process. Each 10 mL sample of blood produced approximately 1.3e7 mononuclear cells, obtained through density gradient centrifugation. The adult hematopoietic stems (CD34+) within the mononuclear fraction were stimulated to expand and differentiate by the growth factors in the medium. The cell cultures were counted every 3 to 5 days with a Countess® Automated Cell Counter.
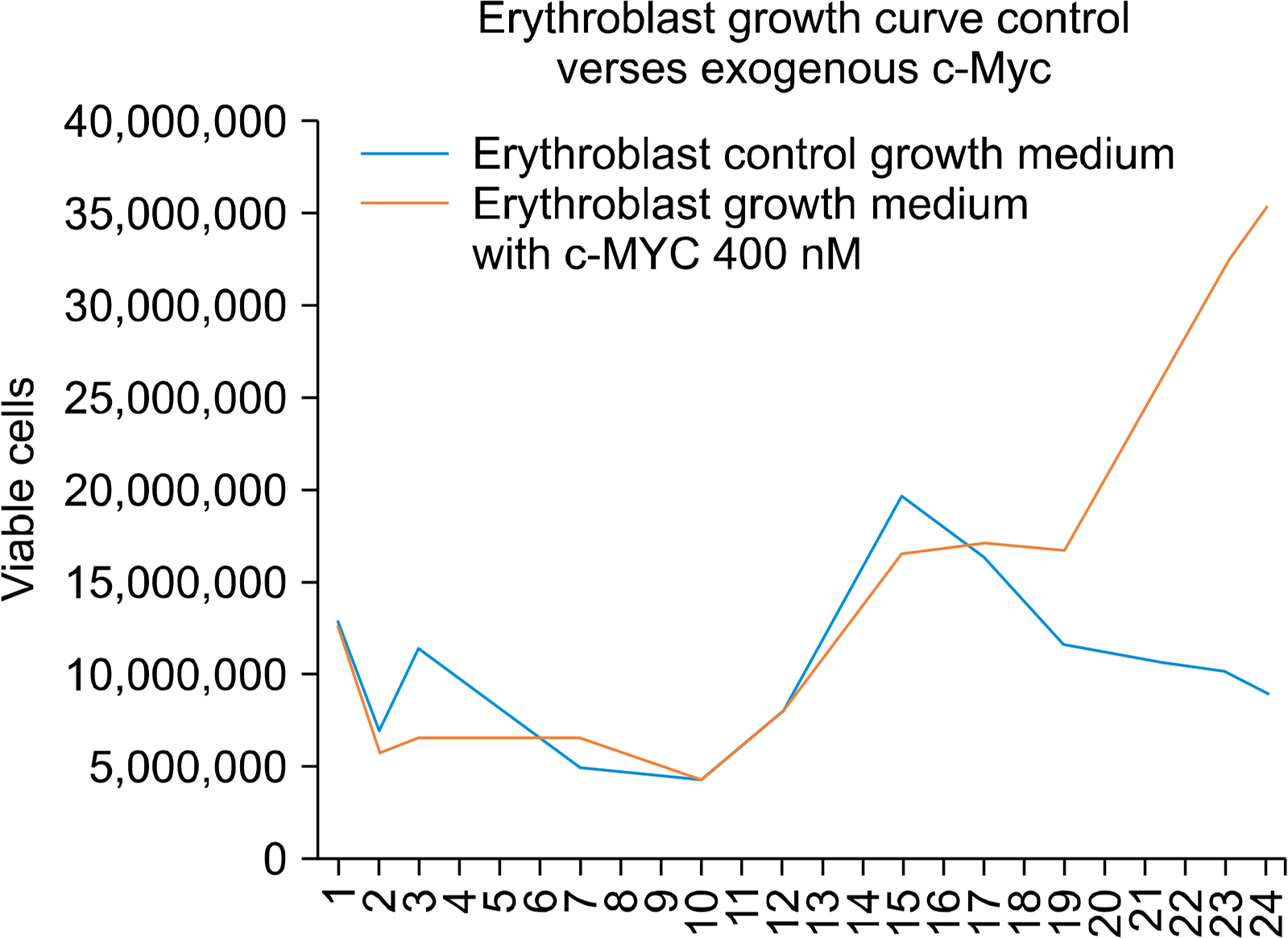
Each arm of the experiment reached a nadir at about day 10, as the non-stem cells died off in culture and the HSCs present were stimulated to expand and differentiate and ultimately overtake the culture (Fig. 1).
Given each experimental arm started with approximately 40,000 HSCs, the c-Myc experimental arm produced a 4 fold more erythroblasts than the control arm.
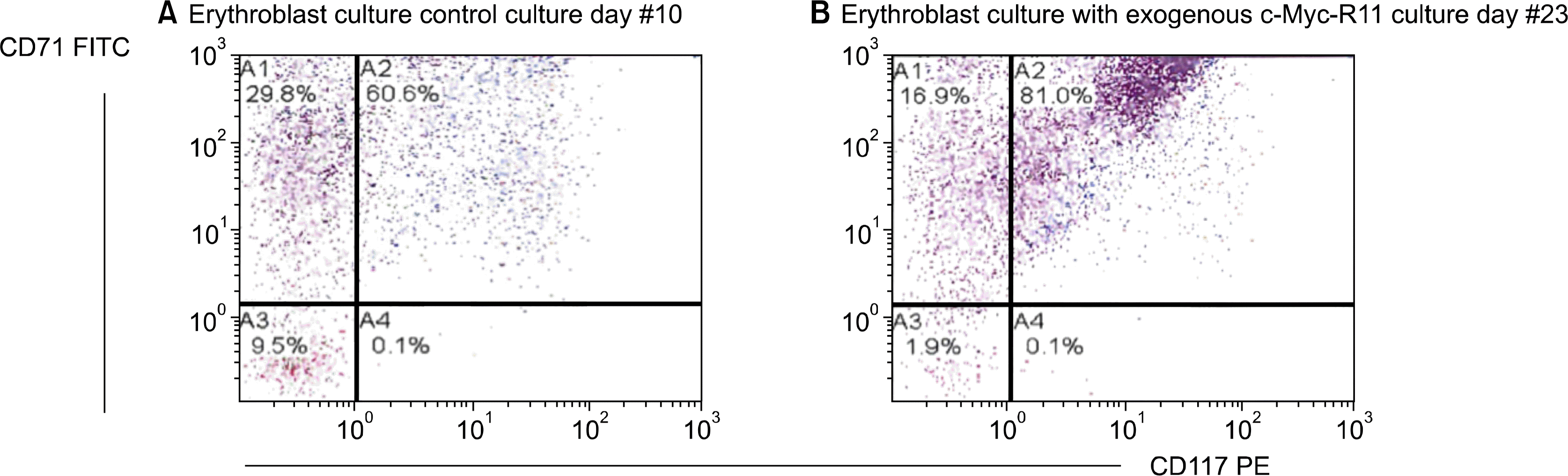
The protocols were evaluated by Flow Cytometry to determine immunophenotyping identification of the maturing erythroid cultured cells. The cells were immunophenotypically analyzed for the presence of CD71 and CD117. CD71 is the transferrin receptor molecule and is present on all cells that have committed to the erythroid cell lineage. CD117 is the c-kit/Stem Cell Factor receptor and is present on immature progenitor cells and is lost as cells differentiate (Fig. 2).
In this experiment, we also observed a block in erythroblast differentiation, which is consistent with the conclusions from a previous studies (21–23), where retroviral overexpression of c-Myc inhibited erythroblast differentiation in murine fetal liver cells and transfected human pluripotent stem cells in vitro and gave rise to continuously growing erythroblast cell lines.
Go to : 
In this experiment we studied the effects of exogenous recombinant c-Myc added to our erythroblast growth medium. We used a c-Myc transcription factor that was attached to a cell penetrating peptide(CPP). CPPs are short peptides that facilitate cellular uptake of proteins into the cell and nucleus. We used a supraphysiologic dose of c-Myc of 400 nM in the treated experiment.
In the c-Myc treated experiment, we observed that 80% of erythroblasts retained the CD117 c-kit expression until the 23rd day of culture. By day 10 in the control group, only 60% retained the CD117 c-kit expression. The retained expression of CD117 c-kit is likely due to a differentiation block by exogenous c-myc.
In the control experiment growth protocol, we observed a 225 fold increase in cells over 23 days which is consistent with other research groups using CD34+ cells isolated from mobilized peripheral blood (25, 26). In our c-Myc treated protocol we observed an 860 fold increase in cells, approximately 4 times more than our control protocol.
