Introduction
Materials and Methods
Cell cultures
Cerebral organoid generation from feeder-free cultured hPSCs
Microsection and immunocytochemistry
Results
Selection and culture of human pluripotent stem cells
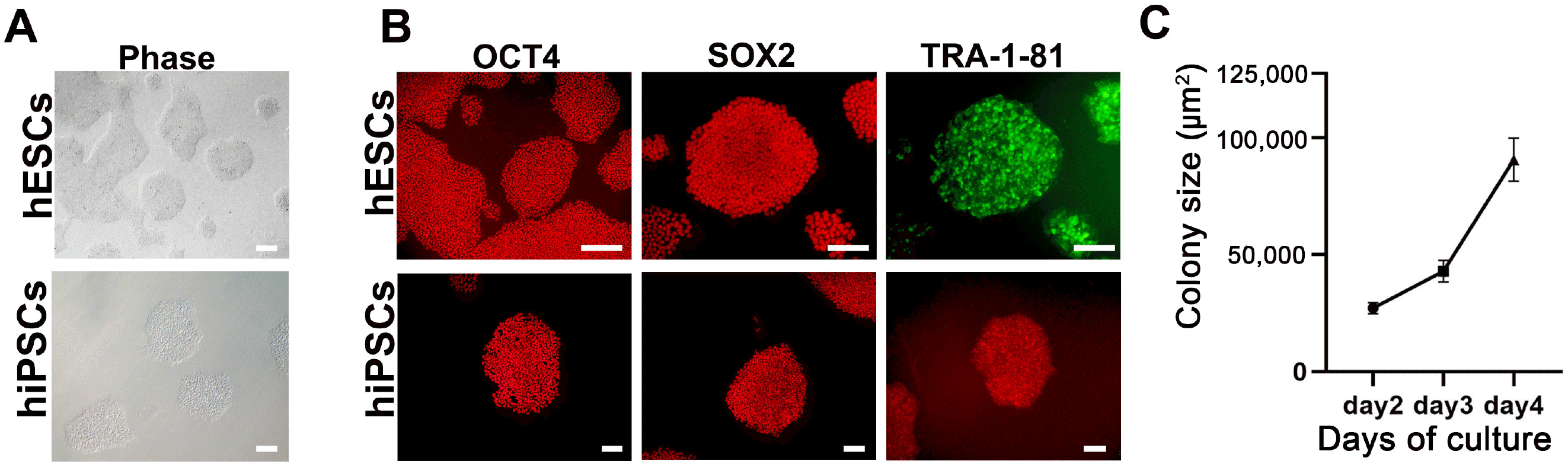 | Fig. 1Characterization of human embryonic stem cells (hESCs) and human induced pluripotent stem cells (hiPSCs). (A) Phase contrast images of hESCs and hiPSCs in feeder-free conditions. Scale bar=200 μm. (B) Immunocytochemical staining of the pluripotency markers OCT4, SOX2, and TRA-1-81 in hESCs (upper) and hiPSCs (lower). Scale bar=200 μm in the upper panels and 100 μm in the lower panels. (C) Growth rate of hESCs from 2 to 4 days after seeding. Growth rate was measured as an increase in colony size (μm2). Error bars represent standard error of the mean. |
Neural lineage induction via embryoid body (EB) formation from hPSCs
