Introduction
Materials and Methods
Ethics statement
ADSC isolation and identification
Strand-specific and high-throughput RNA-Seq library construction
Diabetic wound induction
CD31 immunohistochemistry
RNA overexpression or interference
Western blot assay
Quantitative real-time polymerase chain reaction (RT-qPCR)
Luciferase reporter assay
Statistical analyses
Results
The hypoxic pretreatment increases the therapeutic effect of ADSCs to accelerates diabetic wound closure
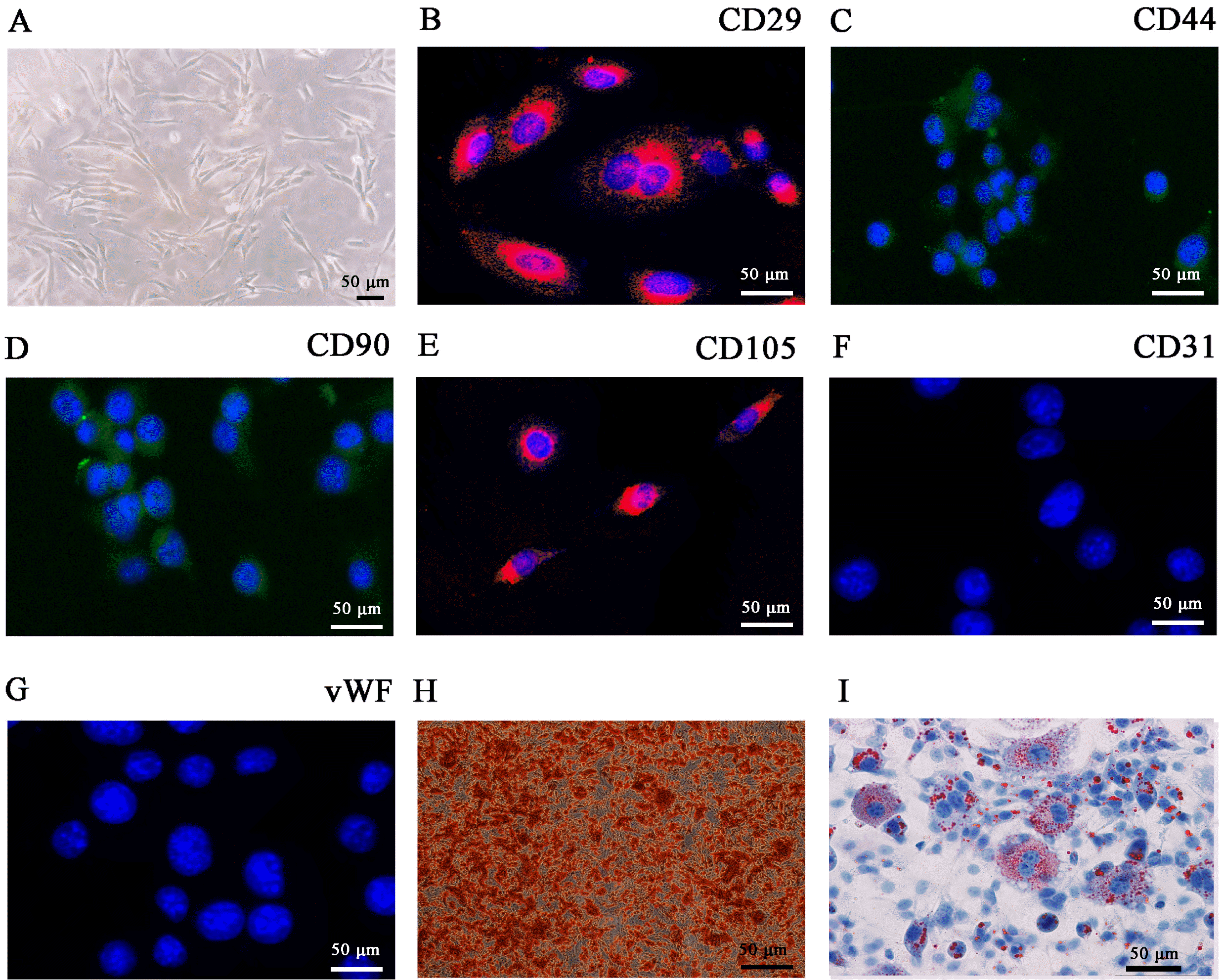 | Fig. 1Characterization of adipose-derived mesenchymal stem cells (ADSCs). (A) ADSCs show a typical cobblestone-like morphology. (B∼G) Immunofluorescence staining of cell surface markers. Antibodies