Abstract
Background
The normal cells derived from human embryonic stem cells (hESCs) are regarded as substitutes for damaged or dysfunctional adult cells. However, tumorigenicity of hESCs remains a major challenge in clinical application of hESC-derived cell transplantation. Previously, we generated monoclonal antibody (MAb) 57-C11 specific to the surface molecule on undifferentiated hESCs. The aim of this study is to prove whether 57-C11-positive hESCs are pluripotent and tumorigenic in immunodeficient mice.
Methods
Undifferentiated hESCs were mixed with retinoic acid (RA)-differentiated hESCs at different ratios prior to 57-C11-mediated separation. To isolate 57-C11-positive hESCs from the mixture, biotinylated 57-C11 and streptavidin-coated magnetic beads were added to the mixture. Unbound 57-C11-negative hESCs were first isolated after applying magnet to the cell mixture, and 57-C11-bound hESCs were then released from the magnetic beads. In order to measure the efficiency of separation, 57-C11-positive or -negative hESCs were counted after isolation. To evaluate the efficiency of teratoma formation in vivo, 57-C11-positive or negative cells were further injected into left and right, respectively, testes of nonobese diabetic/severe combined immunodeficiency (NOD/SCID) mice.
Results
Approximately 77~100% of undifferentiated hESCs were isolated after applying 57-C11-coated magnetic beads to the mixed cell populations. Importantly, teratomas were not observed in NOD/SCID mice after the injection of isolated 57-C11-negative hESCs, whereas teratomas were observed with 57-C11-positive hESCs.
Human embryonic stem cells (hESCs) have been regarded as a potential source of therapeutic cells for many diseases caused by tissue loss or dysfunction (1). Because of the self-renewal and pluripotency of hESCs, however, teratoma formation is a seriously raised concern whenever utilizing hESC-derived differentiated cells (2, 3). The number of hESC-derived cell transplantation studies into animal models have been done, but teratoma formation was not detected depending on experimental conditions (4–6). Longer in vitro differentiation of hESCs seems to reduce the risk of teratoma formation due to low survival rate of hESC-derived cells in animal models. However, survived dopaminergic implants in parkinsonian rats exhibit expanding cores of undifferentiated mitotic neuroepithelial cells, which can be tumorigenic (7). Furthermore, hESC-derived neural precursor cells have repeatedly induced tumors in animal models even though remaining hESCs were not detected (8, 9). Thus, the elimination of remaining undifferentiated hESCs is a major hurdle to hESC-derived cell transplantation.
Previously, we generated a panel of MAbs against surface molecules on undifferentiated hESCs (10). One of the MAbs, 57-C11 (IgG1, k), recognizes the phosphorylated form of adenovirus early-1B-55kDa associated protein 5 (E1B-AP5) or heterogeneous nuclear ribonucleoprotein U-like 1 (hnRNPUL1) (11). E1B-AP5 is a member of the heterogeneous nuclear ribonucleoprotein (hnRNP) family and is ubiquitously expressed in the nucleus and cytoplasm in all tissues (12). However, we showed for the first time that 57-C11-reactive E1B-AP5 is expressed on the cell surface of undifferentiated hESCs and cell surface-expressed E1B-AP5 is downregulated upon the differentiation of hESCs, suggesting that cell surface-expressed E1B-AP5 is a specific surface marker for undifferentiated hESCs (11). In this study, we have tested whether 57-C11 is able to eliminate undifferentiated hESCs from the mixture of undifferentiated and differentiated hESCs. To isolate 57-C11-positive hESCs, 57-C11 was biotinylated and added to the mixture of undifferentiated and differentiated hESCs. Sequentially, streptavidin-coated magnetic beads were added into the same tube. After applying magnet to the mixture, 57-C11-positive and negative hESCs were separated and analyzed. The isolated cells were further validated in in vivo animal model by injecting the isolated cells into right and left testes of the same NOD/SCID mice. The results showed that teratoma formation was not detected with 57-C11-negative hESCs whereas teratoma was detected with 57-C11-positve hESCs. The results indicate that 57-C11-positive hESCs are really pluripotent and tumorigenic while 57-C11-negative hESCs are not. The results also suggest that 57-C11-mediated isolation system will be useful for the elimination of residual undifferentiated hESCs from the preparation of hESC-derived differentiated cells.
H9 hESCs were cultured on irradiated mouse embryonic fibroblast (MEF) as described previously (13, 14). hESCs were fed daily with DMEM/F12 medium supplemented with 20% Knockout serum replacement (Life Technologies, Seoul, Korea), 0.1 mM 2-Mercaptoethanol, 1% Non-essential amino acid, 1 mM glutamine, 100 U/ml penicillin G, 100 μg/ml streptomycin, and 4 ng/ml basic fibroblast growth factor (PeproTech, Rocky Hill, NJ, USA). Feeder-free culture method was performed as described previously (13, 15). Briefly, 10 μM of Y27632 (Sigma-Aldrich, Seoul, Korea) were treated for 1 hour prior to detaching hESCs, the cells were harvested with trypsin-EDTA. After elimination of MEF cells on gelatin coated tissue culture dish, hESCs were cultured in conditioned medium supplemented with 10 μM of Y27632 on tissue culture dish coated with Matrigel (BD Biosciences, Seoul, Korea). For differentiation of hESCs, cells were treated 10−5 M retinoic acids (RA) in culture medium for 10~14 days. Colo205 cells were maintained following recommendation of ATCC.
Antibody was biotinylated by using DSB-XTM biotin labeling kit (Life Technologies) according to manufacturer’s procedure.
