Abstract
Objectives:
Mesenchymal stem cells (MSCs) are adult stem cells which identified by adherence to plastic, expression of cell surface markers including CD44, CD90, CD105, CD106, CD166, and Stro-1, lack of the expression of hematopoietic markers, no immunogenic effect and replacement of damaged tissues. These properties led to development of progressive methods to isolation and characterization of MSCs from various sources for therapeutic applications in regenerative medicine.
Methods:
We isolated MSC-like cells from testis biopsies, ovary, hair follicle and umbilical cord Wharton’s jelly and investigated the expression of specific cell surface antigens using flow cytometry in order to verify stemness properties of these cells.
Results:
All four cell types adhered to plastic culture flask a few days after primary culture. All our cells positively expressed common MSC- specific cell surface markers. Moreover, our results revealed the expression of CD19and CD45 antigens in these cells.
Conclusion:
According to our results, high expression of CD44 in spermatogonial stem cells (SSCs), hair follicle stem cells (HFSCs),granulosa cells (GCs)and Wharton’s jelly- MSCs (WJ-MSCs)may help them to maintain stemness properties. Furthermore, we suggest that CD105+SSCs, HFSCs and WJ-MSCs revealed the osteogenic potential of these cells. Moreover, high expression of CD90 in SSCs and HFSCs may associate to higher growth and differentiation potential of these cells. Further, the presence of CD19 on SSCs and GCs may help them to efficiency in response to trans-membrane signals. Thus, these four types of MSCs may be useful in clinical applications and cell therapy.
Go to : 
Stem cells (SCs) are indefinitely dividing cells that can give rise to more differentiated cell types. SCs are considered as one of the fundamental bases of tissue biology. They replenish tissues that need cell replacement like blood, bone, gametes, epithelia, nervous system, muscle, and numerous other tissues by fresh cells throughout life (1). Terms that frequently have been used to define the differentiation potential of SCs are: totipotent, pluripo-tent, multipotent, oligopotent, and unipotent. Cells in early days after fertilization are totipotent and can give rise to a complete and functional organism. During the development of the embryo, the totipotent cells become specialized more restricted and are considered to be pluripotent, that they can give rise to every cell in the embryo. Pluripotent SCs become increasingly restricted in their lineage potential and generate tissue-specific multipotent SCs, which can differentiate into the cell types of tissues that they are belonging to it. Adult stem cells (ASC) or somatic stem cells are undifferentiated cells that located throughout of the body characterized by self- renewing and multipotency; these cells participate in regeneration of damaged tissues and replenish of dying cells (2). Multi-potent mesenchymal stromal cells or mesenchymal stem cells (MSCs) are adult stem cells that found not only in bone marrow, but obtained from different human organs such as adipose tissue, umbilical cord, synovium, as well as adult human testis (3-5). Based on the minimal criteria of International