were labeled with either fluorescein isothiocyanate (FITC, green) or phycoerythrin (PE, red). CD29, CD44, CD90, and CD105 staining was positive, whereas CD31 and von Wille-brand Factor (vWF) staining was negative. (H, I) Differentiation potential of ADSCs by oil red O (H) and alizarin red staining (I). Scale bar, 50 μm. |
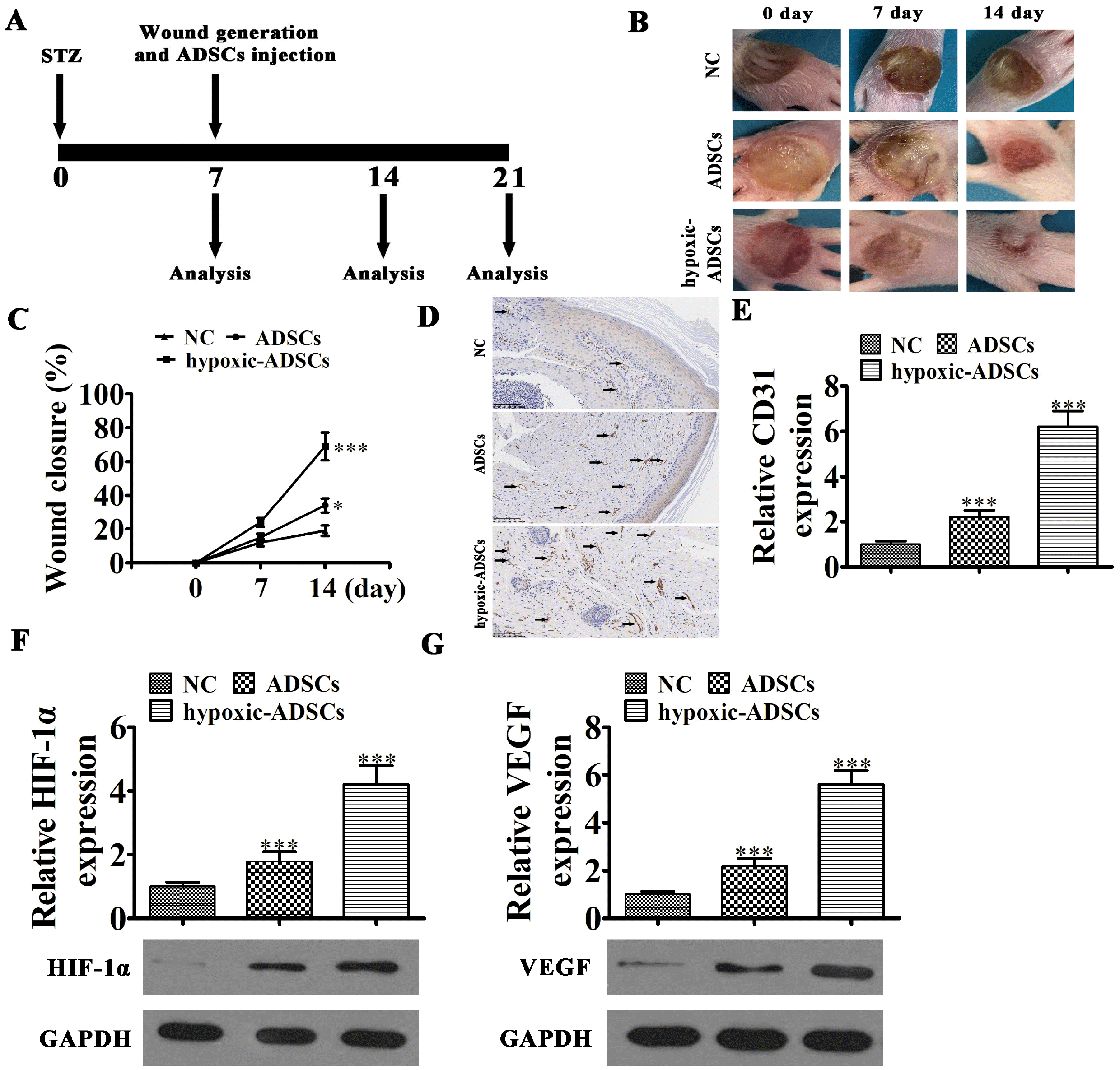 | Fig. 2Wound healing effects of hypoxia-pretreated ADSCs in mice. (A) Schematic of in vivo procedures. (B, C) Wound closure rates were quantified at indicated times after wound generation and ADSC transplantation. Data are presented as the mean±SD. *p<0.05, ***p<0.001 vs. NC group. (D, E) CD31 immunohistochemical staining detection