Cell isolation was carried out by using Dynabeads FlowComp Flexi kit (Life Technologies) according to the manufacturer’s protocol. The cells were detached with dissociation solution for human ES/iPS cells (ReproCELL). To prevent physiological cell damage, dissociation buffer was immediately neutralized by complete medium. The mixture of undifferentiated and RA-treated hESCs were suspended in 500 μl of cold isolation buffer (phosphate buffered saline (PBS), pH 7.4, 5% Knockout serum replacement, 1 μg/ml Doxycyclin, 0.5 mM Mg2+, 1 mM Ca2+) in Eppendorf tube and incubated with 20 μg of biotinylated 57-C11 for 30 min at 4°C with rolling and tilting. Cells were harvested after addition of 500 μl of cold isolation buffer. Cells (2×106) were then resuspended in 500 μl of cold isolation buffer and incubated for 30 min at 4°C with rolling and tilting after addition of 50 μl (7.5×107 beads) of Dynabeads (Life Technologies). 57-C11-positive hESCs were collected by placing the tube in a magnet for 2~3 min. The supernatant containing 57-C11-negative hESCs was carefully removed in the magnet, and the bead/cell complexes were cleaned up by a total of three washes. Cells were then removed from the magnet by incubation of 1 ml of FlowComp Release Buffer (Life Technologies) for 10 min at room temperature (RT) with rolling and tilting. The supernatant with 57-C11-positive hESCs was carefully transferred to a new tube and put on the magnet again to remove residual beads. The supernatant with 57-C11-positive hESCs was subjected to centrifugation at 600 × g for 10 min, and the bead free-cells were resuspended by stepwise addition of a 1:300 mixture of PBS: Matrigel (BD Bioscience) to a final total volume of 50 μl before injection.
The procedure of teratoma formation experiments was performed as described (16). All procedures were carried out under guidelines approved by the Institutional Review Board (SJU-2015-008) and Institutional Animal Care and Use Committee (IACUC No. SJ-20140801) of Sejong University. Male NOD/SCID mice (8~12 weeks) were used for all experiments. All procedures were carried out in a biological safety cabinet under sterile conditions using sterile materials. Injection of hESCs into testes was done under anesthesia. Mice received the following anesthetics and analgesics: Zoletil 50 (30 mg/kg)/Rompun (9.32 mg/kg). For testis injection, a sterile 1 ml syringe with a 31 G needle was injected into testes with hESC suspension (50 μl). After successful injection, the needle was retracted swiftly, the injection site was pressed with a cotton swab for about 1 min, and then a sterile, resorbable wound pad was placed at the injection site. Teratomas were surgically removed at 12~18 weeks postinjection under sterile conditions. The sizes of teratomas were measured by a ruler.
Flow cytometry analysis was performed described as previous study (10). Briefly, the undifferentiated H9, RA-induced differentiated H9 cells or Colo205 cells were incubated with 20 μg of DSB-X biotin-labeled 57-C11. After isolation process described above, the cells were washed with PBS (pH7.4) supplemented with 1% bovine serum albumin (PBA). The cells were incubated with 57-C11 or biotin-labeled 57-C11 for 30 min, followed by fluorescein isothiocyanate-conjugated anti-mIgG or streptavidin-phycoerythrin, depending on the isotypes of the primary antibodies. After washing with PBA, Propidium iodide-negative cells were analyzed by FACScalibur (BD biosciences).
Previously, we found that 57-C11-reactive E1B-AP5 is closely associated with pluripotent and undifferentiated H9 hESCs and the surface expression of 57-C11-reactive E1B-AP5 is downregulated during the differentiation of H9 cells (11). To test whether 57-C11 is able to specifically eliminate undifferentiated H9 cells from the mixture of undifferentiated and differentiated H9 cell population, in this study, we have developed a 57-C11-mediated magnetic cell separation system, based on the specificity of 57-C11 to undifferentiated H9 cells (11). First, 57-C11 was biotinylated and 57-C11-bound H9 cells were analyzed by flow cytometry analysis with streptavidin-phycoerythrin conjugate. As shown in Fig. 1A, H9 cells (106 cells) were readily detected with biotin-labeled 57-C11 at final concentrations of 10 or 30 μg/ml. As compared with that of 10 μg/ml of biotin-labeled 57-C11, 57-C11 binding to H9 cells were not significantly increased with 30 μg/ml of biotin-labeled 57-C11, indicating that 10 μg/ml of biotin-labeled 57-C11 is enough to analyze one million hESCs. To confirm whether biotin-labeled 57-C11/streptavidin-magnetic beads were able to specifically isolate undifferentiated H9 cells from the mixed cell population, CA142 (IgG1, k), a MAb specific to surface antigen on Mycoplasma hyorhinis, was also biotinylated and used as a control isotype antibody (17). When the mixture of undifferentiated H9 hESCs and Colo205 cells were incubated with 10 or 20 μg/ml of biotin-labeled 57-C11 and subjected to the streptavidin-magnetic beads-associated isolation procedure, 57-C11-positive H9 cells were clearly separated with 20 μg/ml of biotin-labeled 57-C11 (data not shown). To reflect the possible scenario of contaminating hESC in mixed cell populations, the mixture of undifferentiated and differentiated hESCs were incubated with 20 μg/ml of biotin-labeled 57-C11 and subjected to the same isolation procedure (Fig. 2A). 57-C11-positive undifferentiated H9 cells were mostly removed with 20 μg/ml of biotin-labeled 57-C11 while the same H9 cells were not removed with the irrelevant antibody CA142 (data not shown and Fig. 1B). Thus, it seems that 57-C11/streptavidin beads is able to specifically isolate 57-C11-positive undifferentiated hESCs from the mixed cell population.
