Introduction
Materials and Methods
Isolation and Culture of human AD-MSCs
Preparation of master cell bank (MCB), working cell bank (WCB) and investigational product (IP)
Quality control testing
Animals and experimental design
Animals
Table 2
High-resolution computed tomography (HRCT)
Hydroxyproline estimation
Histological evaluations
AD-MSCs homing and localization studies
Pro-inflammatory and pro-fibrotic marker analysis in lung
Statistical analysis
Results
Cell characterization and quality parameters
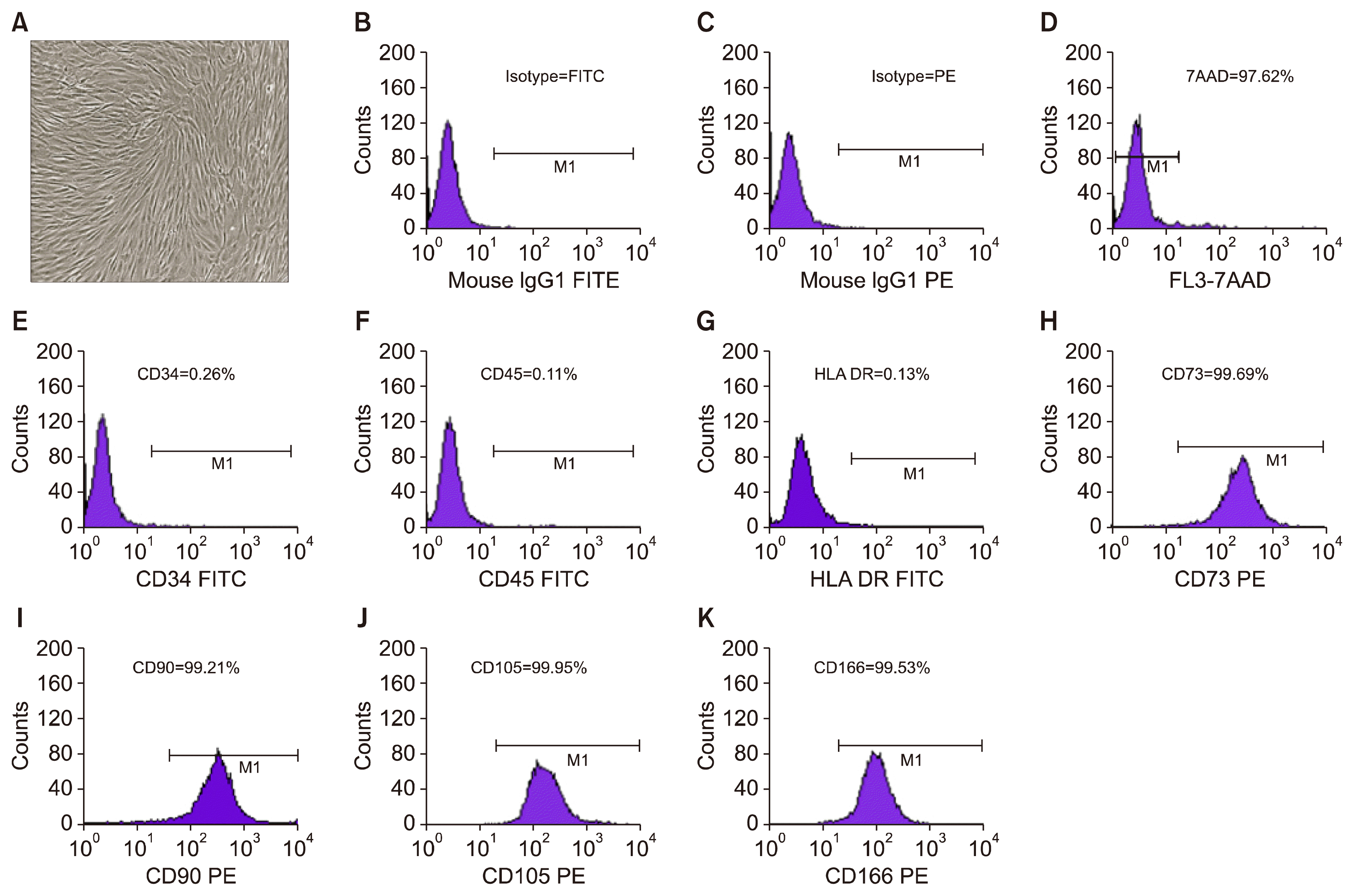 | Fig. 1Morphology and Immunophenotyping of AD-MSCs. (A) AD-MSCs demonstrated long, spindle shaped fibroblast like appearance. (B) Isotype-Fluorescein isothiocynate (FITC). (C) Isotype-Phycoerythrin (PE). (D) Histogram representing flow cytometric