Society of Cellular Therapy (ISCT), human MSCs identified by adherence to plastic and expression of cell surface markers including CD29, CD44, CD90, CD49a-f, CD51, CD73 (SH3), CD105 (SH2), CD106, CD166, and Stro-1 and lack of expression of CD45, CD34, CD14 or CD11b, CD79a orCD19 and HLA-DR surface molecules (6). MSCs have no immunogenic effect and could replace the damaged tissues (7). These properties led to development of progressive methods to isolation and characterization of MSCs from various sources for therapeutic applications in regenerative medicine. In present study, we isolated MSC- like cells from testis biopsies, ovary, hair follicle and umbilical cord Wharton’s jelly and investigated the expression of specific cell surface antigens using flow cytometry in order to verify stemness properties of these cells.
Go to : 
In this study, all samples collected and used for research following informed consent.
Testicular biopsies obtained from azoospermic patients by testicular sperm extraction (TESE). A small portion of the testicular tissue placed in Hank’s balanced salt solution (HBSS) supplemented with penicillin and streptomycin (Biosera, UK) and minced in small pieces. In order to isolation of spermatogonial stem cells from testis, the tissue was digested with 0.25%trypsin (Sigma Aldrich, USA) for 5 minutesat 37°C. The obtained suspension centrifuged at 1500 rpm for 5 minutes and the supernatant discarded and cell pellet cultured in DMEM/F12 (Gibco, USA) supplemented with 20% FBS (Gibco, USA) and 1% penicillin/streptomycin. After 15 days, human spermatogonial stem cell clusters collected and mechanically isolated and cultured in new cell culture flask. Subsequently, the cells subcultured after confluence phase and in passage one the expression of MSC- related cell surface antigens analyzed by flow cytometry.
Granulosa cells (GCs) were collected by transvaginal ultrasound-guided aspiration from infertile women treated byassisted reproduction technology (ART). After aspiration, GCs isolated by enzymatic method, thenfreshly isolated GCs were used for cell culture. The primary isolated GCs were centrifuged at 1500 rpm for 5 minutes. The supernatant discarded and the cell pellet transferred to T25 cell culture flask (orange scientific, Belgium) containing DMEM/F12supplemented with 20% FBS and 1%penicillinandstreptomycinand incubated at 37°C and 5%CO2 inhumidifiedincubator. The medium was changed every 4 days until the cells reached confluence phase. For subculturing, GCs detached by 0.25% trypsin/EDTA and re-plated in the same medium. The GCs analyzed for the expression of MSC- related cell surface antigens in passage one.
Hair follicles obtained from adult male scalp tissue by micropunch technique. Follicles transfer to T25flaskcontainingnormalsaline. The flask was transported to the laboratory in ice containing container. In the laboratory, under the laminar flow hood, follicles removed from normal saline and were transferred into Petri dish containing HBSS supplemented with penicillin and streptomycin. Using a scalpel, the capsule around the hair follicle isolated and the follicles cultured in DMEM/F12 medium supplemented with 20% FBS and 1% penicillin and streptomycin and maintained at 37°C and 5% CO2 in humidified incubator. Sixteen days after culture of hair follicles, the migration and proliferation of first cells around the hair follicle bulge were appeared. In 23th day, the hair follicles removed from the culture flask and the attached cells were subcultured. In day 27, the expression of MSC-related cell surface antigens in hair follicle stem cells (HFCSs) were investigated by flow cytometry.