To examine the applicability and efficiency of this separation strategy, we prepared several pools of undifferentiated and differentiated H9 cells at different ratios (0, 10, 30, 50, or 100% undifferentiated H9 hESCs) and performed the same 57-C11-mediated isolation procedure (Fig. 2A). Approximately 10% of hESCs were recovered from 1×105 cells of differentiated H9 cells after the isolation procedure, suggesting that some cells are isolated from the differentiated H9 cells during the isolation procedure (Fig. 1C). Approximately 91% of hESCs were recovered from 1×105 cells of undifferentiated H9 cells after the isolation procedure (Fig. 1C). Our previous studies showed that approximately 89~96% of undifferentiated H9 cells were 57-C11-positive (11). Therefore, it seems that undifferentiated hESCs are almost completely recovered through the isolation procedure. When undifferentiated and differentiated hESCs were mixed at different ratios, approximately 77~100% of undifferentiated H9 hESCs were recovered after the isolation procedure (Fig. 1C). Thus, the spiking experiments suggest that the recovery rate of 57-C11-positive hESCs from mixed cell populations is approximately 77~100%.
Before we validated whether isolated 57-C11-positive H9 cells were tumorigenic in NOD/SCID mice, we tested how many undifferentiated hESCs were able to form teratomas in NOD/SCID mice. Undifferentiated hESCs rapidly undergo apoptosis after the preparation of single cell suspensions from the colonies. Therefore, more than one million hESCs are recommended to use for teratoma formation in immunodeficient mice (16, 18). When we injected with 1×105, 4×105, 1×106, or 2×106 cells of undifferentiated and RA-differentiated H9 cells into the left and right testes of the same mice, respectively, we found that undifferentiated H9 cells could form teratomas with 4×105 cells under our experimental condition 8~18 weeks after injections while RA-differentiated H9 cells were not able to do (Fig. 3).
The overall scheme of the experiment including magnetic cell separation of 57-C11-positive and -negative H9 hESCs from mixed cell populations and teratoma formation of isolated H9 hESCs in NOD/SCID mice is shown in Fig. 2A. Before the injection of 57-C11-positive and -negative hESCs into NOD/SCID mice, the viability of isolated H9 cells were tested. After the isolation procedure, approximately 85% of 57-C11-negative cells remained alive while only 25% of 57-C11 positive cells remained alive (Fig. 2B). It seems that the releasing processes from magnetic beads are harmful to 57-C11-positive H9 cells. Therefore, the viability of injected hESCs were checked before injection and 1×106 cells of 57-C11-positive and negative hESCs were injected into left and right testes of NOD/SCID mice. Untreated and RA-differentiated H9 cells were also injected into NOD/SCID mice as a control experiment in the same way. When the first set of mice were opened at 12 weeks postinjection, teratoma formation was not observed even with untreated H9 cells (Fig. 4A). When the second set of mice were opened at 18 weeks postinjection, teratoma formation was observed with 57-C11-positive H9 cells or untreated H9 cells whereas it was not observed with 57-C11-negative H9 cells or RA-treated H9 cells (Fig. 4A and 4B). The results suggest that 57-C11-positive hESCs are undifferentiated and pluripotent hESCs whereas 57-C11-negative hESCs are not.
Previously, we generated the MAb 57-C11 that recognizes the phosphorylated form of E1B-AP5 on the surface of undifferentiated hESCs (10, 11). Further studies revealed that the expression of 57-C11-reactive E1B-AP5 has a close relationship with pluripotent marker expression and is downregulated during the differentiation of hESCs (11), suggesting that 57-C11-reactive E1B-AP5 will be a specific surface marker for undifferentiated hESCs. To prove whether 57-C11-reactive E1B-AP5 is a specific surface marker for undifferentiated hESCs in vivo, we isolated 57-C11-positive and -negative hESCs by magnetic separation system and tested the capacity of teratoma formation in NOD/SCID mice. The results showed that 57-C11-positive hESCs had capacity to form teratoma whereas 57-C11-negative hESCs had not, suggesting that 57-C11-positve hESCs are really pluripotent and tumorigenic. The result also suggests that 57-C11 would be a good tool to separate remaining undifferentiated hESCs from the mixed cell population.
In the first set of injection into 2 NOD/SCID mice, teratoma formation was not observed even with untreated H9 cells at 12 weeks postinjection (Fig. 4A). To generate consistent teratomas in immunodeficient mice, many researchers have injected more than one million undifferentiated hESCs. Generally, hESCs rapidly undergo apoptosis after the preparation of single cell suspensions from the colonies even with Y-27632, the selective inhibitor of Rho-associated, coiled-coil containing protein kinase (19). Therefore, we assumed that although injected cells were alive, they were damaged and were not healthy enough to form teratomas at 12 weeks postinjection. In the second set of injection with the same cell preparation, therefore, the hESCs were given more time to recover from the damage. At 18 weeks postinjection, teratomas were observed in both untreated H9 and 57-C11-positive H9 cells. However, the teratoma size of 57-C11-positive hESCs were still smaller than that of untreated hESCs (Fig. 4B). In the first step of the isolation of 57-C11-positive hESCs, 57-C11-positive hESCs were readily separated by streptavidin-magnetic beads from mixed hESC population. After further separation of 57-C11-positive hESCs from magnetic beads, however, only 25% of isolated 57-C11-positive hESCs remained alive (Fig. 3B). Therefore, it is tempting to speculate that 57-C11-positive hESCs suffer from cytotoxic damage during the releasing processes from magnetic beads. More investigation needs to safely separate captured hESCs from magnetic beads.
Acknowledgments
This study was supported in part by the National Research Foundation of Korea (2016918220, 2016008610, and 2016903249). We thank Saerom Lee for helping us illustrate graphic strategy.
References
1. Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998; 282:1145–1147. DOI: 10.1126/science.282.5391.1145. PMID: 9804556.