analysis of 7-AAD labelled cells revealed >98% viability of AD-MSCs. (E~G) Histograms representing flow cytometric analysis of hematopoietic cell markers revealed <0.2% of CD34, CD45 and HLA-DR. (H~K) Histograms representing flow cytometric analysis of AD-MSC positive markers revealed >99% of CD73, CD90, CD105 and CD166 positive cells, 189×239 mm (150×150 DPI). |
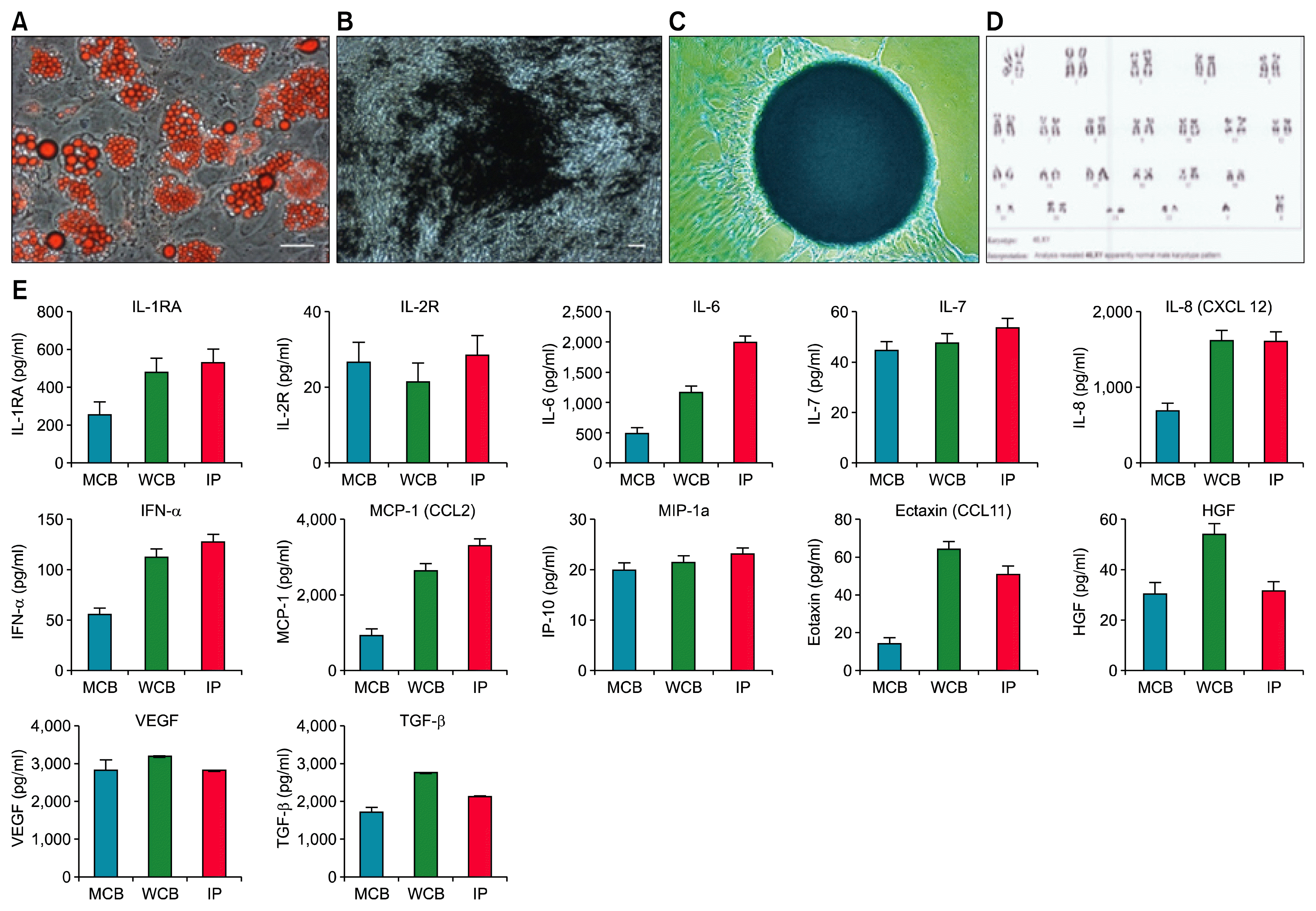 | Fig. 2Representative data of quality control testing for MCB, WCB and IP. (A) Adipogenesis was detected by neutral oil droplet formation stained with Oil Red O at day 21. (B) Osteogenesis was confirmed by mineralized matrix deposition stained with Von-Kossa at day 21. (C) Chondrogenesis was detected by the presence of proteoglycans stained with Alcian Blue staining at day 