Abstract
The interactions between the tumor microenvironment and the tumor cells confers a condition that accelerate or decelerate the development of tumor. Of these cells, mesenchymal stem cells (MSCs) have the potential to modulate the tumor cells. MSCs have been established with double functions, whereby contribute to a tumorigenic or anti-tumor setting. Clinical studies have indicated the potential of MSCs to be used as tool in treating the human cancer cells. One of the advantageous features of MSCs that make them as a well-suited tool for cancer therapy is the natural tumor-trophic migration potential. A key specification of the tumor development has been stablished to be angiogenesis. As a result, manipulation of angiogenesis has become an attractive approach for cancer therapy. This review article will seek to clarify the anti-angiogenesis strategy in modulating the MSCs to treat the tumor cells.
Go to : 
Malignancies and cancer diseases account for a quarter of human death cases (1). The typical therapeutics for chances, including surgery, chemotherapy, and radiotherapy have not been able to be satisfying in treating the cancer patients. Although the methods for treating the malignancies and curing the cancer patients have greatly been improved in last years, in most of the cases there is not a proper response to the traditional therapeutics by the cancer cells. The tumor specificity is regarded to be the most important factor in limiting the efficacy of traditional cancer medications. Hence, it seems necessary for the medicine to search for the most efficient treatment approaches specifically targeting malignancies (2).
Mesenchymal stem cells (MSCs) are considered as the primary option for obtaining the stem cells for using in clinical and experimental applications. These cells have been obtained from various tissues, including brain, heart, and kidney suggesting a potential of promising candidates for several human diseases (3-7). MSCs are able to differentiate toward different cells and can simply be expanded in vitro, and therefore, have attracted the interests for utilizing in treatment options of that human diseases. They have the ability for self-renewal into mesenchymal lineages. MSCs exhibit pathotropic migratory features, establish them as potential candidates for selective delivering the drugs with the aim of tumor therapy (3, 4).
Tumor cells applies angiogenesis to be survived and proliferated (8). A number of growth factors and extracellular matrix proteins are responsible for tumor angiogenesis and, therefore, researchers have been attracted to utilize the targeted anti-angiogenic therapy of treatment of tumors (9, 10). Besides, several stem cells have been modified in vitro for expressing anti-angiogenic factors more efficiently (3). In this review article, we try to clarify the possible applications of MSCs through anti-angiogenic properties to treat cancer patients.
Go to : 
MSCs are considered as the adult stem cells and naturally generated in the human body. MSCs were initially found in the bone marrow (BM) stromal matrix but they are distributed throughout the body (11, 12). MSCs are commonly found in several fetal and adult organs, like amniotic fluid, heart, skeletal muscle, adipose tissue, synovial tissue, placenta, pancreas. According to evidence, organs and tissues containing connective tissue have also MSCs in themselves (13). MSCs are considered as the primitive cells that are basically originating from the mesodermal germ layer and have been regarded as progenitor cells that have the potential to be developed to the connective tissues, skeletal muscle and vascular cells. MSCs have also the potential to be differentiated into cells of the mesodermal lineage, like fat, bone, and cartilage cells, but they can also differentiate into neuroectodermic and endodermic cells (14). MSCs have been considered as an important source in biomedicine due to the multilineage capacity (15). Because of an ease in acquisition, fast proliferative capacity, and the autologous transplantation potential, MSCs have been regarded as the first option of stem cells to be utilized in the regenerative medicine. MSCs may confer advantageous capacity for cell recovery in the harmed tissues (16). These stem cells has been reported to be involved in the modulation of immune cells and, hence, are attributed to be contributing factor in autoimmune disorders (17, 18). Studies have established a tumor specific migration and residence feature for MSCs, suggesting a positive capacity of these cells to be utilized as promising carriers of drugs for the aim of cancer therapy (19). Both pro- and anti-cancer characteristics have been attributed to MSCs (20); nonetheless, if MSCs are monitored efficiently, for example with anticancer agents, they could be applied in cancer therapy.
