The mammalian mammary gland is a complex organ, made up of various cell types that work together for milk synthesis. In females, the mammary gland has a ductal structure that supports formation of the alveolar structure during pregnancy, prior to the onset of lactogenesis (
1,
2). The primary mammary duct invades the mammary fat pad at E17, and formation of a small, branched ductal tree begins at this time and develops its shape (
3). A previous study had established a clonal cell line from primary bovine mammary alveolar cells (MAC-T) for the study of bovine milk production and synthesis (
4). Prolactin was used to induce the MAC-T cell differentiation. The differentiated cells had important characteristics of increases in their beta-casein mRNA abundance as well as number and size of casein secretory vesicles, and the ability to secrete alpha-S- and beta-casein proteins (
4). Dairy proteins have been reported to have favorable effects on oxidative stress and inflammation, as well as conveying human health advantages such as blood pressure, blood lipid, and glucose control (
5,
6). Based on these positive effects of dairy protein, many researchers have attempted to investigate the mechanism of casein production for increasing its content in milk. For example, hormones such as somatotropin, growth hormone, retinoic acid, and prolactin have been shown to increase milk protein synthesis in mammary cell models (
7,
8). However, the cell signaling mechanism that may be involved in mediating the effect of growth hormones on milk production in the mammary gland of lactating dairy cows is still unknown (
9). Knowledge about such hormone-related mechanism would help to define a novel means of steroid receptor-mediated transcriptional repression of a physiologically important gene in mammary gland development and differentiation (
10). The MAC-T cell line has the characteristics of uniform differentiation, immortality, and a population doubling time of approximately 17 h (
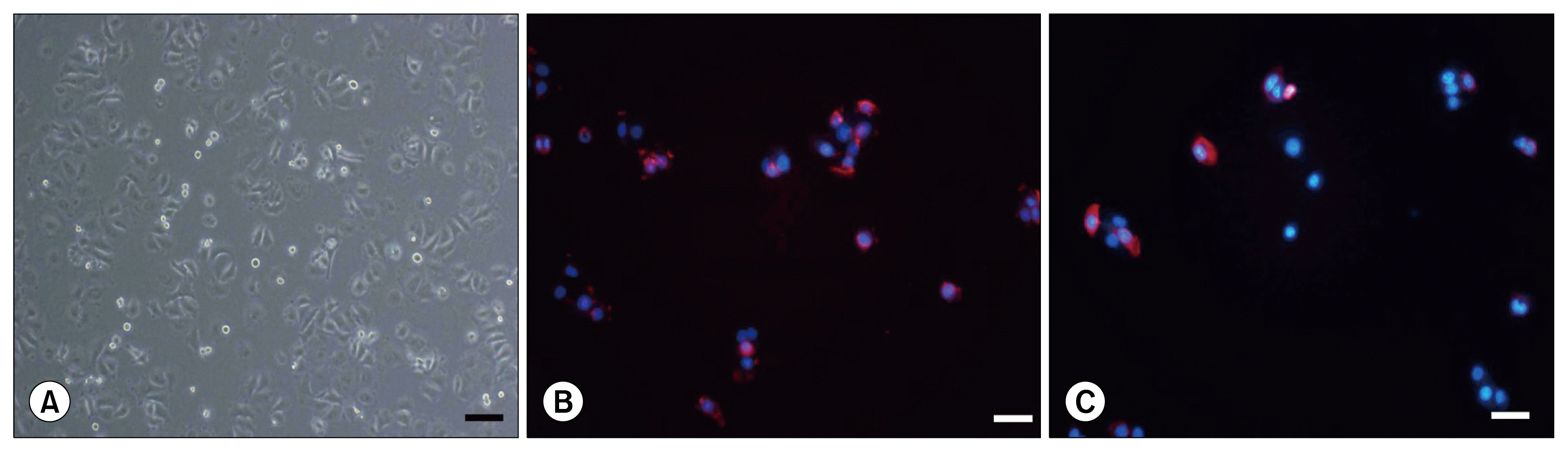
4). These cells may be very useful for dairy protein synthesis studies, particularly of casein. Generally, farm animals such as cattle and horses are difficult to handle for experiments. The mouse, on the other hand, is the most commonly used mammalian research model for laboratory-scale experiments. Hence, it would be beneficial to develop a mouse model of bovine mammary alveolar ducts for laboratory scale-studies. Here, we aimed to generate the bovine mammary gland ductal structure from MAC-T cells transplanted into mice dorsal tissue.





 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download