21. (D) Cytogenetic stability of AD-MSC under prolonged culture. (E) Multiplex Elisa assays for secretome analysis of MCB, WCB and IP showed very high secretion of growth factors, cytokines and chemokines in the conditioned media. Results are representative of three experiments. |
Table 3
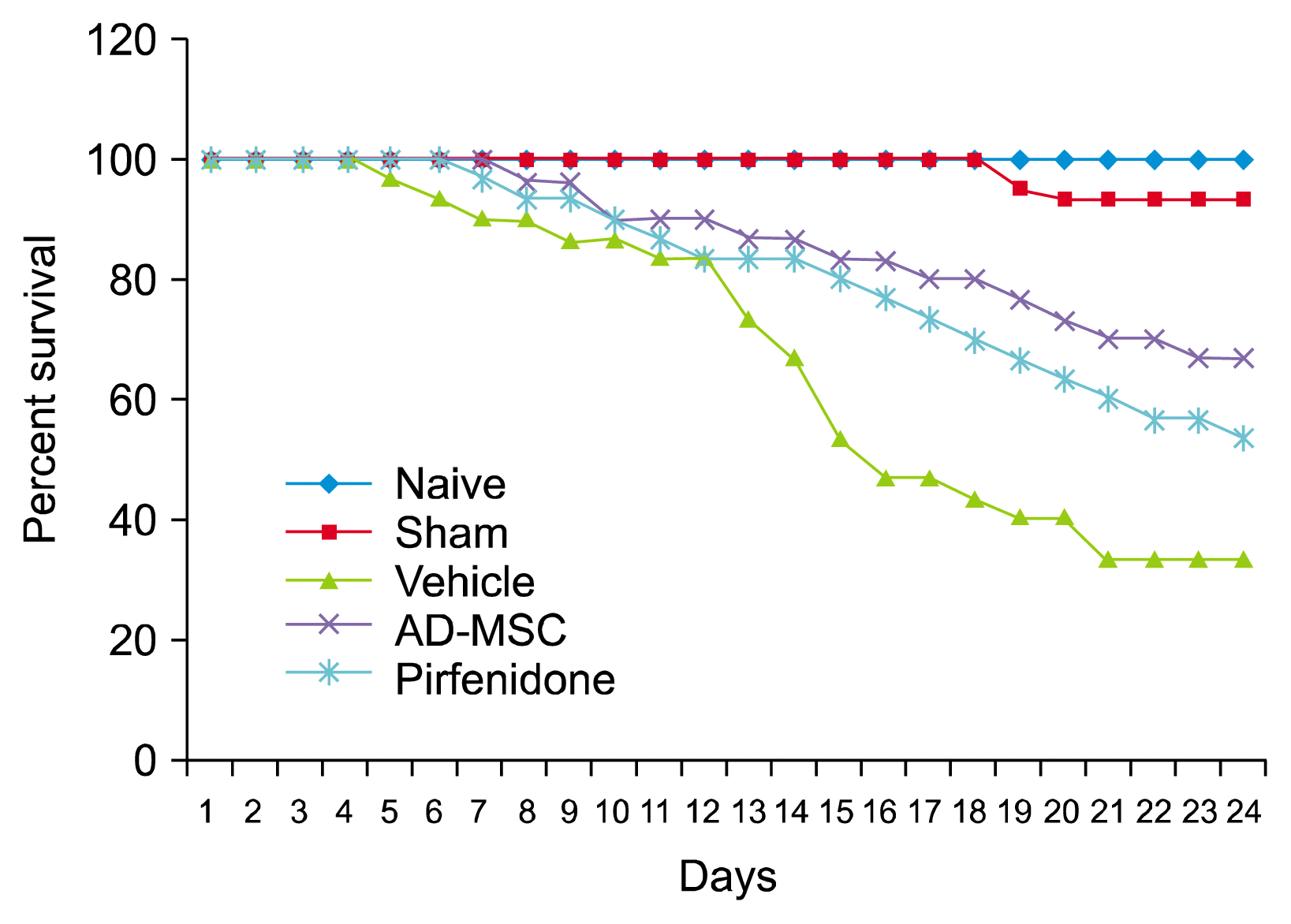
Survivability analysis
AD-MSCs abrogate bleomycin-induced collagen deposition in lungs
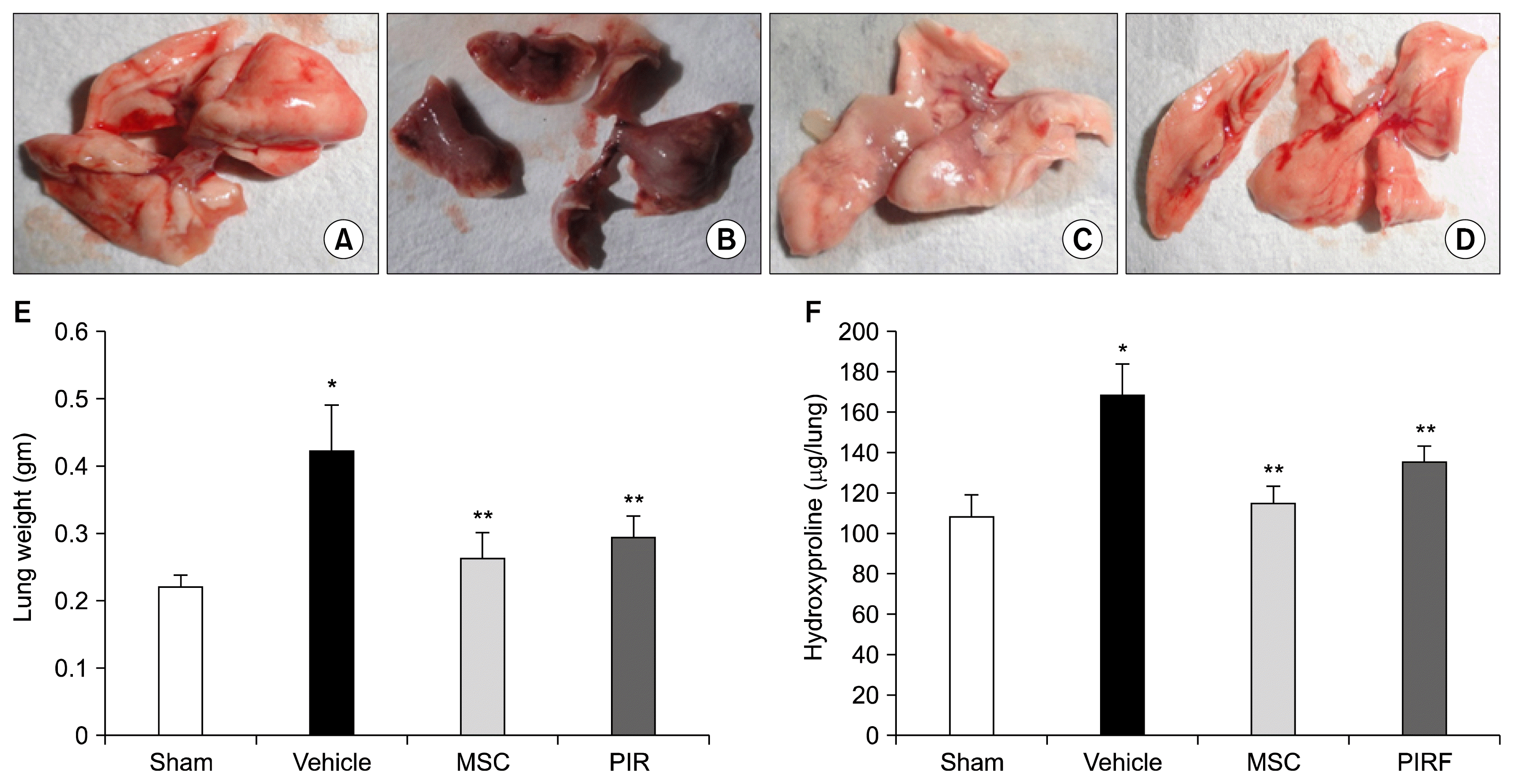 | Fig. 4Representative figure of Gross pathology of A. sham control lung, B. Vehicle control lung with severe fibrosis, C. AD-MSC treated lung with partial reversal of fibrosis with small fibrotic patches, D. pirfenidone treated lung with partial reversal of fibrosis and few fibrotic areas. AD-MSCs abrogate bleomycin-induced collagen