Umbilical cord (UC) is an excellent source of stem cells. Wharton’s jelly (WJ) within the umbilical cord contains stromal fibroblast- like cells characterized by higher proliferation rate and self-renewal capacity and wide multi-potency to differentiation into other cell types in compared to adult stem cells. In order to isolation of Wharton’s jelly derived stem cells, fresh human UC was obtained after full-term birth and transferred to the laboratory in normal saline within 6 hours. The UC submerged in ethanol 70% for 30 second and after removal of the vessels, minced in 5∼6 cm pieces and transferred to Hank’s balanced salt solution (HBSS) and dissected. The obtained suspension centrifuged at 1500 rpm for 5 minutes and the explants were cultured in DMEM/F12 containing 20% FBS and 1% penicillin and streptomycin. After 15 days, the explants removed and the outgrowth cells from the explants isolated and cultured in the same medium. The expression of MSC- related cell surface antigens assessed by flow cytometry.
SSCs, GCs and HFSCs at their first passage and WJ-derived MSCs at their fifth passage, after reaching confluence, detached using 0.25% trypsin/EDTA and centrifuged at 1500 rpm for 5 minutes. The supernatant discarded and the cell pellet resuspended in D-PBS-A containing 1% fetal bovine serum. Cells incubated with 10 μL of primary antibody (1:100 ratios) in 4°C for 45 minutes. Inpresent study, human mesenchymal stem cell marker antibody panel (R&D system, USA) including anti- Stro-1, anti- CD90, anti- CD105, anti- CD106, anti-CD146, anti CD166, anti- CD44, anti-CD45 and anti- CD19 anti-bodieswere used. Then cells centrifuged at 12000 rpm for 15 minutes and washed with flow cytometry washing buffer and secondary antibodies including Goat anti mouse IgG-FITC (Dako, USA) and Goat anti mouse IgM-FITC (Abcam, USA) were added and the tubes were incubated in 4° for 45 minutes in the dark. After a washing step, the cells analyzed by flow cytometry analyzer (BD FACS Calibur).
Go to : 
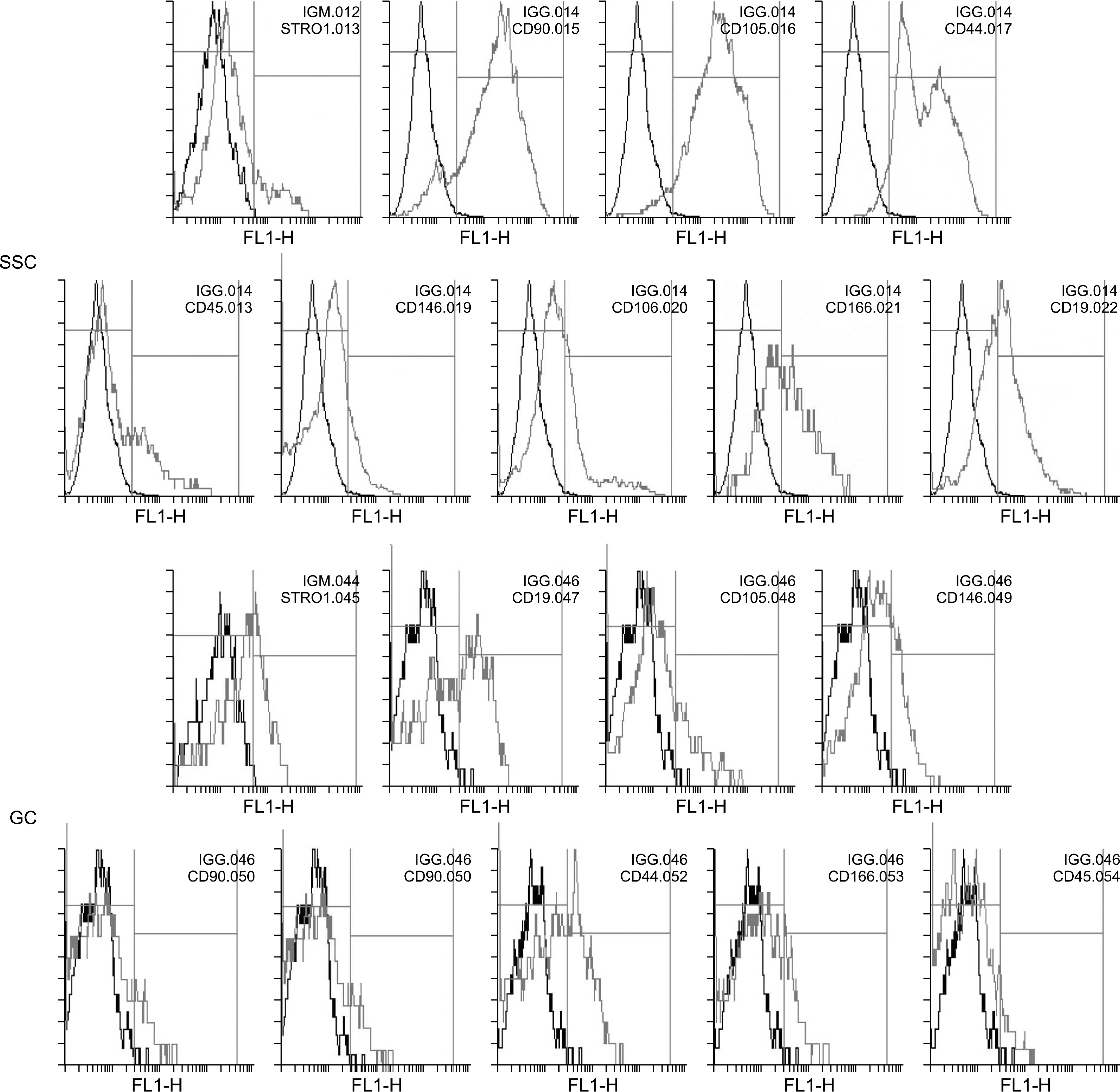
Cells where isolated from testes of azoospermic patients were fibroblast- like cells which adhered to plastic culture flask after 4 days (Fig. 1A). Human spermatogonial stem cell colonies appeared after 10∼17 dayson the fibroblast-like cells (Fig. 1B). The number of SSC colonies varied between one and 10 colony in each culture flask. Then, these colonies collected and cultured in new flasks. SSCs in passage one and after reaching confluence, assessed for the expression of mesenchymal stem cell associated cell surface markers. Flow cytometry results showed that human SSCs highly expressed CD90, CD105 and CD44 and are positive for Stro-1, CD146, CD106 and CD166. Furthermore, the expression of CD19 and CD45 observed in low percentage of these cells (Fig. 2 and 4).