The results of this experiment support the conclusion that supraphysiologic doses of exogenous c-Myc perturb the maturation process of normal adult human erythroblast culture at an immature and highly proliferative state that increases the expansion capacity of the erythroblast culture. It is likely that target genes of c-Myc interfere with the differentiation process of the erythroblast.
The present experiment suggests that exogenous c-Myc can interfere in the differentiation process of lineage committed (lin+) erythroid progenitor cells in an erythroblast expansion culture. It has previously been shown that c-Myc is indispensable in both primitive and adult murine hematopoiesis (22,27). The deletion of c-Myc can result in >30 reduction in differentiating and mature lineage-positive (lin+) hematopoietic progenitor cells (22). The c-Myc deletion also resulted in 3 fold increase in the lineage negative (lin−) progenitors as well as a >20 fold increase in the subpopulation of c-Kit+, lin−, Sca-1+ hematopoietic stem cells. It is likely that c-Myc activity is required for the differentiation process of (lin−) hematopoietic stem cells into lineage positive progenitors. Whereas our study demonstrates that supraphysiologic c-Myc blocks differentiation of c-Kit+, lin+ erythroblasts into the more mature c-Kit-, lin+ erythroblast.
The mass production of red blood cells in order to reach clinically relevant quantities, near the trillion cell level present in a transfusion, will require significant advancement in erythroblast proliferation capacity in culture. We theorize here that this barrier to mass production of erythroid cells may be overcome by maintaining erythroid progenitor cells in an immature and highly proliferative state. Temporary biochemical manipulation with exogenous c-myc may represent one avenue for reaching the trillion-cell level.
Go to : 
ACKNOWLEDGMENTS
This study was supported by NSF grant #1315876, National Science Foundation. We are grateful to the Santa Barbara Cottage Hospital Flow Cytometry laboratory for providing Fluorescence-activated cell sorting services and to our colleagues in the Flow cytometry lab for technical support. We are grateful to the Institutional Review Board at Santa Barbara Cottage Hospital for their oversight in our human studies.
Go to : 
References
1. The 2007 National Blood Collection and Utilization Survey Report. Washington DC: DHHS;2008.
2. Benjamin RJ, Whitaker BI. Boom or bust? Estimating blood demand and supply as the baby boomers age. Transfusion. 2011. 51:670–673.