2. Vogel G. Cell biology. Ready or not? Human ES cells head toward the clinic. Science. 2005; 308:1534–1538. DOI: 10.1126/science.308.5728.1534. PMID: 15947149.
3. Hentze H, Graichen R, Colman A. Cell therapy and the safety of embryonic stem cell-derived grafts. Trends Biotechnol. 2007; 25:24–32. DOI: 10.1016/j.tibtech.2006.10.010.

4. Zhang SC, Wernig M, Duncan ID, Brüstle O, Thomson JA. In vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nat Biotechnol. 2001; 19:1129–1133. DOI: 10.1038/nbt1201-1129. PMID: 11731781.

5. Laflamme MA, Gold J, Xu C, Hassanipour M, Rosler E, Police S, Muskheli V, Murry CE. Formation of human myocardium in the rat heart from human embryonic stem cells. Am J Pathol. 2005; 167:663–671. DOI: 10.1016/S0002-9440(10)62041-X. PMID: 16127147. PMCID: 1698736.

6. Brederlau A, Correia AS, Anisimov SV, Elmi M, Paul G, Roybon L, Morizane A, Bergquist F, Riebe I, Nannmark U, Carta M, Hanse E, Takahashi J, Sasai Y, Funa K, Brundin P, Eriksson PS, Li JY. Transplantation of human embryonic stem cell-derived cells to a rat model of Parkinson’s disease: effect of in vitro differentiation on graft survival and teratoma formation. Stem Cells. 2006; 24:1433–1440. DOI: 10.1634/stemcells.2005-0393. PMID: 16556709.