Isolated GCs during of the first day of culture, adhered to plastic culture flask and were round shaped cells emitted long cytoplasmic extensions (Fig. 1C). Light microscopy visualizations showed that GCs exhibited fibroblast-like morphology after seven days of culture (Fig. 1D). GCs examined for the expression of mesenchymal stem cells associated markers in their first passage. These cells were positive for Stro-1, CD105, CD146, CD90, CD106, CD44 and CD166 markers. Moreover, the expression of CD45 and CD19 markers observed in 8.8% and 41% of cells, respectively.
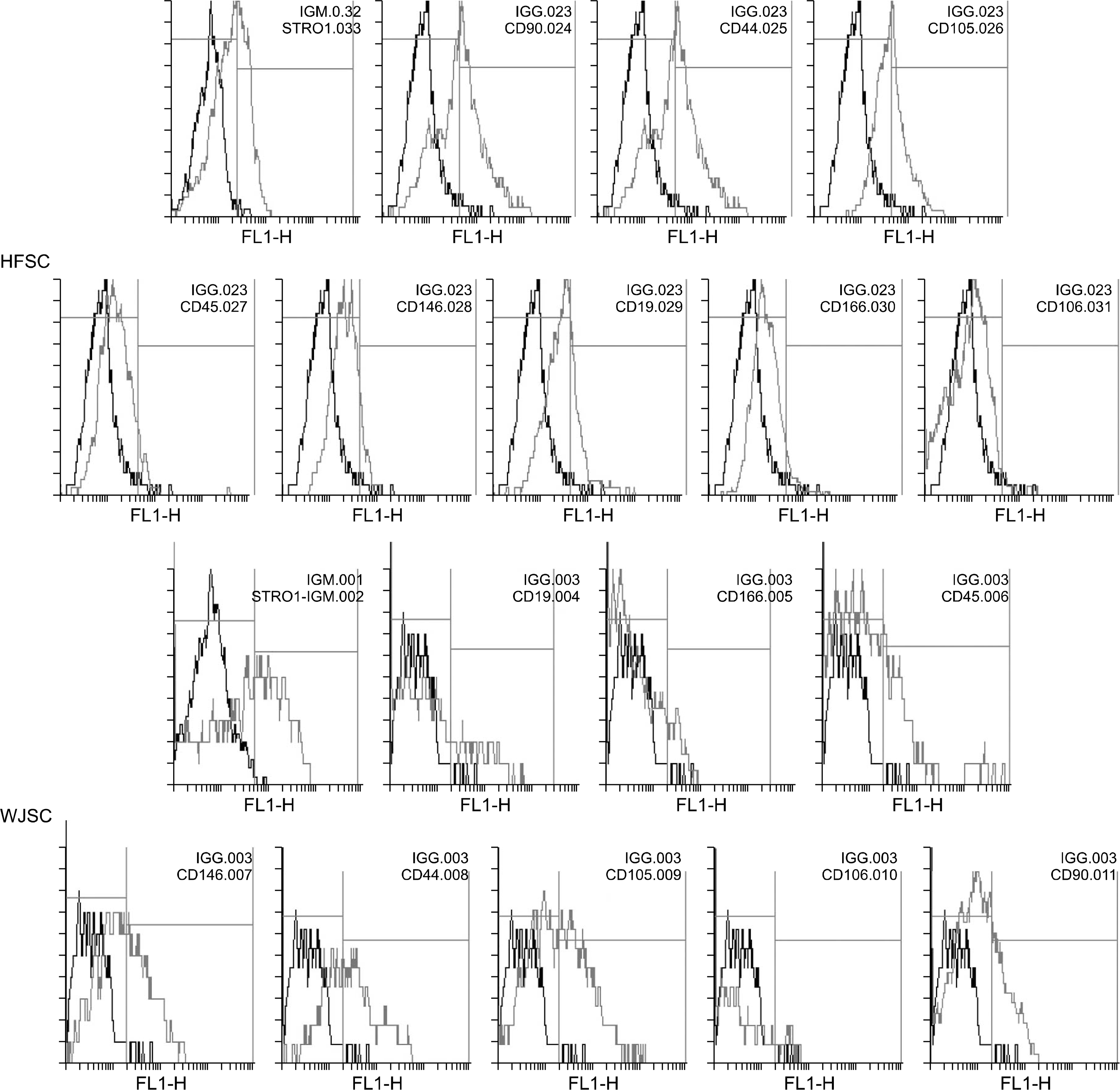
Human hair follicles decapsulated and cultured in un-coated culture flasks. After the first 15 days, the primary cells observed around the hair follicles were somewhat amorphous and mostly round shaped (Fig. 1E). In twentieth day of culture, the cells became elongated and in the next days of culture turn to fibroblast- like cells (Fig. 1F). HFSCs expressed Stro-1, CD90, CD44, CD105, CD146, CD166 and CD106 markers; these cells showed low expression of CD19 and CD45 surface markers (Fig. 3 and 4).
The outgrowth cells from human umbilical cord ex-plants displayed a spindle- shape and fibroblast- like morphology (Fig. 1; GandH). The immunophenotyping of UC- derived stromal cells indicated that these cells positive for common surface markers of MSCs and expressed Stro-1, CD44, CD90, CD105, CD106, CD146 and CD166 markers. Moreover, these cells revealed some levels of expression of CD19 and CD45 markers (Fig. 3 and 4).
Go to : 
Mesenchymal stem cells (MSCs) are regenerative population of cells in developed organs with potential of differentiation into osteocytes, adipocytes and chondrocytes in vitro. They are excellent candidate for cell therapy through their low immunogenicity, ease accessibility, broad differentiation potential and immunomodulatory effects (8). Multipotent mesenchymal stem cells derived from bone marrow (BM) first characterized and described in 1976 by Friedenstein et al. (9). Several researches demonstrated that BM-MSCs express important stem cell surface markers such as CD44, CD90, CD105 (SH2), CD166 and CD73 (SH3) and are negative for hematopoietic markers including CD14, CD34 and CD45 (10). Recent studies focused on discovery of MSC- Like cells from other tissues. In present study, we demonstrated that MSCs isolated from various tissues of the human body including SSCs, GCs, HFSCs and WJ- MSCs expressed BM- derived MSC surface markers.
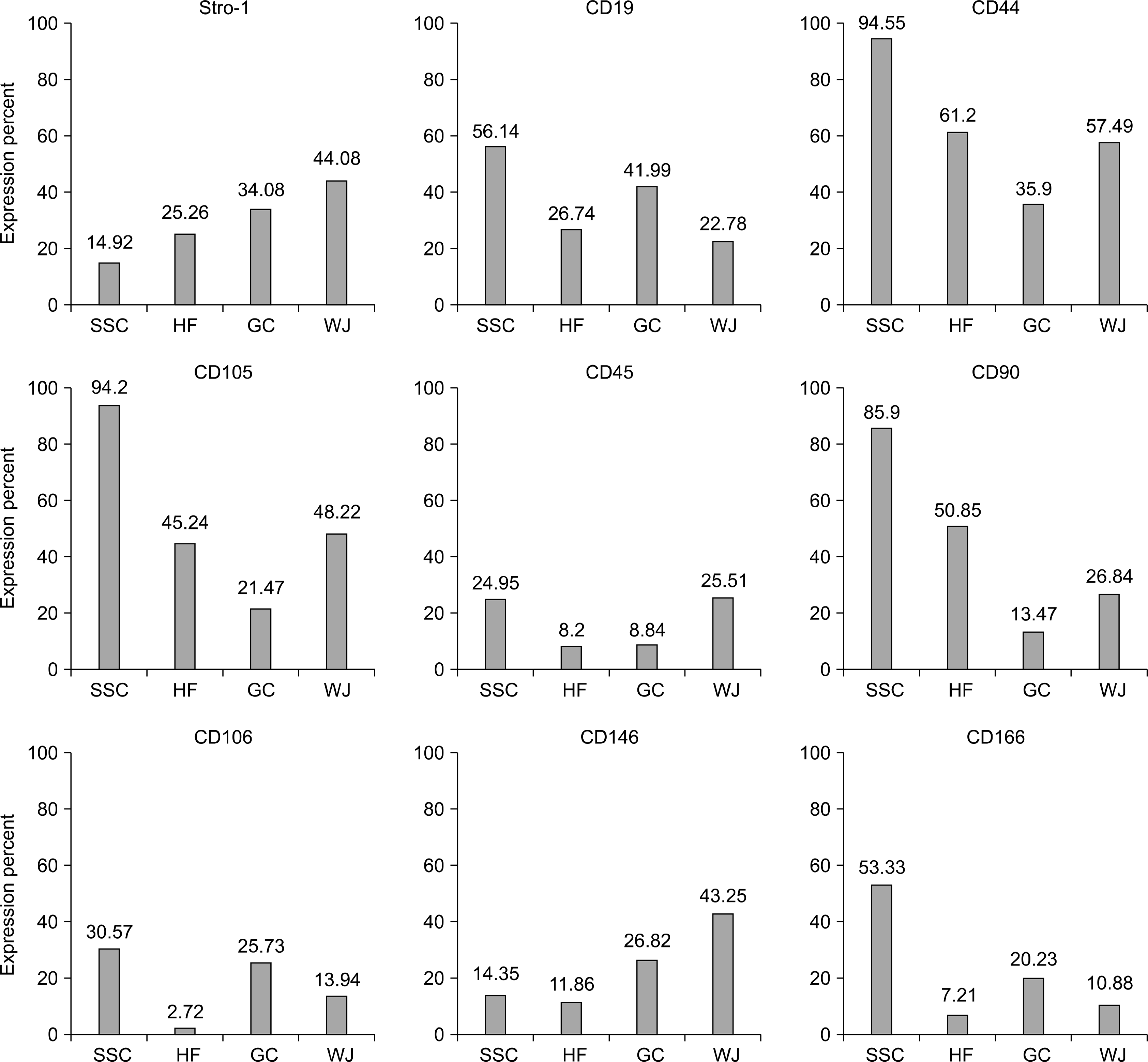
In this study we showed that, CD90 expressed by high percentage in SSCs (85.9%) and HFSCs (50.85%). Cluster differentiation 90 (CD90) which also known as Thy-1 is a cell surface anchored glycoprotein, expressed on human mesenchymal stem cells (MSCs) and have a role in cell-cell and cell- matrix interactions as well as cell motility (11). Our study also showed that GCs and WJ- MSCs expressed CD90 on 13.47% and 26.84% of cells, respectively. According to the expression of CD90 marker in SSCs, HFSCs, GCs and WJ- MSCs, this protein may have a role in stem cell growth and differentiation. CD44 or homing cell adhesion molecule (HCAM) also known as hyaluro-nate receptor is widely expressed on many