deposition in lungs. (E) A reduction in lung weight is observed in the AD-MSC treated group when compared to the vehicle control group. All values are averages±S.E.M. (F) Levels of collagen deposition in the lung are determined by hydroxyproline estimation. Vehicle control mice show increased levels of hydroxyproline due to bleomycin-induced lung damage. On treatment with AD-MSCs a reduction in levels of hydroxyproline was observed. All values are averages±S.E.M. Results presented are representative of 3 separate experiments. 90×120 mm (150×150 DPI). |
AD-MSCs attenuate fibrosis and maintain alveolar architecture of diseased lungs
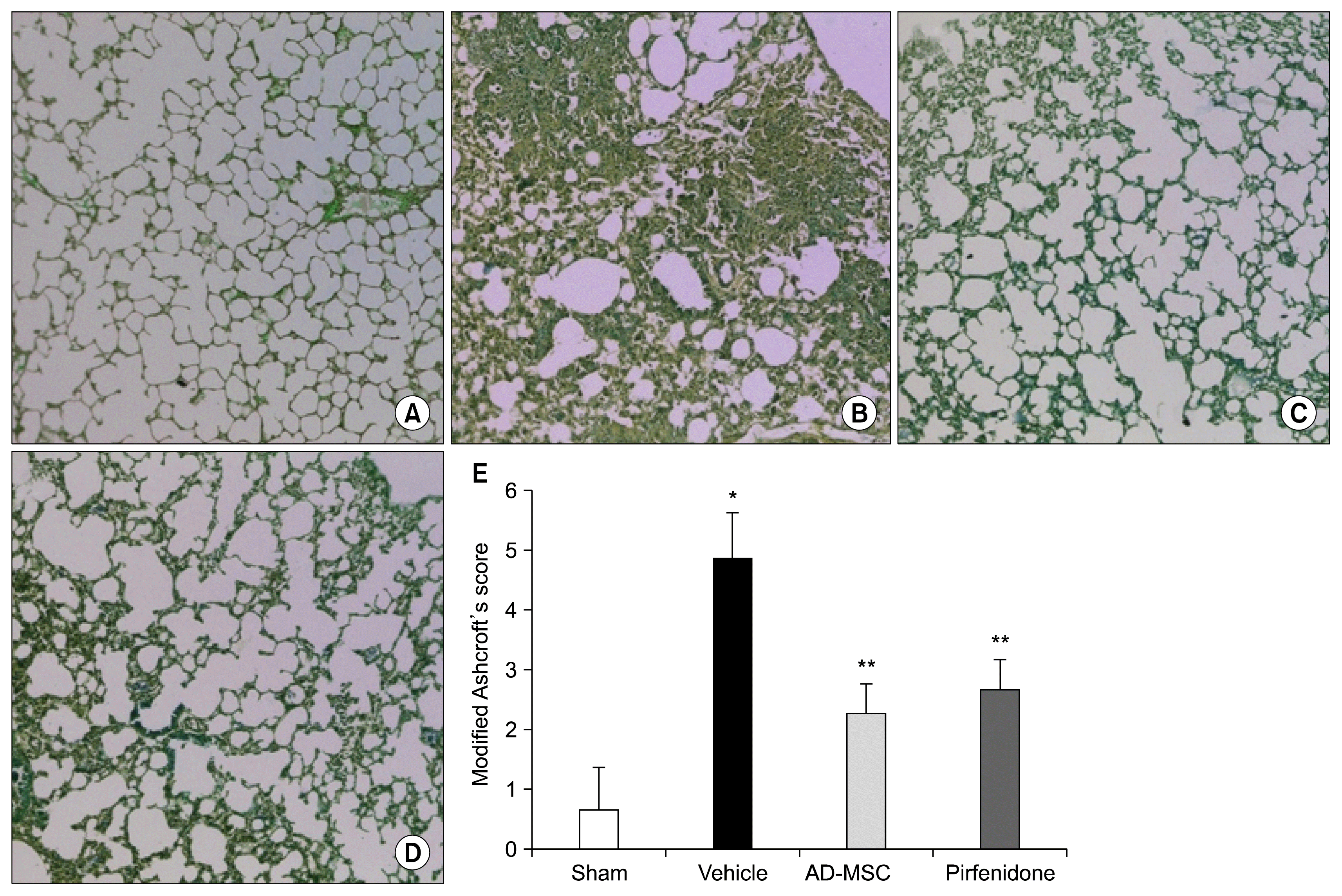 | Fig. 5Representative images of histopathology. AD-MSCs markedly inhibit bleomycin-induced histological