7. Roy NS, Cleren C, Singh SK, Yang L, Beal MF, Goldman SA. Functional engraftment of human ES cell-derived dopaminergic neurons enriched by coculture with telomerase-immortalized midbrain astrocytes. Nat Med. 2006; 12:1259–1268. DOI: 10.1038/nm1495. PMID: 17057709.

8. Doi D, Morizane A, Kikuchi T, Onoe H, Hayashi T, Kawasaki T, Motono M, Sasai Y, Saiki H, Gomi M, Yoshikawa T, Hayashi H, Shinoyama M, Refaat MM, Suemori H, Miyamoto S, Takahashi J. Prolonged maturation culture favors a reduction in the tumorigenicity and the dopaminergic function of human ESC-derived neural cells in a primate model of Parkinson’s disease. Stem Cells. 2012; 30:935–945. DOI: 10.1002/stem.1060. PMID: 22328536.

9. Seminatore C, Polentes J, Ellman D, Kozubenko N, Itier V, Tine S, Tritschler L, Brenot M, Guidou E, Blondeau J, Lhuillier M, Bugi A, Aubry L, Jendelova P, Sykova E, Perrier AL, Finsen B, Onteniente B. The postischemic environment differentially impacts teratoma or tumor formation after transplantation of human embryonic stem cell-derived neural progenitors. Stroke. 2010; 41:153–159. DOI: 10.1161/STROKEAHA.109.563015.

10. Choi HS, Kim H, Won A, Kim JJ, Son CY, Kim KS, Ko JH, Lee MY, Kim CH, Ryu CJ. Development of a decoy immunization strategy to identify cell-surface molecules expressed on undifferentiated human embryonic stem cells. Cell Tissue Res. 2008; 333:197–206. DOI: 10.1007/s00441-008-0632-6. PMID: 18560898.

11. Choi HS, Kim WT, Kim H, Kim JJ, Ko JY, Lee SW, Jang YJ, Kim SJ, Lee MJ, Jung HS, Kzhyshkowska J, Um SJ, Lee MY, Lee SH, Kim CH, Ryu CJ. Identification and characterization of adenovirus early region 1B-associated protein 5 as a surface marker on undifferentiated human embryonic stem cells. Stem Cells Dev. 2011; 20:609–620. DOI: 10.1089/scd.2010.0265.

12. Gabler S, Schütt H, Groitl P, Wolf H, Shenk T, Dobner T. E1B 55-kilodalton-associated protein: a cellular protein with RNA-binding activity implicated in nucleocytoplasmic transport of adenovirus and cellular mRNAs. J Virol. 1998; 72:7960–7971. PMID: 9733834. PMCID: 110131.

13. Kim WT, Seo Choi H, Min Lee H, Jang YJ, Ryu CJ. B-cell receptor-associated protein 31 regulates human embryonic stem cell adhesion, stemness, and survival via control of epithelial cell adhesion molecule. Stem Cells. 2014; 32:2626–2641. DOI: 10.1002/stem.1765. PMID: 24898727.

14. Kim WT, Choi HS, Hwang HJ, Jung HS, Ryu CJ. Epitope mapping of antibodies suggests the novel membrane topology of B-cell receptor associated protein 31 on the cell surface of embryonic stem cells: the novel membrane topology of BAP31. PLoS One. 2015; 10:e0130670. DOI: 10.1371/journal.pone.0130670. PMID: 26102500. PMCID: 4478030.

15. Choi HS, Lee HM, Jang YJ, Kim CH, Ryu CJ. Heterogeneous nuclear ribonucleoprotein A2/B1 regulates the self-renewal and pluripotency of human embryonic stem cells via the control of the G1/S transition. Stem Cells. 2013; 31:2647–2658. DOI: 10.1002/stem.1366. PMID: 23495120.

16. Hentze H, Soong PL, Wang ST, Phillips BW, Putti TC, Dunn NR. Teratoma formation by human embryonic stem cells: evaluation of essential parameters for future safety studies. Stem Cell Res. 2009; 2:198–210. DOI: 10.1016/j.scr.2009.02.002. PMID: 19393593.

17. Choi HS, Lee HM, Kim WT, Kim MK, Chang HJ, Lee HR, Joh JW, Kim DS, Ryu CJ. Detection of mycoplasma infection in circulating tumor cells in patients with hepatocellular carcinoma. Biochem Biophys Res Commun. 2014; 446:620–625. DOI: 10.1016/j.bbrc.2014.03.024. PMID: 24637212.