cell types of stem cells (12). CD44 which expressed by 94.55% of SSCs, 61.2% of HFSCs, 57.49% of WJ-MSCs and 35.9% of GCs have considered that functions in cell adhesion, migration, homing, proliferation and apoptosis of stem cells and have role in stemness maintenance by involving in contact between stem cells/progenitor cells and their cellular niche (13, 14). Other MSC marker, CD105 (endoglin) also identified as SH2, is a component of the receptor complex of transforming growth factor- beta (TGF-β) which involved in cell proliferation, differentiation and migration (15). Our data showed that CD105 expressed on 94.2% of SSCs, 48.22% of WJ- MSCs, 45.24% of HFSCs and 21.47% of GCs. It is found that CD105+ MSCs are capable of differentiation toward adipogenic, osteogenic and chondrogenic lineages (16, 17). Vascular cell adhesion molecule-1 (VCAM-1) or CD106 is a member of the immunoglobulin (Ig) super-family which interacts with the integrin very late antigen-4 (VLA4). The VCAMl/VLA4 interaction mediates both adhesion and signal transduction (18). Furthermore, CD106 plays a role in T and B lymphocytes life cycle and biological function and is necessary for homing (19). Some studies demonstrated that VCAM1 act as an environmental sensor for responding to chemokines involved in tissue repair (20). As we shown in this study, CD106expressed in low percentage of our cells. In the case of CD146 stem cell marker, CD146 expressed on 43.25%, 26.82%, 14.35% and 11.86% of WJ-MSCs, GCs, SSCs and HFSCs, respectively. CD146 or M-CAM mediates cell-cell interactions and migration in endothelial cells (21). The intracellular tail of CD146 binds to ezrinradixin- moesin (ERM) protein that connects actin filaments with plasma membranes, therefore mediating cytoskeleton remodeling (22). Furthermore, CD146 is important for endothelial cell migration and angiogenesis (23), and promotes intermediate trophoblast invasion during pregnancy establishment (24). CD146 also involved in signal transduction and regulation of cellular functions (25). CD166 or ALCAM by acting as a homophilic adhesion protein and heterophilic binding to CD6 in hematopoiesis, play an important role in the immune response and in the nervous system (26). ALCAM express during organ development in the central and peripheral nervous system, sensory organs, hematopoiesis, endothelial and epithelial lineage (27). Homophilic (ALCAM-ALCAM) adhesion was shown to play important role in tight cell-to-cell interaction and regulation of stem cell differentiation (28). CD166 expressed on 53.33% of SSCs and have low expression pattern in GCs, WJ-MSCs and HFSCs. Stro-1 identified as acell surface antigen present in human bone marrow stromal cells. The Stro-1 positive populations are considerably enriched in clonogenic cells that were able to both generate CFU-Fs (colony-forming units-fibroblasts) and differentiate intomultiple mesenchymal lineages in vitro (29). Our data showed that stro-1 antigen have higher expression on WJ-MSC compared to GCs, HFSCs and SSCs. In fact, the expression of endoglin and stro-1 considered as phonotypic marker of mesenchymal stromal cell multi-potency (30, 31). The more primitive Stro-1positive MSCs have been shown to exert more