Go to : 
Several sources of adult tissues have been identified for MSCs extraction and the harvesting of MSCs is not subject to the ethical issues (3). MSCs are potentially able to develop to various tissue types that might be either within or across the germ lines (21). BM-derived MSCs have been attributed with the highest level of lineage plasticity, that can be differentiated to the almost all cell types (22, 23). That notwithstanding, the available data obtained from the preclinical studies have indicated that the BM-derived MSC may not be the best source for utilization in the clinic. Invasive procedures are used for the harvesting of BM that yields a little number of cells. On the other side, the number, differentiation capacity, and life time of BM-derived MSC are decreased as the individuals become older (24, 25). Adipose tissue and umbilical cord blood are considered as two alternative sources to harvest MSCs. Recently, MSCs obtained from adipose tissue have gained high attention to be used in preclinical and clinical evaluations, since tissue sampling is simple, the initial cell numbers is high, and the proliferation potential is satisfactory (26). However, phenotypes of MSCs, and the expansion and differentiation capacity of these cells originating from the adipose tissue are similar to those obtained from BM (27).
Furthermore, the umbilical cord blood as well as the wharton’s jelly have been implied to have a plenty of MSCs (28). The cells extracted from placenta in adherent layer, which is considered a noninvasive and simple approach, have been reported to have a fibroblastiod morphology, which represent similar surface molecules as BM-derived MSCs, such as CD90, CD13, CD49e, CD29, and CD54 (29). Umbilical cord blood-derived MSCs show the ability to proliferate at a higher rate in comparison to the BM and adipose tissue-derived MSCs (27, 30), which is probably because of high telomerase activity in the umbilical cord blood-derived MSCs (31).
Go to : 
As the first step in the homing, MSC needs to migrate from the BM to the circulation and move toward other tissues (Fig. 1). It has been indicated that MSCs can mobilize from the BM as well as other tissues to the circulation upon the various injury situations, including hypoxia, normoxia, and inflammation (32, 33). It is not completely divulged that how MSCs migrate from the BM and cross through the endothelium and how home in the tissues. That notwithstanding, it is clear that a common role of MSCs is to home in and repair the injured organs. The wound healing ability of MSCs initiates with their migration to the signals released from the injured organs (34). Various inflammatory factors released from wounds have also been identified in the tumor microenvironment that have been attributed to the migration of MSCs (35). Among the mediators/receptors involved in the migration of MSCs are MCP-1/CCR2, SDF-1/CXCR4, HGF/c-Met, SCF-c-Kit, VEGF/VEGFR, PDGF/PDGFR, and HMGB1/RAGE (36). The involvement of the stromal cell-derived factor SDF-1 and the related receptor, CXC chemokine receptor-4 (CXCR4), in the migration of MSCs identified in surveys that either the receptor or the cytokine was knocked down (37-39). Furthermore, it was demonstrated that inhibition of CXCR4 and SDF-1 in mice cause marked decrease in the migration of exogenous stem cells to target organs (37). It has also been indicated that the blockade of CXCR4 resulted in an impaired homing of endogenous MSC into tumor sites, development of MSCs into myofibroblasts, and reduced MSC survival (40). It has been revealed that chemokines like CXCL12, CXCL13, CXCL16, and their receptors play a role in the mutual mobilization of MSCs to BM and generate the BM niche. In the efficient homing of MSC into the BM, CXCL16 play an important role. CCL22 has been shown to play the strongest chemotactic impression in the migration of MSCs from the BM into the blood (41). Tumor tissues represent CCL2 and CXCL16, suggesting that they are playing a vital role in the mobilization and migration of MSCs into tumor sites (42-44).
Matrix metalloproteinase (MMPs) enzymes poses proteinases function, which is needed for proteolytical cleavage of precursor proteins, such as adhesion molecules, growth factors, cytokines, as well as some receptors. It has been demonstrated that MMP-1 and tissue inhibitor of metalloproteinase-3 (TIMP3) play role in the migration of MSCs across the BM endothelium (45). Plus, increased MMP-2 levels in the serum was attributed to the C1q complement -mediated migration of umbilical cord blood-derived MSC into the wounded tissue (46).
It was demonstrated that MSCs were recruited to the regions of irradiation, and local irradiation may increase the MSC specificity to be homed in particular tissues (47). These observations about the migratory characteristics of MSCs testify the promising potentials for designing the therapeutic tools that exploit the tumoritropic features of MSCs through engineering these cells towards potent cells via delivering the compounds against tumors.
