1. Benyon RC, Arthur MJ. 2001; Extracellular matrix degradation and the role of hepatic stellate cells. Semin Liver Dis. 21:373–384. DOI:
10.1055/s-2001-17552. PMID:
11586466.

2. Brew K, Dinakarpandian D, Nagase H. 2000; Tissue inhibitors of metalloproteinases: evolution, structure and function. Biochim Biophys Acta. 1477:267–283. DOI:
10.1016/S0167-4838(99)00279-4. PMID:
10708863.

3. Herrera MB, Bruno S, Buttiglieri S, Tetta C, Gatti S, Deregibus MC, Bussolati B, Camussi G. 2006; Isolation and characterization of a stem cell population from adult human liver. Stem Cells. 24:2840–2850. DOI:
10.1634/stemcells.2006-0114. PMID:
16945998.

4. Lee JH, Park HJ, Jang IK, Kim HE, Lee DH, Park JK, Lee SK, Yoon HH. 2014; In vitro differentiation of human liver-derived stem cells with mesenchymal characteristics into immature hepatocyte-like cells. Transplant Proc. 46:1633–1637. DOI:
10.1016/j.transproceed.2013.12.070. PMID:
24935339.

5. Jung KH, Shin HP, Lee S, Lim YJ, Hwang SH, Han H, Park HK, Chung JH, Yim SV. 2009; Effect of human umbilical cord blood-derived mesenchymal stem cells in a cirrhotic rat model. Liver Int. 29:898–909. DOI:
10.1111/j.1478-3231.2009.02031.x. PMID:
19422480.

6. Peng L, Xie DY, Lin BL, Liu J, Zhu HP, Xie C, Zheng YB, Gao ZL. 2011; Autologous bone marrow mesenchymal stem cell transplantation in liver failure patients caused by hepatitis B: short-term and long-term outcomes. Hepatology. 54:820–828. DOI:
10.1002/hep.24434. PMID:
21608000.

7. Mohamadnejad M, Alimoghaddam K, Bagheri M, Ashrafi M, Abdollahzadeh L, Akhlaghpoor S, Bashtar M, Ghavamzadeh A, Malekzadeh R. 2013; Randomized placebo-controlled trial of mesenchymal stem cell transplantation in decompensated cirrhosis. Liver Int. 33:1490–1496. DOI:
10.1111/liv.12228. PMID:
23763455.

8. Lee JH, Park HJ, Kim YA, Lee DH, Noh JK, Kwon CH, Jung SM, Lee SK. 2012; Differentiation and major histocompatibility complex antigen expression in human liver-derived stem cells. Transplant Proc. 44:1113–1115. DOI:
10.1016/j.transproceed.2012.03.008. PMID:
22564639.

9. Lee JH, Park HJ, Kim YA, Lee DH, Noh JK, Kwon CH, Jung SM, Lee SK. 2012; The phenotypic characteristic of liver-derived stem cells from adult human deceased donor liver. Transplant Proc. 44:1110–1112. DOI:
10.1016/j.transproceed.2012.02.020. PMID:
22564638.

10. Brenner DA, Waterboer T, Choi SK, Lindquist JN, Stefanovic B, Burchardt E, Yamauchi M, Gillan A, Rippe RA. 2000; New aspects of hepatic fibrosis. J Hepatol. 32:32–38. DOI:
10.1016/S0168-8278(00)80413-4. PMID:
10728792.

11. Reeves HL, Friedman SL. 2002; Activation of hepatic stellate cells--a key issue in liver fibrosis. Front Biosci. 7:d808–d826. DOI:
10.2741/A813. PMID:
11897564.
12. Aimes RT, Quigley JP. 1995; Matrix metalloproteinase-2 is an interstitial collagenase. Inhibitor-free enzyme catalyzes the cleavage of collagen fibrils and soluble native type I collagen generating the specific 3/4- and 1/4-length fragments. J Biol Chem. 270:5872–5876. DOI:
10.1074/jbc.270.11.5872. PMID:
7890717.
13. Benyon RC, Iredale JP, Goddard S, Winwood PJ, Arthur MJ. 1996; Expression of tissue inhibitor of metalloproteinases 1 and 2 is increased in fibrotic human liver. Gastroenterology. 110:821–831. DOI:
10.1053/gast.1996.v110.pm8608892. PMID:
8608892.