powerful immunosuppressive effects in vitro and greater homing ability in animal models (32). The human CD19 antigen which identified as B4 antigen of human B lymphocytes is a transmembrane glycoprotein belonging to immunoglubolin (Ig) superfamily (33, 34) and is a specific biomarker of normal and neoplastic B cells (35). Previous studies showed that CD19 deficiency in human and mouse leading to hypo responsiveness to transmembrane signals (36). Our results showed that this protein expressed in 56.14% of SSCs, 26.74% of HFSCs, 41.99% of GCs and 22.78% of WJ-MSCs. Finally, in present study, we showed that CD45 expressed between 8 to 26 percent of our cells. CD45 is a transmembrane protein tyrosine phosphatase that expressed on all leukocytes including hematopoietic stem and progenitor cells. CD45 regulates lymphocyte maturation stages, specifically in their activation and proliferation. CD45 play multiple roles in regulation of autonomous motility of bone marrow progenitor cells and their retention (37). Our results revealed that SSCs were CD44+/CD90+/CD105+/CD166+. Previous studies demonstrated that SSCs from testis biopsies express CD90, CD105, CD166 and Stro-1 (5). In present study, HFSCs positively expressed CD44, CD90 and CD105. Similarly, the expression of CD90 and CD105 reported in stem cells isolated from human hair follicles (38). Immunophenotyping of GCs revealed that these cells positively expressed Stro-1, CD19, CD44, CD106, CD146 and CD166. Other reports showed that GCs identified as CD44+/CD90+/CD105+/CD166+ cells which highly express CD105 and CD166 (39). Finally, in this study we showed that, WJ-derived MSCs expressed Stro-1, CD44, CD105 and CD146. Previous studies revealed a positive response pattern expression of CD44, CD90, CD105, CD146 and CD166 detected at 4th passage (40).
Go to : 
In present study we aimed to isolate MSC-like cells from various sources and compare the expression of MSC specific cell surface markers in these cells. All four types of our cells including SSCs, HFSCs, GCs and WJ- derived MSCs exhibited the ability to attach plastic culture flask and expressed the CD44, CD90, CD105, CD106, CD146, CD166 and Stro-1 cell surface antigens which considered as common characteristics for mesenchymal stem cells. As we described above, high expression of CD44 in SSCs, HFSCs, GCs and WJ-MSCs may help them to maintain stemness properties. Furthermore, we suggest that the high expression of CD105 in SSCs, HFSCs and WJ-MSCs may have role osteogenic differentiation potential in these cells. Moreover, considerably high expression of CD90 in SSCs and HFSCs may associate to higher growth and differentiation potential of these cells. Further, although MSC identified as CD19− cells, we suggest that the presence of CD19 on SSCs and GCs may help them to efficiency in response to transmembrane signals. Thus, these four types of MSCs may be useful in clinical applications and cell therapy.
Go to : 
ACKNOWLEDGMENTS
This work supported by ACECR East Azarbaijan branch, Azarbaijan ART center. Our group special thanks to Royan institute’s flow cytometry lab.
Go to : 
References
1. Morrison SJ, Spradling AC. Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cell. 2008. 132:598–611.