18. Choo AB, Tan HL, Ang SN, Fong WJ, Chin A, Lo J, Zheng L, Hentze H, Philp RJ, Oh SK, Yap M. Selection against undifferentiated human embryonic stem cells by a cytotoxic antibody recognizing podocalyxin-like protein-1. Stem Cells. 2008; 26:1454–1463. DOI: 10.1634/stemcells.2007-0576. PMID: 18356574.

19. Watanabe K, Ueno M, Kamiya D, Nishiyama A, Matsumura M, Wataya T, Takahashi JB, Nishikawa S, Muguruma K, Sasai Y. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat Biotechnol. 2007; 25:681–686. DOI: 10.1038/nbt1310. PMID: 17529971.

Fig. 1
Characterization of biotinlyated 57-C11 antibody. (A) Biotinylation efficiency of 57-C11 was tested by flow cytometry analysis. H9 cells were reacted with 10 and 30 μg/ml of un-biotinylated or biotinylated 57-C11. The binding of antibody was measured with FITC-conjugated anti-mIgG or PE-conjugated streptavidin. (B) Flow cytometry analysis of H9 cells with 57-C11. After isolation with 20 μg/ml of biotinylated 57-C11 or CA142 from the mixed cells, the surface binding for 57-C11 was confirmed in 57-C11-positive/negative and CA142-positive/negative cells, respectively. (C) Recovery rate of undifferentiated H9 cells after isolation with 57-C11. Undifferentiated and RA-differentiated H9 cells were mixed at different ratios and undifferentiated H9 cells were recovered with 57-C11-mediated isolation procedure. The 57-C11-mediated isolation procedure nonspecifically recovered approximately 10% cells (1.08×104 cells) from 10×104 RA-differentiated H9 cells. Therefore, the number was subtracted from all the numbers of recovered cells in the calculation of the recovery rate.
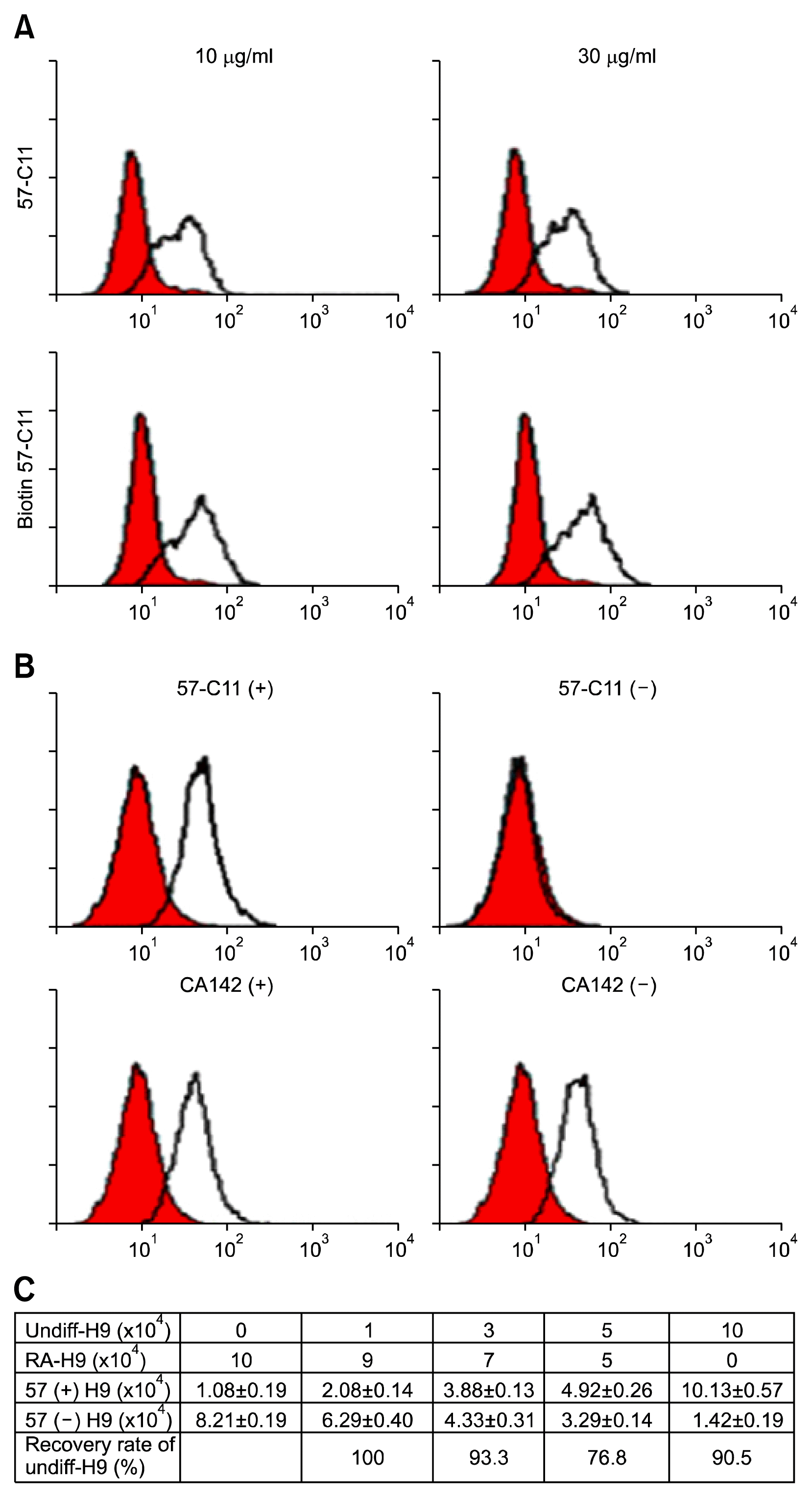
Fig. 2
Strategy of isolation of undifferentiated hESCs with 57-C11. (A) The model of isolation process with biotinylated 57-C11 using Dynabeads. (B) The live cells were counted after the isolation with 57-C11 from the mixture of undifferentiated and RA-induced differentiated H9 cells. The percentage of live cells was estimated by flow cytometry analysis using PI-negative cell population.