14. Knittel T, Mehde M, Grundmann A, Saile B, Scharf JG, Ramadori G. 2000; Expression of matrix metalloproteinases and their inhibitors during hepatic tissue repair in the rat. Histochem Cell Biol. 113:443–453. DOI:
10.1007/s004180000150. PMID:
10933221.

15. Murphy FR, Issa R, Zhou X, Ratnarajah S, Nagase H, Arthur MJ, Benyon C, Iredale JP. 2002; Inhibition of apoptosis of activated hepatic stellate cells by tissue inhibitor of metalloproteinase-1 is mediated via effects on matrix metalloproteinase inhibition: implications for reversibility of liver fibrosis. J Biol Chem. 277:11069–11076. DOI:
10.1074/jbc.M111490200. PMID:
11796725.

16. Yoshiji H, Kuriyama S, Yoshii J, Ikenaka Y, Noguchi R, Nakatani T, Tsujinoue H, Yanase K, Namisaki T, Imazu H, Fukui H. 2002; Tissue inhibitor of metalloproteinases-1 attenuates spontaneous liver fibrosis resolution in the transgenic mouse. Hepatology. 36:850–860. DOI:
10.1053/jhep.2002.35625. PMID:
12297832.

17. Khuu DN, Scheers I, Ehnert S, Jazouli N, Nyabi O, Buc-Calderon P, Meulemans A, Nussler A, Sokal E, Najimi M. 2011; In vitro differentiated adult human liver progenitor cells display mature hepatic metabolic functions: a potential tool for in vitro pharmacotoxicological testing. Cell Transplant. 20:287–302. DOI:
10.3727/096368910X516655. PMID:
20719066.

18. Najimi M, Khuu DN, Lysy PA, Jazouli N, Abarca J, Sempoux C, Sokal EM. 2007; Adult-derived human liver mesenchymal-like cells as a potential progenitor reservoir of hepatocytes? Cell Transplant. 16:717–728. DOI:
10.3727/000000007783465154. PMID:
18019361.

19. Scheers I, Maerckx C, Khuu DN, Marcelle S, Decottignies A, Najimi M, Sokal E. 2012; Adult-derived human liver progenitor cells in long-term culture maintain appropriate gatekeeper mechanisms against transformation. Cell Transplant. 21:2241–2255. DOI:
10.3727/096368912X639026. PMID:
22525602.

20. Fouraschen SM, Pan Q, de Ruiter PE, Farid WR, Kazemier G, Kwekkeboom J, Ijzermans JN, Metselaar HJ, Tilanus HW, de Jonge J, van der Laan LJ. 2012; Secreted factors of human liver-derived mesenchymal stem cells promote liver regeneration early after partial hepatectomy. Stem Cells Dev. 21:2410–2419. DOI:
10.1089/scd.2011.0560. PMID:
22455365.

21. Pan Q, Fouraschen SM, Kaya FS, Verstegen MM, Pescatori M, Stubbs AP, van Ijcken W, van der Sloot A, Smits R, Kwekkeboom J, Metselaar HJ, Kazemier G, de Jonge J, Tilanus HW, Wagemaker G, Janssen HL, van der Laan LJ. 2011; Mobilization of hepatic mesenchymal stem cells from human liver grafts. Liver Transpl. 17:596–609. DOI:
10.1002/lt.22260. PMID:
21506248.

22. Terai S, Tsuchiya A. 2017; Status of and candidates for cell therapy in liver cirrhosis: overcoming the "point of no return" in advanced liver cirrhosis. J Gastroenterol. 52:129–140. DOI:
10.1007/s00535-016-1258-1. PMID:
27631592.

23. Parekkadan B, van Poll D, Megeed Z, Kobayashi N, Tilles AW, Berthiaume F, Yarmush ML. 2007; Immunomodulation of activated hepatic stellate cells by mesenchymal stem cells. Biochem Biophys Res Commun. 363:247–252. DOI:
10.1016/j.bbrc.2007.05.150. PMID:
17869217. PMCID:
PMC2096777.