2. Avasthi S, Srivastava RN, Singh A, Srivastava M. Stem Cell: past, present and future: a review article. Internet Journal of Medical Update. 2008. 3:22–30.

3. Sakaguchi Y, Sekiya I, Yagishita K, Muneta T. Comparison of human stem cells derived from various mesenchymal tissues: superiority of synovium as a cell source. Arthritis Rheum. 2005. 52:2521–2529.

4. Erices A, Conget P, Minguell JJ. Mesenchymal progenitor cells in human umbilical cord blood. Br J Haematol. 2000. 109:235–242.

5. Gonzalez R, Griparic L, Vargas V, Burgee K, Santacruz P, Anderson R, Schiewe M, Silva F, Patel A. A putative mesenchymal stem cells population isolated from adult human testes. Biochem Biophys Res Commun. 2009. 385:570–575.

6. Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006. 8:315–317.

7. Xu J, Woods CR, Mora AL, Joodi R, Brigham KL, Iyer S, Rojas M. Prevention of endotoxin-induced systemic response by bone marrow-derived mesenchymal stem cells in mice. Am J Physiol Lung Cell Mol Physiol. 2007. 293:L131–L141.

8. Helmy KY, Patel SA, Silverio K, Pliner L, Rameshwar P. Stem cells and regenerative medicine: accomplishments to date and future promise. Ther Deliv. 2010. 1:693–705.

9. Friedenstein AJ, Gorskaja JF, Kulagina NN. Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp Hematol. 1976. 4:267–274.
10. Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999. 284:143–147.

11. Rege TA, Hagood JS. Thy-1 as a regulator of cell-cell and cell-matrix interactions in axon regeneration, apoptosis, adhesion, migration, cancer, and fibrosis. FASEB J. 2006. 20:1045–1054.

12. Schieker M, Pautke C, Reitz K, Hemraj I, Neth P, Mutschler W, Milz S. The use of four-colour immunofluorescence techniques to identify mesenchymal stem cells. J Anat. 2004. 204:133–139.

13. Zhu H, Mitsuhashi N, Klein A, Barsky LW, Weinberg K, Barr ML, Demetriou A, Wu GD. The role of the hyaluronan receptor CD44 in mesenchymal stem cell migration in the extracellular matrix. Stem Cells. 2006. 24:928–935.

14. Knudson W, Loeser RF. CD44 and integrin matrix receptors participate in cartilage homeostasis. Cell Mol Life Sci. 2002. 59:36–44.

15. Aslan H, Zilberman Y, Kandel L, Liebergall M, Oskouian RJ, Gazit D, Gazit Z. Osteogenic differentiation of non-cultured immunoisolated bone marrow-derived CD105+ cells. Stem Cells. 2006. 24:1728–1737.

16. Roura S, Farré J, Soler-Botija C, Llach A, Hove-Madsen L, Cairó JJ, Gòdia F, Cinca J, Bayes-Genis A. Effect of aging on the pluripotential capacity of human CD105+ mesenchymal stem cells. Eur J Heart Fail. 2006. 8:555–563.

17. Barry FP, Boynton RE, Haynesworth S, Murphy JM, Zaia J. The monoclonal antibody SH-2, raised against human mesenchymal stem cells, recognizes an epitope on endoglin (CD105). Biochem Biophys Res Commun. 1999. 265:134–139.

18. Pepinsky B, Hession C, Chen LL, Moy P, Burkly L, Jakubowski A, Chow EP, Benjamin C, Chi-Rosso G, Luhowskyj S, et al. Structure/function studies on vascular cell adhesion molecule-1. J Biol Chem. 1992. 267:17820–17826.