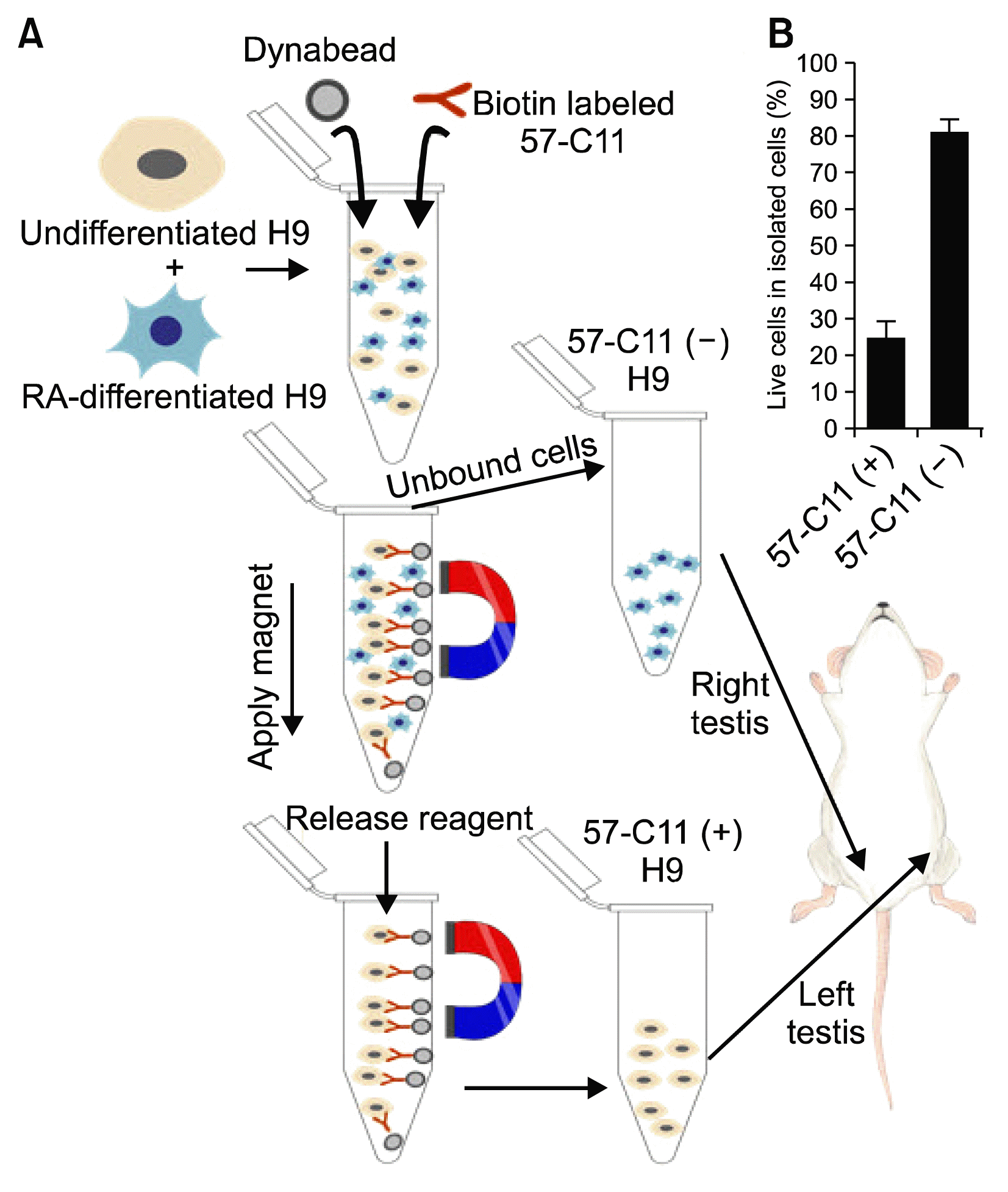
Fig. 3
Teratoma formation of H9 cells. 0.1, 0.4, 1, and 2×106 of undifferentiated H9 or RA-induced differentiated H9 cells were injected into the right or left testis of NOD/SICD mice, respectively. The teratomas were recovered 8~18 weeks after injection.
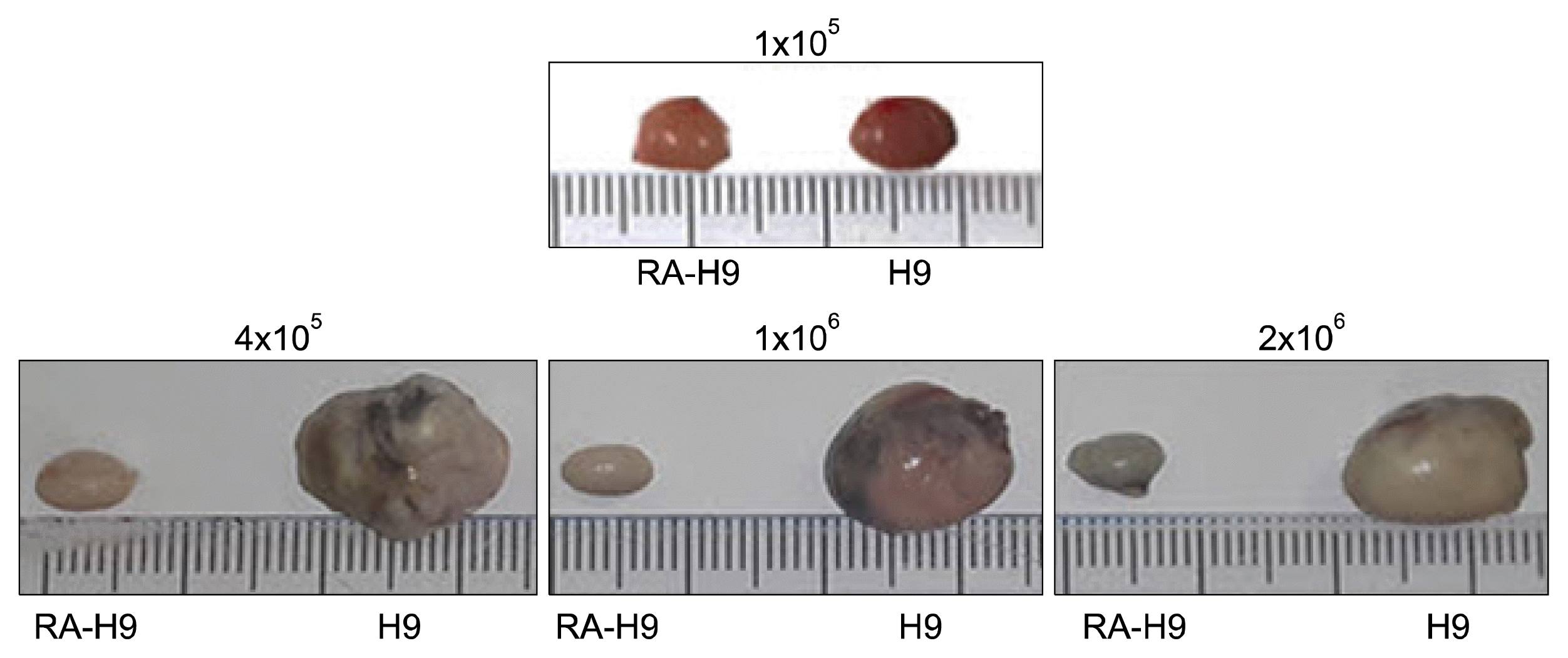
Fig. 4
Teratoma formation of 57-C11-positive cells. (A) Table of teratoma formation of 57-C11-positive cells at 12 or 18 weeks after injection. (B) After the isolation of 57-C11 positive cells from the mixture of undifferentiated and RA-induced differentiated H9 cells, the 1×106 cells of 57-C11-positive or -negative cells were injected into the left or right testis of NOD/SCID mice. The teratomas were recovered 18 weeks postinjection and measured size and weight before fixation. The undifferentiated H9 (H9) and RA-induced differentiated H9 (RA) cells were used as positive and negative controls, respectively.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download