24. Zhao DC, Lei JX, Chen R, Yu WH, Zhang XM, Li SN, Xiang P. 2005; Bone marrow-derived mesenchymal stem cells protect against experimental liver fibrosis in rats. World J Gastroenterol. 11:3431–3440. DOI:
10.3748/wjg.v11.i22.3431. PMID:
15948250. PMCID:
PMC4315999.

25. Chang YJ, Liu JW, Lin PC, Sun LY, Peng CW, Luo GH, Chen TM, Lee RP, Lin SZ, Harn HJ, Chiou TW. 2009; Mesenchymal stem cells facilitate recovery from chemically induced liver damage and decrease liver fibrosis. Life Sci. 85:517–525. DOI:
10.1016/j.lfs.2009.08.003. PMID:
19686763.

26. Tanimoto H, Terai S, Taro T, Murata Y, Fujisawa K, Yamamoto N, Sakaida I. 2013; Improvement of liver fibrosis by infusion of cultured cells derived from human bone marrow. Cell Tissue Res. 354:717–728. DOI:
10.1007/s00441-013-1727-2. PMID:
24104560.

27. Herrera MB, Fonsato V, Bruno S, Grange C, Gilbo N, Romagnoli R, Tetta C, Camussi G. 2013; Human liver stem cells improve liver injury in a model of fulminant liver failure. Hepatology. 57:311–319. DOI:
10.1002/hep.25986. PMID:
22829291.

28. Berardis S, Lombard C, Evraerts J, El Taghdouini A, Rosseels V, Sancho-Bru P, Lozano JJ, van Grunsven L, Sokal E, Najimi M. 2014; Gene expression profiling and secretome analysis differentiate adult-derived human liver stem/progenitor cells and human hepatic stellate cells. PLoS One. 9:e86137. DOI:
10.1371/journal.pone.0086137. PMID:
24516514. PMCID:
PMC3906387.

29. Rockey DC, Chung JJ. 1994; Interferon gamma inhibits lipocyte activation and extracellular matrix mRNA expression during experimental liver injury: implications for treatment of hepatic fibrosis. J Investig Med. 42:660–670. PMID:
8521029.
30. Jang IK, Lee JH, Yoon HH, Park HJ, Kim YA, Lee DH, Lee SH, Lee SK. 2015; Suppression of T-cell proliferation by and B7-H1 expression on human liver-derived stem cells. Transplant Proc. 47:784–786. DOI:
10.1016/j.transproceed.2014.12.046. PMID:
25891731.