19. Kwee L, Baldwin HS, Shen HM, Stewart CL, Buck C, Buck CA, Labow MA. Defective development of the embryonic and extraembryonic circulatory systems in vascular cell adhesion molecule (VCAM-1) deficient mice. Development. 1995. 121:489–503.

20. Kundrotas G. Surface markers distinguishing mesenchymal stem cells from fibroblasts. ActaMedica Lituanica. 2012. 19:75–79.

21. Ouhtit A, Gaur RL, Abd Elmageed ZY, Fernando A, Thouta R, Trappey AK, Abdraboh ME, El-Sayyad HI, Rao P, Raj MG. Towards understanding the mode of action of the multifaceted cell adhesion receptor CD146. Biochim Biophys Acta. 2009. 1795:130–136.

22. Tremmel M, Matzke A, Albrecht I, Laib AM, Olaku V, Ballmer-Hofer K, Christofori G, Héroult M, Augustin HG, Ponta H, Orian-Rousseau V. A CD44v6 peptide reveals a role of CD44 in VEGFR-2 signaling and angiogenesis. Blood. 2009. 114:5236–5244.

23. Kang Y, Wang F, Feng J, Yang D, Yang X, Yan X. Knockdown of CD146 reduces the migration and proliferation of human endothelial cells. Cell Res. 2006. 16:313–318.

24. Liu Q, Zhang B, Zhao X, Zhang Y, Liu Y, Yan X. Blockade of adhesion molecule CD146 causes pregnancy failure in mice. J Cell Physiol. 2008. 215:621–626.

26. Ohneda O, Ohneda K, Arai F, Lee J, Miyamoto T, Fukushima Y, Dowbenko D, Lasky LA, Suda T. ALCAM (CD166): its role in hematopoietic and endothelial development. Blood. 2001. 98:2134–2142.

27. Swart GW. Activated leukocyte cell adhesion molecule (CD166/ALCAM): developmental and mechanistic aspects of cell clustering and cell migration. Eur J Cell Biol. 2002. 81:313–321.

28. Fujiwara H, Tatsumi K, Kosaka K, Sato Y, Higuchi T, Yoshioka S, Maeda M, Ueda M, Fujii S. Human blastocysts and endometrial epithelial cells express activated leukocyte cell adhesion molecule (ALCAM/CD166). J Clin Endocrinol Metab. 2003. 88:3437–3443.

29. Simmons PJ, Torok-Storb B. Identification of stromal cell precursors in human bone marrow by a novel monoclonal antibody, STRO-1. Blood. 1991. 78:55–62.

30. Mareschi K, Ferrero I, Rustichelli D, Aschero S, Gammaitoni L, Aglietta M, Madon E, Fagioli F. Expansion of mesenchymal stem cells isolated from pediatric and adult donor bone marrow. J Cell Biochem. 2006. 97:744–754.

31. Halfon S, Abramov N, Grinblat B, Ginis I. Markers distinguishing mesenchymal stem cells from fibroblasts are down-regulated with passaging. Stem Cells Dev. 2011. 20:53–66.

32. Bensidhoum M, Chapel A, Francois S, Demarquay C, Mazurier C, Fouillard L, Bouchet S, Bertho JM, Gourmelon P, Aigueperse J, Charbord P, Gorin NC, Thierry D, Lopez M. Homing of in vitro expanded Stro-1− or Stro-1+ human mesenchymal stem cells into the NOD/SCID mouse and their role in supporting human CD34 cell engraftment. Blood. 2004. 103:3313–3319.

33. Carter RH, Barrington RA. Signaling by the CD19/CD21 complex on B cells. Curr Dir Autoimmun. 2004. 7:4–32.

34. Thierry-Mieg D, Thierry-Mieg J. AceView: a comprehensive cDNA-supported gene and transcripts annotation. Genome Biol . 2006. 7(Suppl 1):S12.1–14.
35. Wang K, Wei G, Liu D. CD19: a biomarker for B cell development,lymphoma diagnosis and therapy. Experimental Hematology and Oncology. 2012. 1:1–7.
36. Poe JC, Minard-Colin V, Kountikov EI, Haas KM, Tedder TF. A c-Myc and surface CD19 signaling amplification loop promotes B cell lymphoma development and progression in mice. J Immunol. 2012. 189:2318–2325.

37. Shivtiel S, Kollet O, Lapid K, Schajnovitz A, Goichberg P, Kalinkovich A, Shezen E, Tesio M, Netzer N, Petit I, Sharir A, Lapidot T. CD45 regulates retention, motility, and numbers of hematopoietic progenitors, and affects osteoclast remodeling of metaphyseal trabecules. J Exp Med. 2008. 205:2381–2395.

38. Wang Y, Liu J, Tan X, Li G, Gao Y, Liu X, Zhang L, Li Y. Induced pluripotent stem cells from human hair follicle mesenchymal stem cells. Stem Cell Rev. 2013. 9:451–460.

Go to : 




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download