Go to : 
MSCs show different kinds of activities in various microenvironments because of the diversity in the signaling pathways involved in the stimulation of MSCs. In the clinical studies, employed MSCs are primarily naïve, obtained from normal tissues, and are usually harvested in vitro. Such naïve MSCs have the ability to interfere with the tumor cell growth in co-cultures with tumor cells. It was observed that naïve MSCs could decrease the expansion of leukemia cells. Such blocking property of naïve MSCs was seen to be dose-dependent, as the naïve MSCs was increased, the rate of inhibition of tumor growth was increased. It was suggested that naïve MSCs might decrease the expansion of tumor cells through mediators released from MSCs, such as Dickkopf-related protein 1 (DKK1), an inhibitor of Wnt signaling pathway in tumor cells (48). Interfering in the Wnt pathway was associated with the decreased expression of Cyclin D2 and c-Myc as well as increased expression of P27KIP1 and P21CIP1, that culminated in the interruption of tumor cell cycle (49-52). On the other side, naïve MSCs have the potential to activate the apoptosis in tumor cells (53) via increasing the expression of caspase 3 (50). Furthermore, naïve MSCs have the potential to decrease the expansion of tumor cells via inhibiting the angiogenesis. In this way, naïve MSCs increase the apoptosis rate in vascular endothelial cells and interfere with angiogenesis (54, 55).
On the other side, tumor supporting activities of MSCs have also been reported (56). An equilibrium between anti-inflammatory and pro-inflammatory phenotypes of MSCs determine the influence of MSCs on the progression or suppression of tumor cells (57). It has been shown that MSCs inside tumors might undergo functional modulations to alter from an anti-tumorigenic phenotype to pro-tumorigenic MSC (58, 59). MSCs with anti-inflammatory properties are involved in contributing to tumor development by several mechanisms. Tumor perturbations has been shown to be associated with recruitment of MSCs to tumor sites (60). MSCs are able to regress immune responses against tumor cells (61), induce angiogenesis in tumors (62, 63), promote epithelial-to-mesenchymal transition (EMT) resulting in metastasis (64, 65), and induce a resistance in tumor cells to different therapeutics (66). Furthermore, it has been indicated that MSCs promote tumor progression through modulating the metabolic settings of tumor cells. Particularly, prostaglandin E2 secreted by MSCs is able to abrogate the apoptosis of lymphoblastic leukemic cells (67). MSCs produce lactate in a tumor microenvironment with high oxidative stress conditions that is uptaken via tumor cells, resulting in promoted migratory ability of tumor cells (68). Naïve MSCs have also been attributed with the increased angiogenesis in the colon cancer cell lines (69, 70), differentiation of MSCs to vascular endothelial cells in melanoma (71), increasing the development of cancer stem cells (CSCs) that promote the tumorigenesis and metastasis (72) in breast cancer (73), enhanced proliferation of gastric cancer cell lines (72, 74). Moreover, naïve MSCs might support tumor progression through increasing the migratory abilities of tumor cells via production of chemokines, like CXCR4 (75), CCL5 (76, 77), intercellular adhesion molecules (ICAMs), and vascular cell adhesion molecules (VCAMs) (78). Moreover, by suppressing the immune responses, MSCs has been observed to enhance the tumor development (79).
In addition, MSCs generate a wide range of chemokines, cytokines, and growth factors that mediate paracrine- or autocrine functions in the tumor development (80). These factors are able to modulate the tumor microenvironment to a tumor supporting settings. Several inflammatory mediators secreted by MSCs, including CXCL1, CXCL2 or CXCL12, are able to indirectly enhance tumor development in several tumor models (81, 82). In the same way, MSCs-derived inflammatory chemokines and cytokines, such as IL-8 and IL-6, contribute the tumor development in colon cancer (83) and breast cancer (73) models. By a paracrine mechanism via IL-6 and CXCL7 secretion, MSCs was shown to migrate into breast cancer xenografts and promoted the development of CSC subpopulations (73).
It seems that MSCs may represent bidirectional behaviors on tumor development, and various studies have reported a disagreement on the MSCs effect on the tumor progression or inhibition. However, it is worthy to mention that there is a general agreement that MSCs are frequently contribute to the tumor development in comparison to suppressing tumor growth, which is widely attributed to the regenerative functions and immune system suppression by MSCs (84, 85).