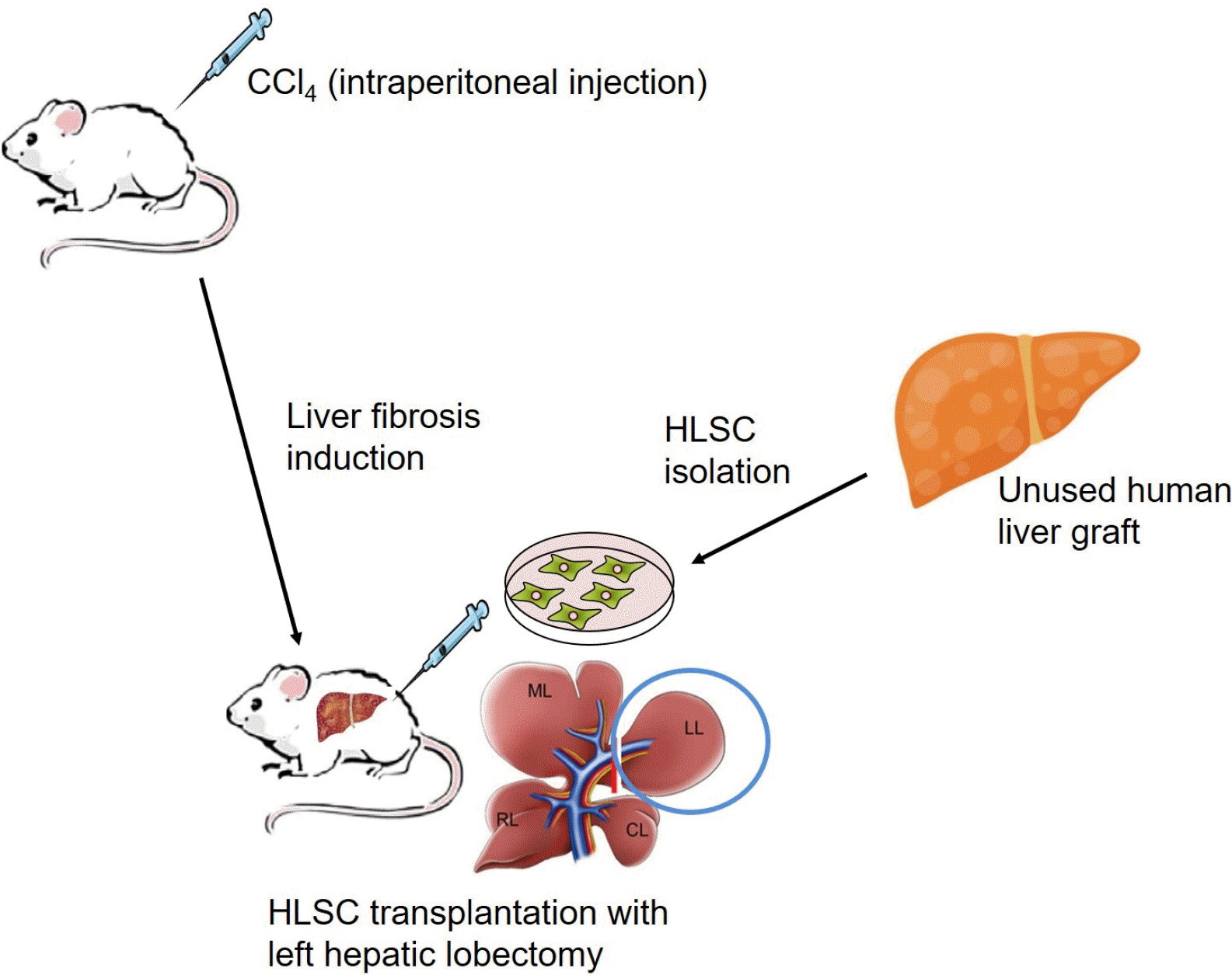
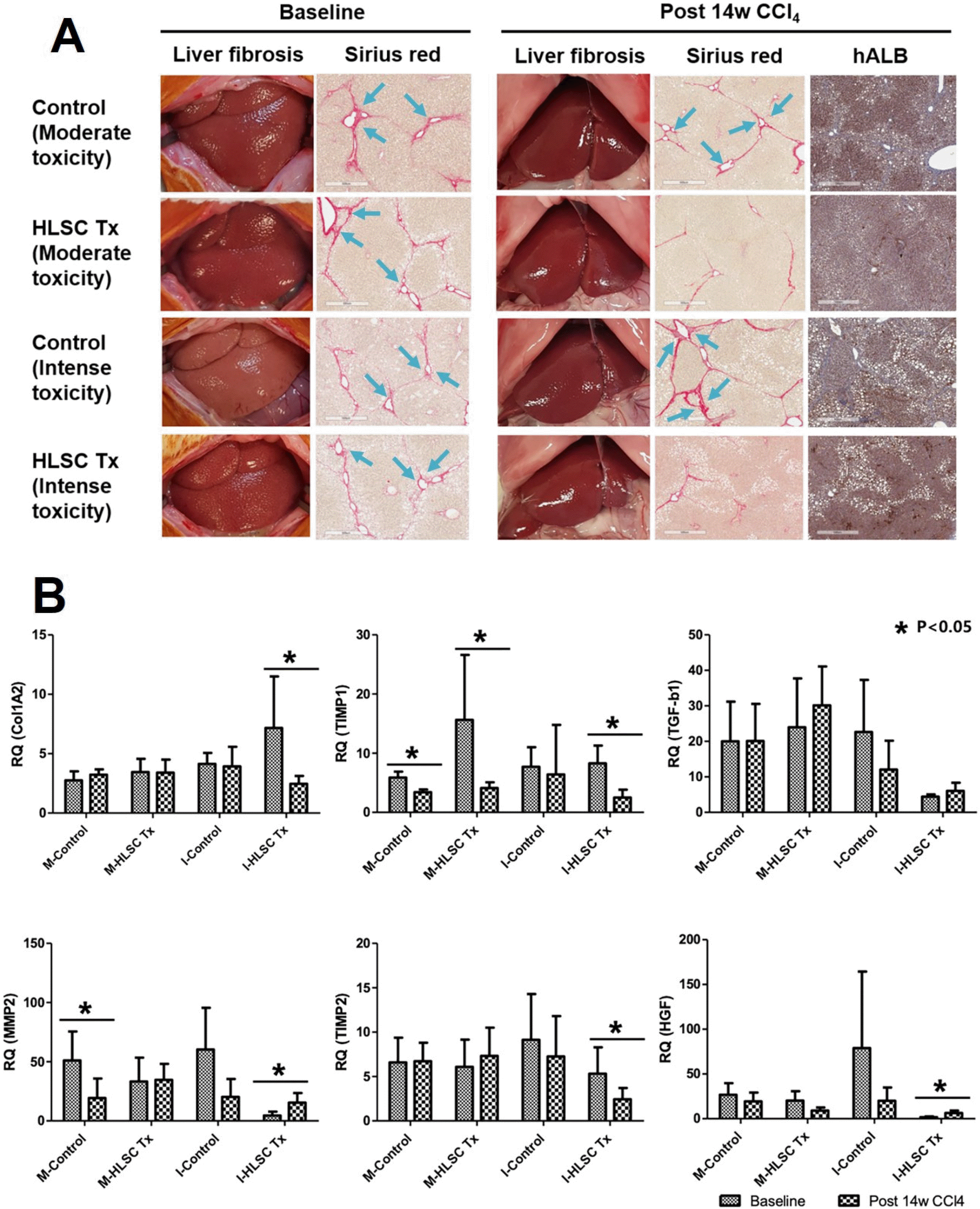