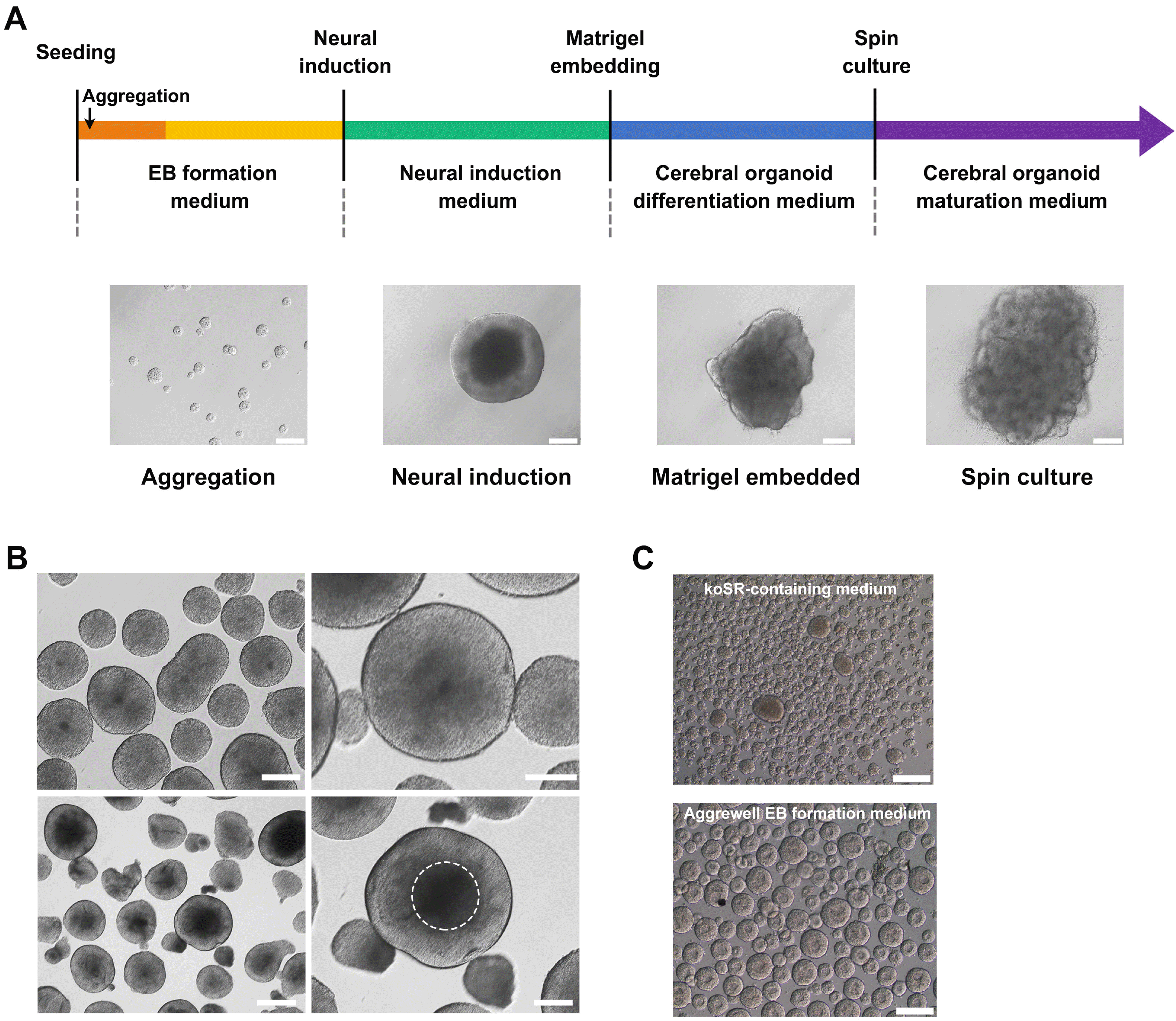 | Fig. 2Brain organoid differentiation protocol using human pluripotent stem cells (hPSCs). (A) Schematic illustration of the generation of brain organoids. Representative images characterizing each stage are shown. Scale bar=200 μm. (B) Morphology of embryoid bodies (EBs) in EB formation medium, based on TeSR medium (upper left), and their enlarged images (upper right). EBs committed to the neural lineage showed a transparent neuroepithelium-like region at the periphery of the structure (lower left), which is depicted with a white dotted circle (lower right). Scale bar=500 μm (lower left), 200 μm (lower right). (C) Comparison of koSR-containing medium (hESC medium) and Aggrewell EB medium for the size of EBs differentiated from hESCs. Scale bars=200 μm. |
Embedding of EBs into Matrigel and subsequent culture for cerebral organoid formation