Go to : 
Extracellular Vesicles (EVs), like exosomes, are membrane-enclosed vesicle with a size of 40∼1,000 nm that are produced by several cells, such as MSCs (86). EVs primarily encompass microRNAs (miRNAs) as well as proteins, which is encapsulated by a lipid bilayer membrane (87-90). Secreted EVs are able to move toward other cells and bind them in order to deliver several signals to the targets (91). MSC-derived EVs are able to modulate several processes in the tumor cells, including metastasis, angiogenesis, and proliferation (92) as well as control immune cells (93, 94). Indeed, MSC-derived EVs might promote the tumor development or repress tumor progression (95).
Umbilical cord derived MSC-EVs was shown to meliorate the oxidative stress and cell apoptosis as well as promote cell proliferation in rats with cisplatin-induced nephrotoxicity (96). Additionally, Zhu et al. (97) indicated that MSC-derived EVs promoted tumor growth through promoting the expression of vascular endothelial growth factor (VEGF) in tumor cells by induction of the extracellular signal-regulated kinase1/2 (ERK1/2) pathway. Therefore, MSCs are able to promote tumor growth and angiogenesis by secreting EVs.
On the other hand, it was shown that BM-derived MSCs-EV caused cell-cycle arrest in the G0/G1 phase and induced apoptosis in tumor cells (98). Additionally, MSCs-EV interrupted the angiogenesis by suppressing the expression of VEGF in tumor cells (99). Furthermore, MSC-derived EVs containing miRNAs targeting VEGF were observed to suppress angiogenesis in human nasopharyngeal carcinoma cells (100). miR-143, which has been investigated in several malignancies (101), was indicated to be harbored by MSC-derived EVs an reduced the migration of osteosarcoma cells (102). As a consequence, MSC-derived EVs are able to repress the tumor cell proliferation and angiogenesis.
Go to : 
Naïve MSCs (have not undergone any manipulation) have been reported to possess antitumor properties. This antitumor activity has been attributed to the mediators produced by MSC that have the potential to decrease the proliferation of tumor cells in different cancers, including breast adenocarcinoma, melanoma, hepatoma, lung cancer, and glioma (52, 103-105). In a mouse model of Kaposi’s sarcoma, the BM-derived MSCs were administered intravenously that resulted in the homing of these cells in the tumor sites and prevented the progression of tumor (106). Studies in vitro and in the mouse model of melanoma indicated that MSCs possess the ability to repress the angiogenesis (55). Furthermore, MSC administration to the melanoma mice model subcutaneously eventuated in increased apoptosis rate and decreased expansion of tumor (55). In addition, umbilical cord blood-derived MSCs overexpressing CD44 and CD133 were reported to increase apoptosis rate in the glioblastoma multiforme cells in vitro (107). As well, umbilical cord blood-derived MSCs administration resulted in underexpression of XIAP activating caspase-3 and caspase-9, leading to increased apoptosis rate in the glioma cells (108). To comply with these observations, umbilical cord blood-derived MSCs as well as MSCs from other sources indicated reduced expansion of the glioblastoma multiforme cells through tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) (109).
Go to : 
In the embryonic development period, neovascularization or new blood vessel formation from pre-existing vessels is regarded as a major occurrence that leads to the construction of vasculature system that contains endothelial cells (ECs) (110). During the physiological situations, vasculogenesis is seldomly observed in the adults. That notwithstanding, angiogenesis may be seen in adults as a physiological event during pregnancy as well as the cyclical growth of vessels in the ovarian corpus luteum (111, 112). In the pathological conditions, such as tissue repair during wound healing and tumor growth, neovascula-rization is considered as a normal phenomenon (113). However, it should be noted that the structure and composition of the tumor vessels are different from those occurred in the normal vessels. It has been shown that ECs of tumor tissues express higher levels of CD109, CD137, CD276, and placenta growth factor (PlGF) compared with the ECs originating from the normal non-tumor organs. In spite of a bulk of studies with respect to the cellular and molecular characteristics of ECs from different tumors, there are disagreements with respect to the origin of ECs in the tumor conditions (114). It has been reported that endothelial progenitor cells (EPCs) are actively involved in the angiogenesis process of tumors (114). However, it is clear that the initiation of angiogenesis requires a divergence toward a profile of increased angiogenesis activators, while inhibited process of angiogenesis suppression. The critical stimulating mediators of angiogenesis include vascular endothelial growth factor (VEGF)-A, fibroblast growth factor (FGF), and hepatocyte growth factor (HGF), PlGF, and MMPs (115, 116). Adversely, thrombospondins, endostatin, angiostatin, and interleukin (IL)-12 are important endogenous inhibitors of angiogenesis (117). Taken together, angiogenesis is thought to be involved in the progression and development of tumors.