3. Surgenor DM, Wallace EL, Hao SH, Chapman RH. Collection and transfusion of blood in the United States, 1982–1988. N Engl J Med. 1990. 322:1646–1651.

4. Wallace EL, Surgenor DM, Hao HS, An J, Chapman RH, Churchill WH. Collection and transfusion of blood and blood components in the United States, 1989. Transfusion. 1993. 33:139–144.

5. Wallace EL, Churchill WH, Surgenor DM, An J, Cho G, McGurk S, Murphy L. Collection and transfusion of blood and blood components in the United States, 1992. Transfusion. 1995. 35:802–812.

6. Sullivan MT, McCullough J, Schreiber GB, Wallace EL. Blood collection and transfusion in the United States in 1997. Transfusion. 2002. 42:1253–1260.

7. Sullivan MT, Wallace EL. Blood collection and transfusion in the United States in 1999. Transfusion. 2005. 45:141–148.

8. Blood Facts and Statistics. American Red Cross. www.redcrossblood.org/learn-about-blood/blood-facts-and-statistics.
9. Stephenson JR, Axelrad AA, McLeod DL, Shreeve MM. Induction of colonies of hemoglobin-synthesizing cells by erythropoietin in vitro. Proc Natl Acad Sci U S A. 1971. 68:1542–1546.

10. Nathan DG, Chess L, Hillman DG, Clarke B, Breard J, Merler E, Housman DE. Human erythroid burst-forming unit: T-cell requirement for proliferation in vitro. J Exp Med. 1978. 147:324–339.

11. Brendt P, Rehfeld I, Kamphausen A, Kreissig C, Peters J. Lipopolysaccharide interference in erythropoiesis in mice. Anaesthesia. 2012. 67:493–500.

12. Richmond TD, Chohan M, Barber DL. Turning cells red: signal transduction mediated by erythropoietin. Trends Cell Biol. 2005. 15:146–155.

13. Lodish H, Flygare J, Chou S. From stem cell to erythroblast: regulation of red cell production at multiple levels by multiple hormones. IUBMB Life. 2010. 62:492–496.

14. Spangler R, Sytkowski AJ. c-myc is an erythropoietin early response gene in normal erythroid cells: evidence for a protein kinase C-mediated signal. Blood. 1992. 79:52–57.

15. Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002. 2:489–501.

16. Grandori C, Cowley SM, James LP, Eisenman RN. The Myc/Max/Mad network and the transcriptional control of cell behavior. Annu Rev Cell Dev Biol. 2000. 16:653–699.

17. Pelengaris S, Khan M, Evan G. c-MYC: more than just a matter of life and death. Nat Rev Cancer. 2002. 2:764–776.

18. Knoepfler PS, Zhang XY, Cheng PF, Gafken PR, McMahon SB, Eisenman RN. Myc influences global chromatin structure. EMBO J. 2006. 25:2723–2734.

19. Dang CV, O'Donnell KA, Zeller KI, Nguyen T, Osthus RC, Li F. The c-Myc target gene network. Semin Cancer Biol. 2006. 16:253–264.

21. Hoffman B, Amanullah A, Shafarenko M, Liebermann DA. The proto-oncogene c-myc in hematopoietic development and leukemogenesis. Oncogene. 2002. 21:3414–3421.

22. Wilson A, Murphy MJ, Oskarsson T, Kaloulis K, Bettess MD, Oser GM, Pasche AC, Knabenhans C, Macdonald HR, Trumpp A. c-Myc controls the balance between hematopoietic stem cell self-renewal and differentiation. Genes Dev. 2004. 18:2747–2763.

23. Acosta JC, Ferrándiz N, Bretones G, Torrano V, Blanco R, Richard C, O'Connell B, Sedivy J, Delgado MD, León J. Myc inhibits p27-induced erythroid differentiation of leukemia cells by repressing erythroid master genes without reversing p27-mediated cell cycle arrest. Mol Cell Biol. 2008. 28:7286–7295.

24. Data Generated in-house STEMCELL Technologies Inc. 570 West Seventh Avenue, Suite 400, Vancouver, BC, V5Z 1B3, Canada.
25. Carlile GW, Smith DH, Wiedmann M. Caspase-3 has a nonapoptotic function in erythroid maturation. Blood. 2004. 103:4310–4316.

Go to : 




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download