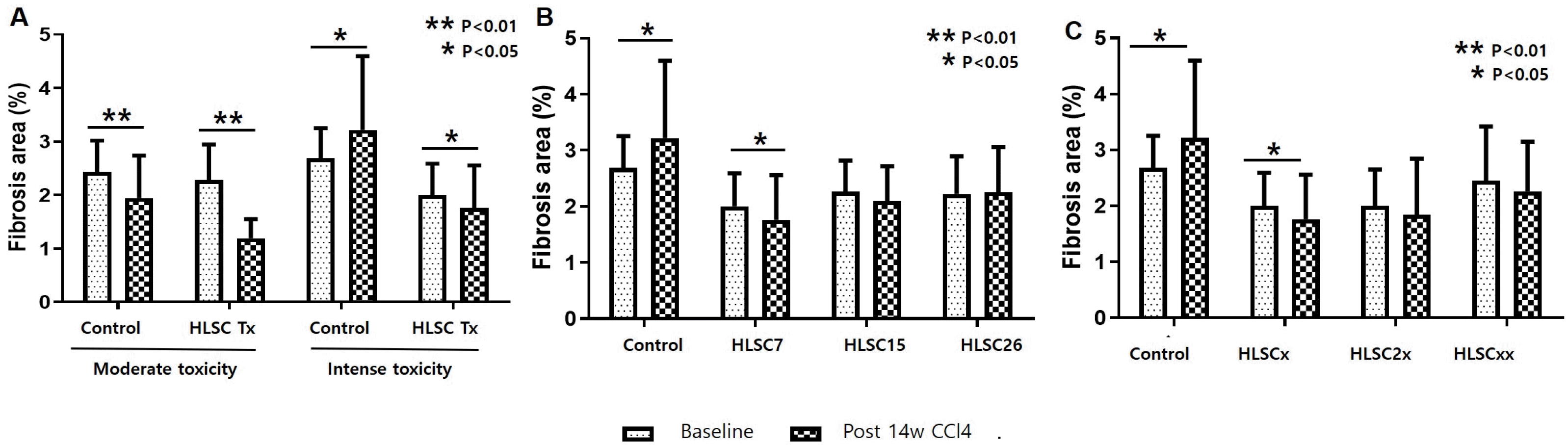
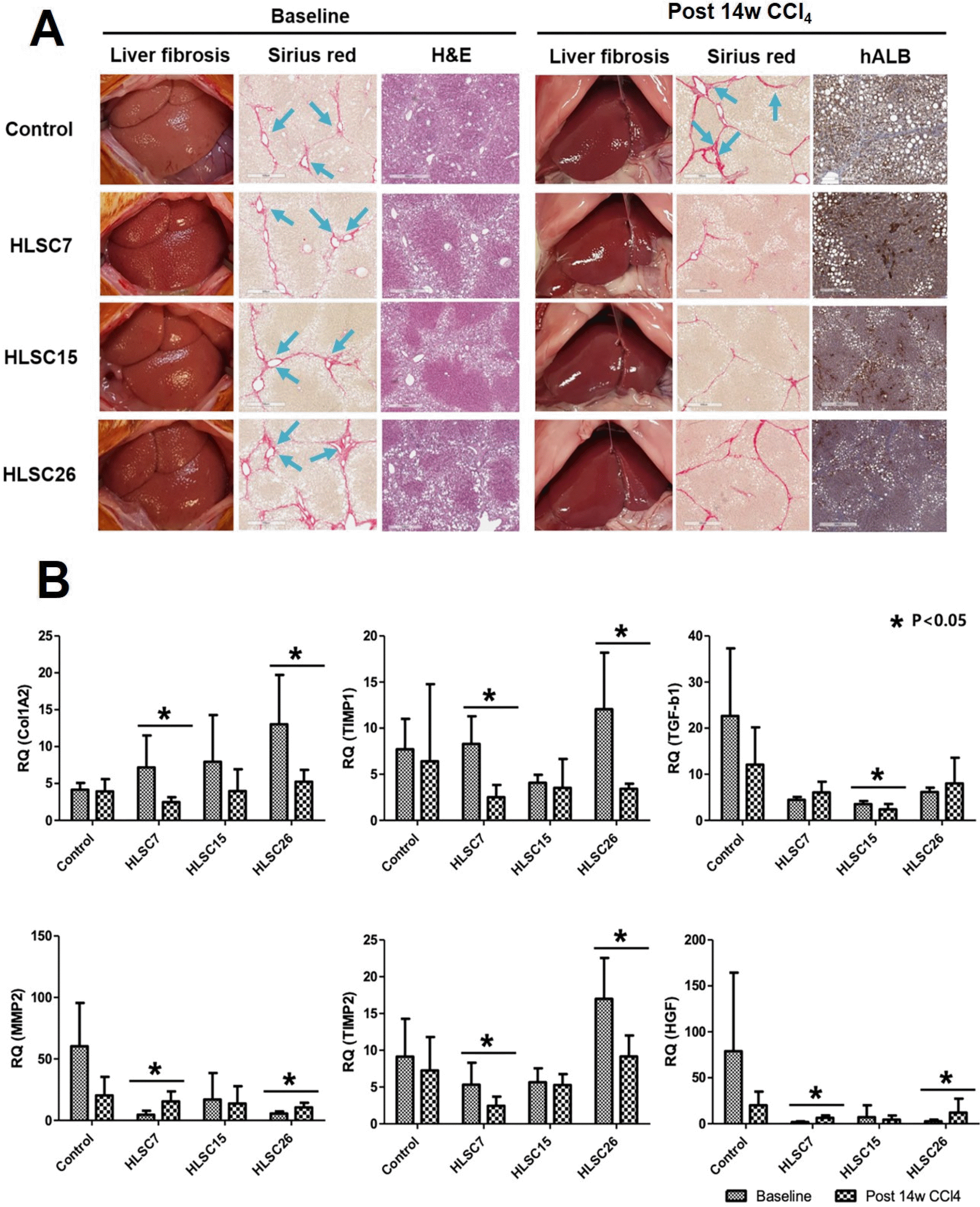




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download