Noting to the participation of the angiogenesis process in the development of tumors, it seems that the molecules involved in the tumor angiogenesis can be targeted as a therapeutic strategy. Inhibition or blocking of the growth factors or signaling pathways prerequisite for the development and progression of ECs is regarded as a logical mindset for prevention of tumor vasculogenesis and, therefore, tumor repression (113, 118, 119). VEGF is considered as an critical angiogenetic factor which is involved in both physiological and pathological situations (120). Genetic alterations in the VEGF gene have been reported to cause defects in the normal development angiogenesis and death in the embryonic period (120). Hypoxia inducible factor (HIF)-1α, through binding to the VEGF promoter, regulates the expression of VEGF (121). A hypoxic condition in various regions of tumors results in the increased expression of VEGF in tumor niche, leading to an enhanced blood supply and expedited expansion of tumor cells (122). Furthermore, VEGF is normally overexpressed in the microenvironment of numerous human tumors (123); however, VEGF receptors of VEGFR1, VEGFR2, and VEGFR3 have been shown to be highly expressed in the tumor associated ECs (124, 125).
The tumor mice model were administered with an monoclonal antibody targeting the VEGF that manifested as a remarkable deceleration in the tumor growth (126). Bevacizumab is a humanized monoclonal antibody to neutralize VEGF (127), was the first anti-angiogenic monoclonal antibody approved by the FDA (Food & Drug Administration) in 2003 for the therapy of metastatic colorectal cancer patients (128, 129). In addition, the promising effects of bevacizumab in treating patients with non-small cell lung cancer (NSCLC) (130) and metastatic breast cancer (131) were established. Upon these successful experiences, some other factors inhibiting the VEGF pathway, target either VEGF or its receptor, have been under clinical trial studies. As a soluble receptor of VEGF, VEGF-TrapR1R2, which carries the functional parts of the VEGFR1 and VEGFR2 (132), has been shown to have the ability to neutralize the circulating VEGF and be utilized in tumor therapy. In addition, it has been observed that VEGF-TrapR1R2 possesses better anti-tumor effects in relation to DC101, which is a VEGF receptor blocker. Studies have shown that the inhibition of VEGF signaling pathway, through the components that blocks the VEGF receptor, are efficiently involved in the repression of the tumor progression (133, 134). Receptor tyrosine kinase inhibitors (RTKIs), which targets molecules in the VEGF signaling pathways, including linifanib (135, 136), cabozantinib (137), axitinib (138), tivozatinib (139), vendatanib (140), sunitinib (141), pazopanib (142), and sorafenib (143) has been shown to be promising in the treatment of various tumors. In spite of promising effects of these therapeutics in the clinical trials, the detailed mechanisms underlying their beneficial anti-angiogenic effects have not fully been disclosed. Preclinical models have revealed that the inhibition of angiogenesis has been the main mechanism of angiogenesis inhibitors that target VEGF pathway. That notwithstanding, vascular normalization has also been implicated as another mechanism of anti-angiogenetic agents. This mechanism imply to the targeting of the non-functional vessels in tumors, that implement positive functions through decreased blood flow to the tumor, or efficient drug delivery to the tumor cells (144). Therefore, progression in designing agents with potential targeting of VEGF pathway and, therefore suppressing the angiogenesis, provided prosperous therapeutic strategy for tumors that have the potential to be further improved in combination with other tools and approaches.
Vascular disrupting agents (VDAs) have been considered as another class of anti-angiogenetic compounds above and beyond the VEGF inhibitors. To implement their anti-angiogenic functions to prevent tumor development, VDAs cause vascular collapse, manifesting as hypoxia, which in turn cause tumor necrosis (145). ASA404 is a VDA that has been shown to cause apoptosis in the ECs, and, thereupon, diminished blood flow to the tumor cells. Clinical studies is currently assessing the efficacy of ASA404 in the NSCLC patients (146).