abnormalities. (A) Representative images of Masson’s trichrome stained sections of the lungs showing collagen staining in sham operated mice. (B) Represents lung of bleomycine induced mice with vehicle treatment, lung section revealed marked fibrosis and loss of architecture. (C) AD-MSC treated lung of bleomycin induced mice revealed attenuation in fibrosis with mild thickening of alveolar septa and protection against lung fibrosis and maintenance of alveolar architecture. (D) Pirfenidone treated lung with thick alveolar septa and moderately high collagen deposition. (E) Histological appearances of the Masson’s trichrome stained sections were scored for fibrosis using the Ashcroft’s modified scoring criteria. AD-MSC treated lung sections were compared with sham operated and vehicle treated lung. Results showed that Ashcroft’s modified score in AD-MSC and Pirfenidone treated lung were significantly lowered than vehicle treated lung (p<0.01). All values are averages±S.E.M. 190×240 mm (150×150 DPI). |
Homing and engraftment potential of systemically administered AD-MSCs at the site of injury
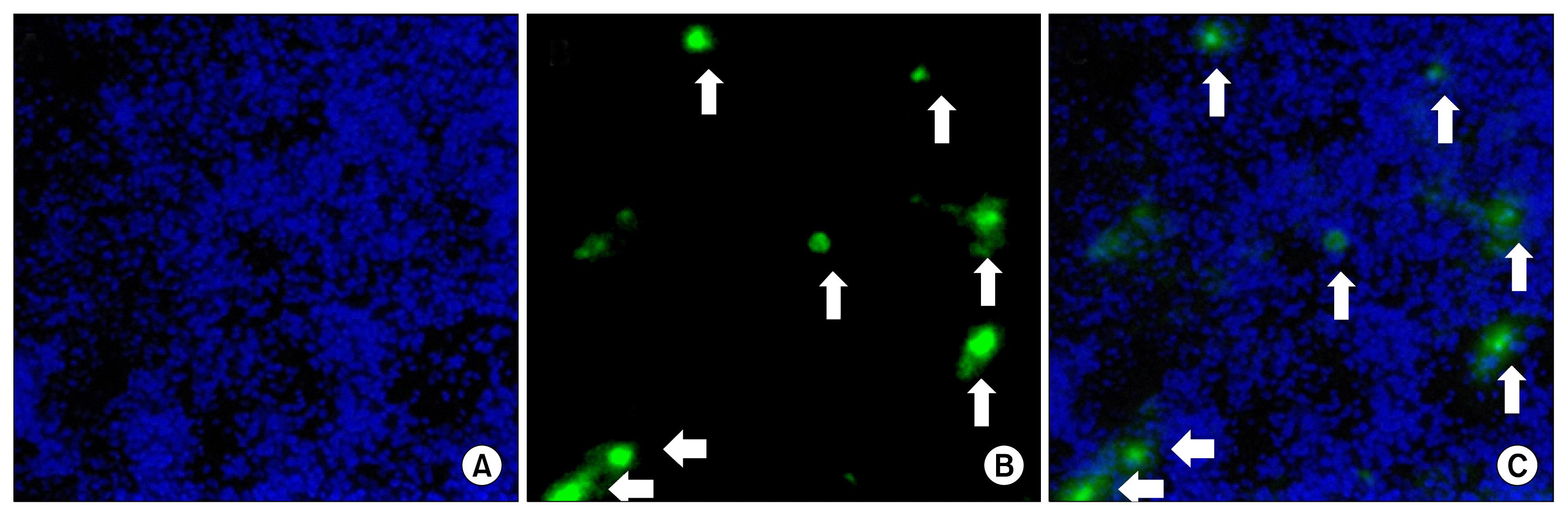 | Fig. 6Homing and engraftment potential of AD-MSCs at the site of injury. (A) Fluorescence photomicrographs of cryo-sections showing nuclear staining of mice lungs with Hoechst 33342. Nucleus is stained blue. (B) Fluorescence photomicrographs of cryo-sections showing engraftment of PKH-67 tagged AD-MSCs at the site of injury in lungs on day 24. AD-MSCs are indicated by green fluorescence. (C) Merged image showing localization of AD-MSCs in the lung parenchyma. 99×244 mm (150×150 DPI). |