the angiogenesis as black arrow show. Data are presented as mean±SD. ***p<0.001 vs. NC group. (F, G) RT-qPCR and western blot detection show the expression HIF-1α and VEGF in mRNA and protein level from tissue surrounding wounds in the diabetic mouse model. Data are presented as the mean±SD. ***p<0.001 vs. NC group. |
Circ-Gcap14 played an important role in hypoxic-ADSC accelerated diabetic wound closure, and enhanced angiogenic expression in our mouse model
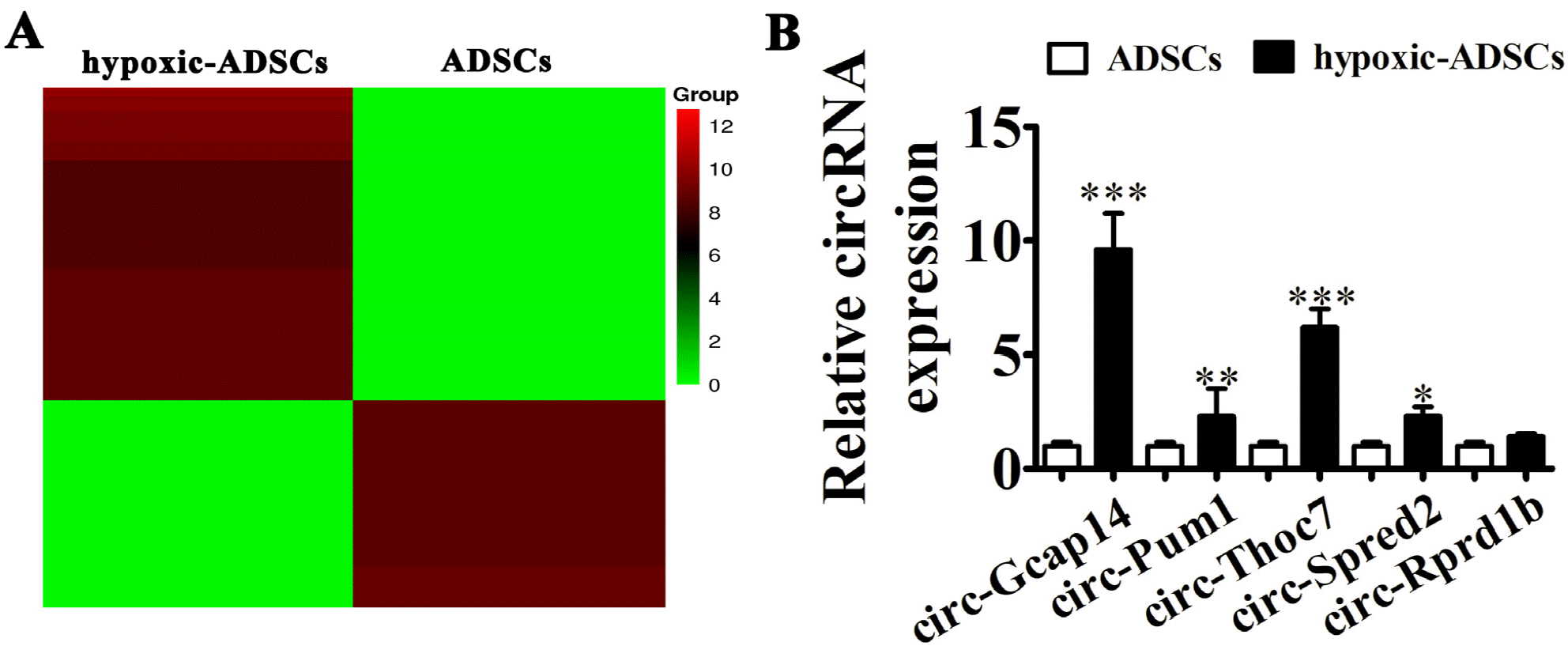 | Fig. 3Circ-Gcap14 is upregulated in hypoxia-pretreated ADSCs (hypoxic-ADSCs). (A) Heat map of upregulated and downregulated circRNAs with a ≥1.5-fold difference between hypoxic- and wild-type