Go to : 
Angiogenesis is applied by tumors to proceed the process of growth (8). Angiogenesis can be inhibited through the exertion of MSCs as vehicles to deliver anti-angiogenetic drugs to the tumors and, therefore, limit the tumor progression. Such engendered MSCs have displayed a tropism to cancer organs and can deliver anti-angiogenic drugs to the tumor sites, while having little adverse effects (147). However, it should be noted that a systemic supply of anti-angiogenic drugs for a long time may present with adverse effects, such as drug toxicity, as well as decreased blood supply of the tissues, which in turn results in the poor delivery of chemotherapeutic drugs to the target sites (148).
Dysregulated balance of pro-angiogenic and anti-angiogenic mediators as well as growth factors in the tumor microenvironment has been attributed to the tumor-mediated angiogenesis (9, 149). As an endogenous inhibitor of angiogenesis in the tumors, endostatin has been exerted as an important anti-angiogenic agent in several malignancies (150). In a study, the human placenta-derived MSCs were manipulated to deliver endostatin and were injected into nude mice. These MSCs, which expressed endostatin, were observed to be resided in the tumor site and a significant reduction in the tumor size was reported without considerable systemic toxicity and adverse effects. The reduced tumor size was reported to be due to increased apoptosis in the tumor cells and blocked angiogenesis (150). Furthermore, a phase II clinical trial reported that delivery of anti-angiogenic compounds resulted in the correction of the abnormal structure and malfunction of the blood vessels, culminating in a remarkable decrease in the brain edema (151). This correction in the vessel structure was reported to be due to decreased vessel diameter and permeability (152, 153). Intratumoral administration of MSCs has been shown to be accompanied with the residence of these cells in the tumor sites and interfering with vasculogenesis, implying to a beneficial potential of MSCs for targeted anti-angiogenic drug delivery (154). The genetically-modified human adipose-derived MSCs expressing the interferon γ-induced protein 10 kDa (IP-10), which is a powerful chemoattractant, in a mouse model of metastatic melanoma resulted in inhibited tumor cell growth and limited angiogenesis and significantly associated with increased survival of mice (155).
Go to : 
Estrogen, a female sex hormone, has been shown to stimulate angiogenesis through acting directly on ECs, as well as indirectly on endometrial cells. It has also been reported that estrogen triggers the expression of VEGF in the uterine stromal cells (156, 157). ER has been established as a potential target of various drugs for inhibition of the breast cancer progression (158). The ligation of estrogen to the estrogen receptor (ER) causes an increased proliferation; thereupon, endocrine cancer therapy attempts to prevent the binding of estrogen to the ER and block the aftermath signaling. Tamoxifen and raloxifene are considered as selective estrogen receptor modulators (SERMs) that are competitive inhibitors of estrogen and directly bind to ER. Interfering with the estrogen pathway has been indicated as an efficient strategy for the therapy of hormone responsive breast cancers, while conferring little toxic side effects (159). High expression of ERβ has been observed in the prostate (160). Implying to the possible response of prostate cancer to SERMs (161). Moreover, promising outcomes have been established about the estrogen therapy in the ovarian cancer patients (162). As well, the Women’s Health Initiative (WHI) trial indicated that estrogen is involved in the colon carcinogenesis (163). As a result, with respect to the role of estrogen and ERs in the process of angiogenesis and the promising outcomes of estrogen/ER pathway blockade, it seems that MSCs can be exerted as machine for the targeted delivery of drugs that interfere with this pathway. Moreover, monoclonal antibodies targeting ERs can be evaluated to inhibit angiogenesis in the tumor microenvironment. SERMs have been attributed with a number of adverse effects in the tissues that are not the target of such compounds. Hence, strategies for targeting the estrogen/ER pathways needs to be optimized to obtain best results. This is a hypothesis by the authors, requiring studies in the animal models as well as clinical trials to be validated.
It has been demonstrated that progesterone prevents the proliferation of ECs in vitro and interferes with the cell cycle in the human dermal endothelial cells (164). Progesterone receptor is expressed on the endometrial ECs and may play a role in the suppression of VEGF-induced proliferation of ECs (165). Therefore, targeting the progesterone pathway through manipulated MSCs seems potential therapeutic strategy for cancer treatment, a hypothesis that needs to be extensively studied.