AD-MSCs exert their mechanism by down-regulating pro-inflammatory and pro-fibrotic transcripts in damaged lungs
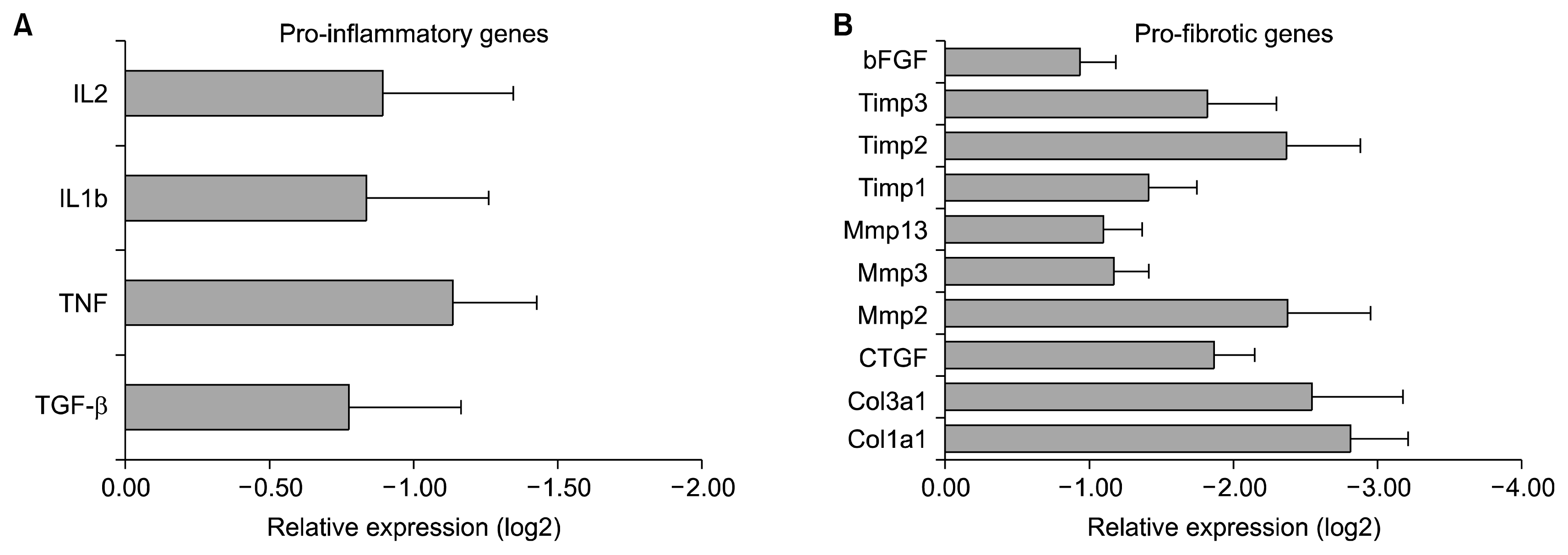 | Fig. 7Representative graph for AD-MSCs downregulate bleomycin-induced expression of pro-inflammatory and pro-fibrotic transcripts in damaged lungs. RNA isolated from tissue sections of lungs of mice from various groups was subjected to RTQ-PCR analyses using appropriate primers for: (A) Pro-inflammatory genes-TGFβ, TNF, IL1b, IL2. (B) Pro-fibrotic genes –Col1a1, Col3a1, CTGF, bFGF, Mmp2, Mmp3, Mmp13, Timp1, Timp2, Timp3. Gapdh was used as the loading control. Results presented are normalized to loading control. The results are presented as the log2 fold change of mRNA expression as compared to the amount present in vehicle control samples. All values are averages±S.E.M. 190×240 mm (150×150 DPI). |