ADSCs. (B) RT-qPCR shows circRNA expression in both hypoxic- and wild-type ADSCs. Data are presented as the mean±SD. **p<0.01, ***p<0.001 vs. ADSCs group. |
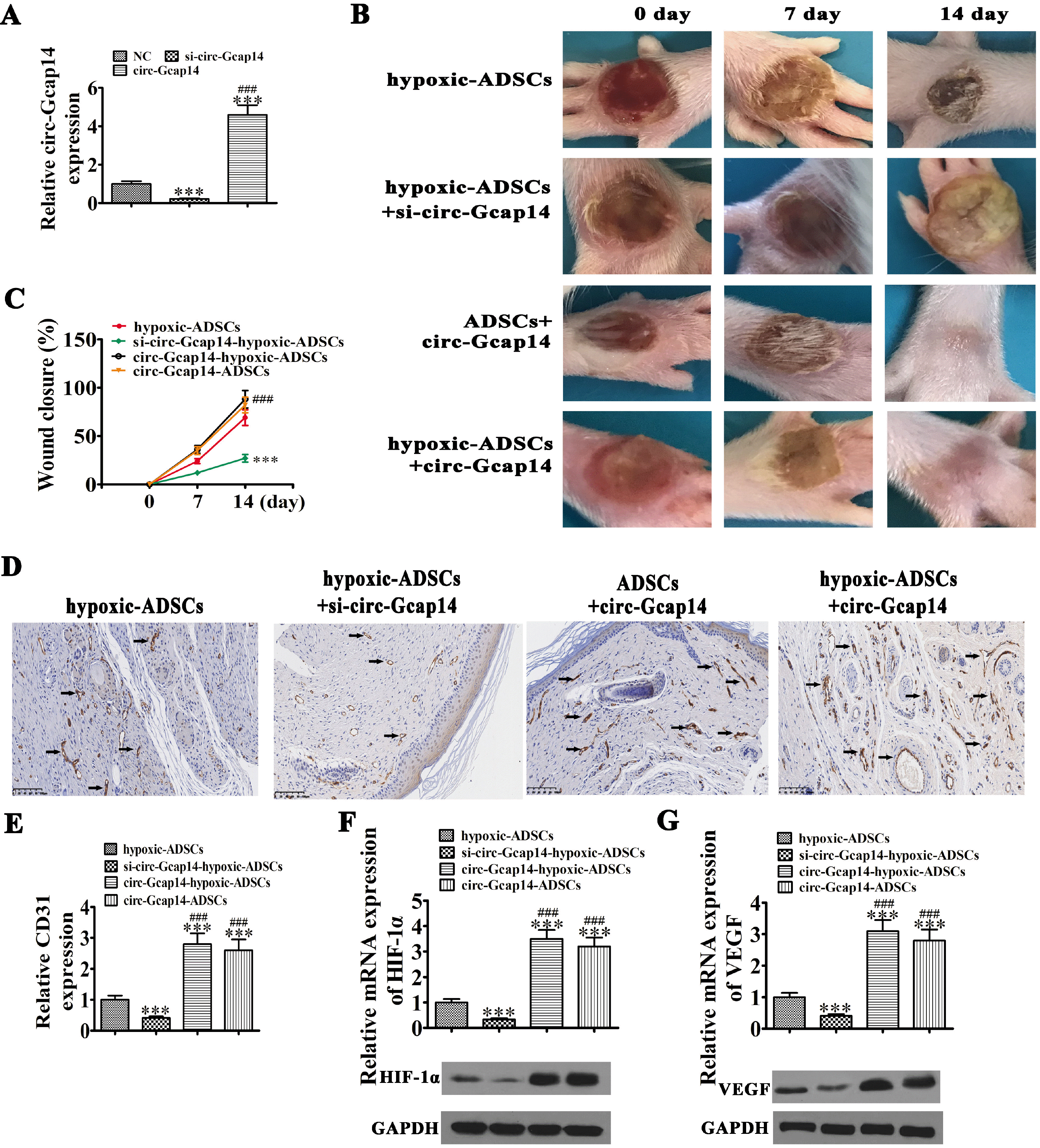 | Fig. 4Circ-Gcap14 plays an important role in ADSC mediated diabetic wound closure. (A) RT-qPCR shows circ-Gcap14 expression in ADSCs upon circ-Gcap14 downregulation or overexpression/silencing. Data are presented as the mean±SD. ***p<0.001 vs. NC. ###p<0.001 vs. si-circ-Gcap14. (B, C) The rate of wound closure was quantified at indicated times after ADSC transplantation. Data are presented as the mean±SD. ***p<0.001 vs. hypoxic-ADSC. ###p<0.001 vs. si-circ-Gcap14-hypoxic-ADSC. (D, E) CD31 immunohistochemical