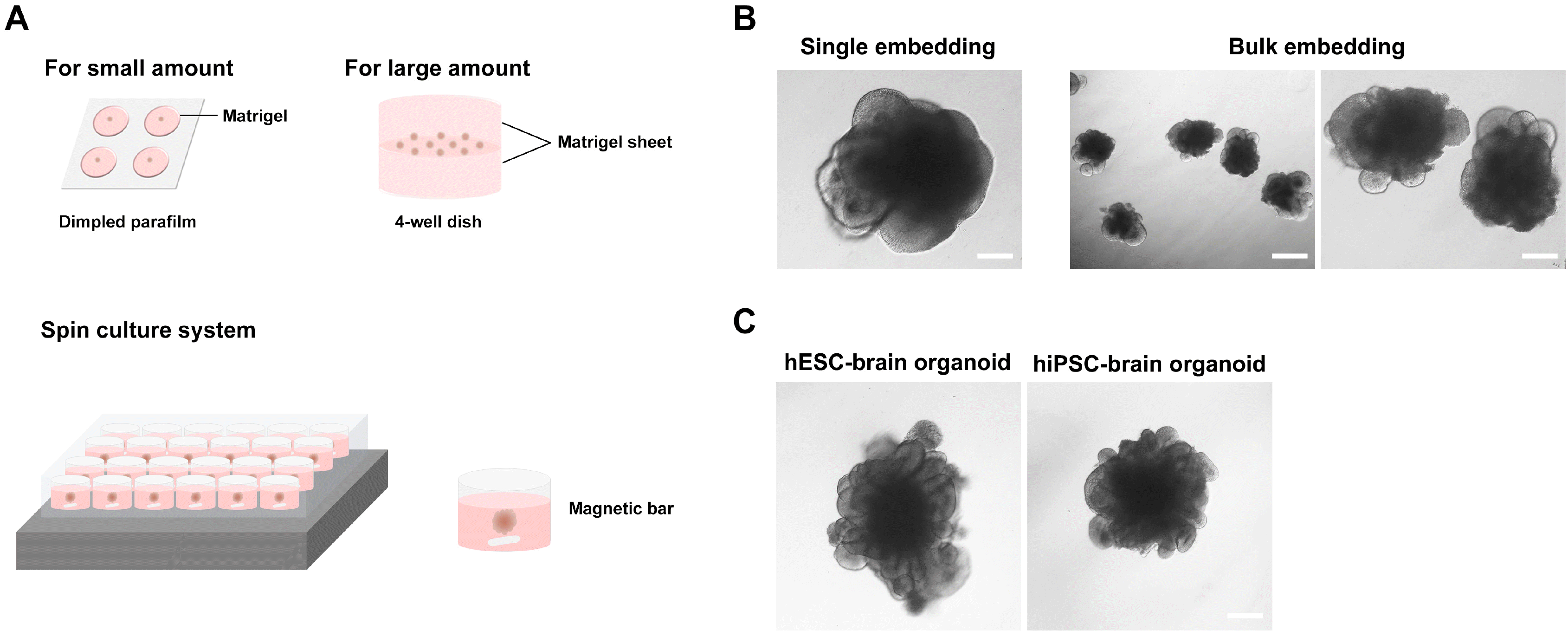 | Fig. 3Matrigel embedding culture and morphology of early-stage brain organoids. (A) Matrigel embedding process for small or large amount of embryoid bodies (EBs) and the spin culture system. (B) The neural folding stage of hESC-derived EBs at day 6 after Matrigel embedding with single (small amount) or bulk (large amount) EBs. Scale bar=200 μm (left and right) and 500 μm (middle). (C) Brain organoids with multiple neural rosette-like structures derived from human embryonic stem cells and (day 19) and human induced pluripotent stem cells (day 20). Scale bar=500 μm. |
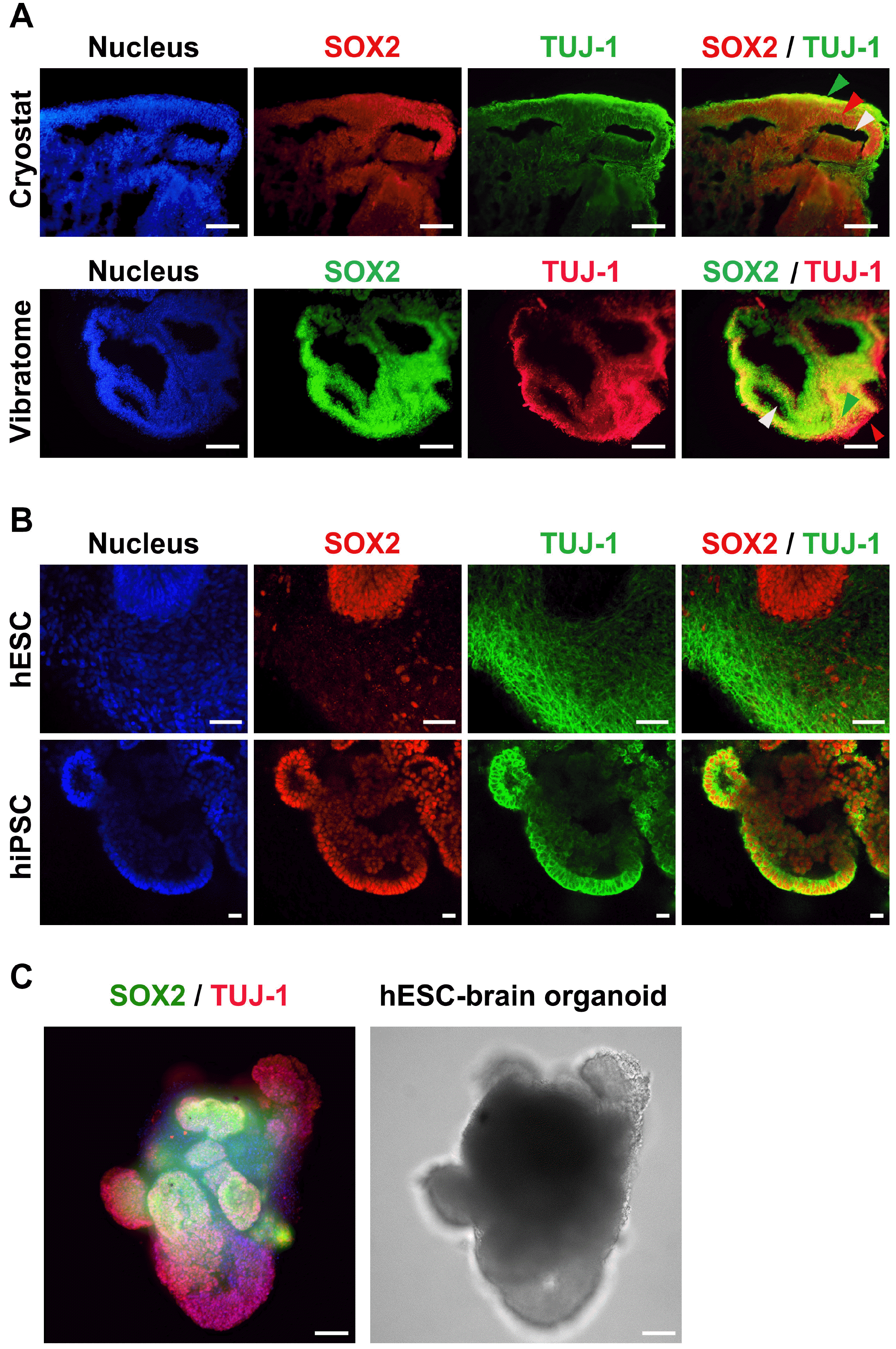 | Fig. 4Characterization of human embryonic stem cell (hESC)- and human induced pluripotent stem cell (hiPSC)-derived brain organoids. (A) 2D immunocytochemistry staining of the early neuron marker TUJ-1 and the neural progenitor marker SOX2 in hPSC-derived organoids. A Cryostat (upper) and Vibratome (lower) were used for tissue sectioning. Scale bar=100 μm (upper panels) and 200 μm (lower panels). Green arrow indicates cortical plate, red arrow indicates ventricular zone, and white arrow indicates ventricle (Cryostat sample). Green arrow indicates ventricular zone, red arrow indicates cortical plate, and white arrow indicates ventricle (Vibratome sample). (B) Confocal images of 3D immunocytochemistry for the early neuron marker TUJ-1 and the neural progenitor marker SOX2 in hESC- and hiPSC-derived organoids. Scale bar=50 μm (upper panels) and 20 μm (lower panels). (C) Representative 3D image of whole brain organoids, which were stained with TUJ-1 and SOX2 (at day 12 hESC-derived). The image was captured using high contents screening (HCS). Scale bars=200 μm. |




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download