HRCT analysis
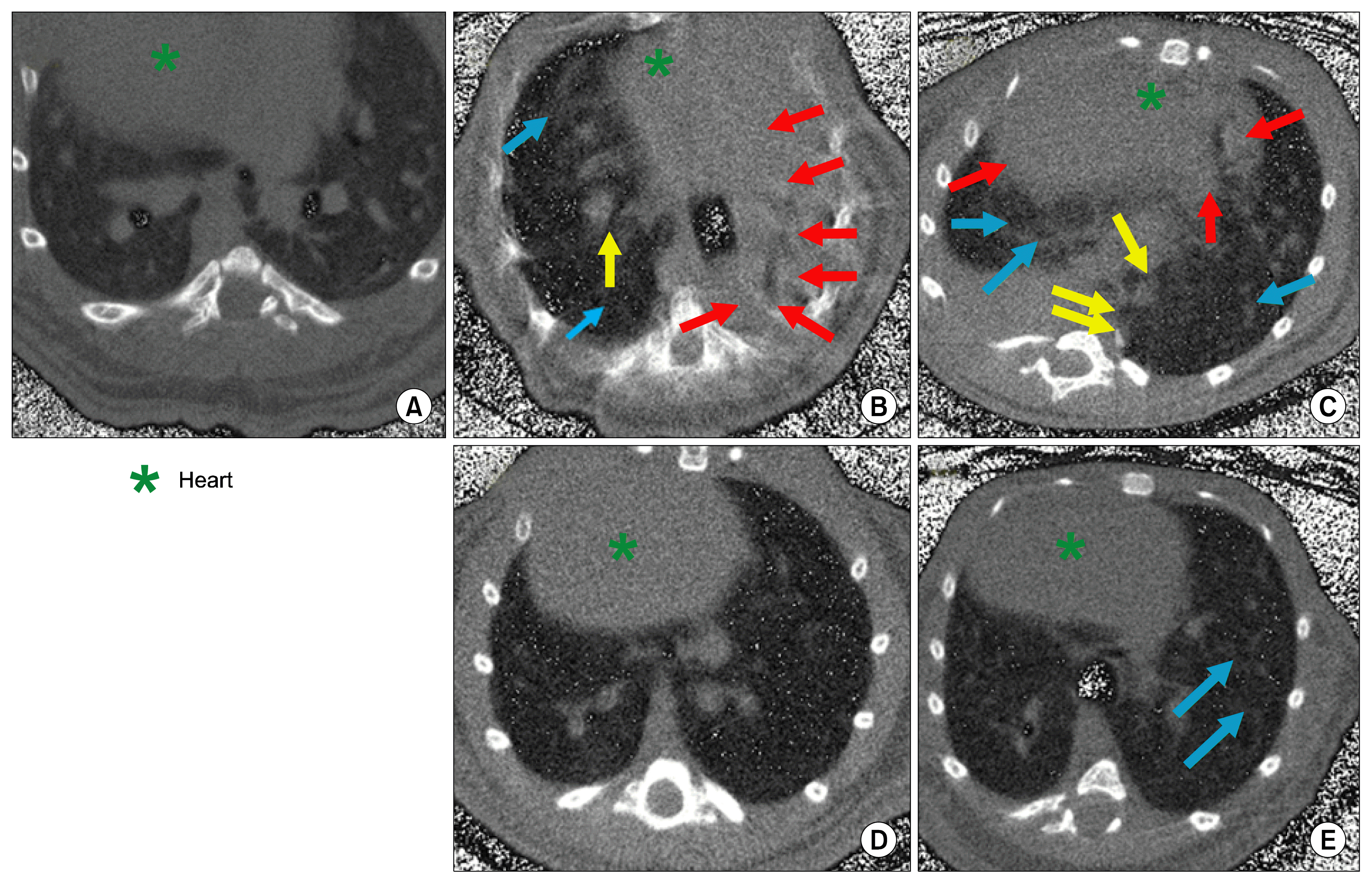 | Fig. 8Representative picture of HRCT scan (A) Naïve control animal with intact lung architecture, (B, C) Lungs of Vehicle control animals with distinct presence of severe fibrotic lesions (red arrow), consolidation of lung (yellow arrow) and ground glass opacities (blue arrow), (D) Lungs of AD-MSC treated animals showing that AD-MSC protect and reverse bleomycin induced lung fibrosis, (E) Lungs of Pirfenidone treated animals showing that pirfenidone protect and reverse bleomycin induced lung fibrosis with few ground glass opacities (blue arrow). |




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download