staining detection the angiogenesis as black arrow show. Data are presented as the mean±SD. ***p<0.001 vs. hypoxic-ADSC. ###p<0.001 vs. si-circ-Gcap14-hypoxic-ADSC. (F, G) RT-qPCR and western blot detection shows the expression of HIF-1α and VEGF in both mRNA and protein level from tissue surrounding wounds. Data are presented as the mean±SD. ***p< 0.001 vs. hypoxic-ADSC. ###p<0.001 vs. si-circ-Gcap14-hypoxic-ADSC. |
The expression of circ-Gcap14 increased HIF-1α level by adsorption miR-18a-5p
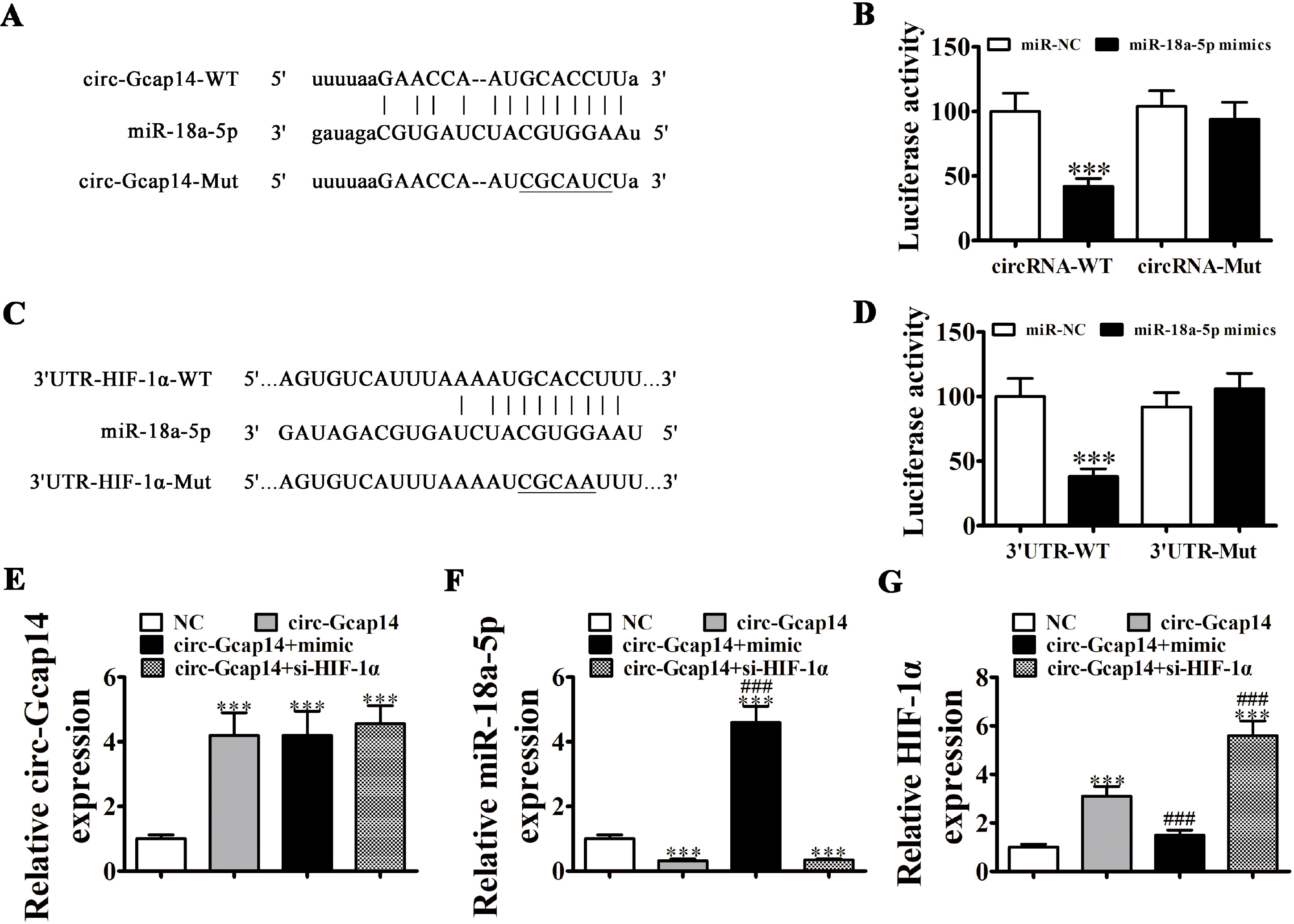 | Fig. 5miR-18a-5p and HIF-1α are downstream targets of circ-Gcap14. (A) Predicted binding sites for miR-18a-5p in circ-Gcap14. The mutated circ-Gcap14 is also shown. (B) Relative luciferase activity at 48 h post-transfection of HEK293T cells with miR-18a-5p mimics/NC or circ-Gcap14 wild-type/mutant. Data are presented as the mean±SD. ***p<0.001. (C) Binding site prediction of miR-18a-5p in the HIF-1α 3’UTR. The mutant version of the 3’-UTR-HIF-1α is shown. (D) Relative luciferase activity at 48 h post-transfection of HEK293T cells with miR-18a-5p mimic/NC or 3’UTR-HIF-1α wild-type/mutant. Data are presented as the mean±SD. ***p<0.001. (E∼G) RT-qPCR shows circ-Gcap14 (E), miR-18a-5p (F) and HIF-1α expression (G). Data are presented as the mean±SD. ***p<0.001 vs. NC group. ###p<0.001 vs. circ-Gcap14. |




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download