The corpus luteum is considered as a temporary endocrine tissue in ovaries that are involved in the prosperous gestation of mammals. When a follicle undergone the rupture process upon ovulation, the corpus luteum is then converted to debris and undergoes a number of modifications, such as differentiation and growth, which rely on the angiogenesis process. It has been reported that some endogenous stimulatory and inhibitory compounds exist in the corpus luteum to regulate the angiogenesis (166). Follicular cells of ovaries produce the pigment epithelium–derived factor (PEDF), which is a physiological inhibitor of angiogenesis (167). PEDF possess an anti-angiogenic function in the corpus luteum, implying to its possible utility as an anti-angiogenesis therapy of cancer in the future, probably through delivering via MSCs as vehicles.
Go to : 
The therapeutic application of MSCs has been associated with multiple challenges that may limit the clinical efficacy of this approach. Among the drawbacks in exertion of MSCs are inappropriate homing of MSCs in the body and little accumulation of these cells in the target tumor (168). Additionally, MSCs may lose their therapeutic potential, gain immunogenicity properties, and obtain tumorigenic characteristics during physiological differentiation into mesenchymal lineages (169). It has also been shown that endogenous MSCs are able to produce several mediators and activate signaling pathways to promote the angiogenesis in tumor cells, like colorectal cancer (69) and melanoma (170). However, a study analyzed clinical 36 trials that used BM-derived MSCs through intravascular delivery in different subjects, including graft versus host disease, Crohn’s disease, ischemic stroke, cardiomyopathy, myocardial infarction, and healthy volunteers. This study indicated no associationship between the MSCs therapy and tumorigenic risk. Moreover, no severe adverse effects of the MSC therapy were observed (171). Most of the clinical trials have evaluated the safety and tolerability of the MSC therapy in cancers. Nonetheless, the adverse effects of MSC therapy in promotion of tumor, instead of suppressing it, has little been studied. The tumorigenic evaluation of MSC therapy needs long time follow-up of the patients, as carcinogenesis is a gradual process.
Go to : 
Several beneficial properties have been proposed for MSCs that make them appropriate candidates for cell-based therapeutic tool in cancer therapy. First, MSCs are able to mobilize and preferentially home in tumor tissues and interact with various cells present in tumor microenvironment. Furthermore, MSCs are easily available, have no or little immunogenic features, can be simply manipulated in vitro. Little have been surveyed with respect to the preclinical evaluation of the MSC potential to be exerted as vehicles to locally deliver/express a single therapeutic compound in cancers. Despite a promising experience in the promoted survival of cancer cases through anti-angiogenic therapy, the pooled findings testify a limited overall survival improvement (172, 173). These limitations stem from several issues. First, tumor microenvironment may exert mechanisms to resist against anti-angiogenetic molecules and, therefore, limit the efficacy of such therapeutics (174). Second, anti-angiogenetic drugs may develop vascular collapse that may result in a hypoxic state, which in turn may result in radio-resistance, chemo-resistance and anti-angiogenesis resistance (175, 176). These adverse effects may culminate in tumor metastasis and, therefore, negatively impress the clinical exertion of anti-angiogenic drugs. In spite of an improvement in our knowledge of MSCs in recent years, little has been attempted with respect to the employment of these cells to downmodulate the angiogenesis in the clinics. Therefore, filling this gape requires developing state-of-the-art technologies and new concepts in exertion of anti-angiogenic compounds conferring little side effects. To attain this end, engineering MSCs to specifically deliver the angiogenesis inhibitors could be still promising, particularly when combined with other therapeutics and targeting other pathways simultaneously. As well, searching for novel molecular targets to diminish the angiogenesis could be a part of answer to this gap.
Go to : 
Notes
Author Contributions
MG and ShGh; Developed the main idea, participated in manuscript drafting, and read the manuscript critically. DR; Developed the main idea, participated in manuscript drafting, and read the manuscript critically. MK and MA; Developed the main idea, participated in manuscript drafting, and read the manuscript critically. MM; Draw the figure, participated in manuscript drafting, and read the manuscript critically. AS and HZ; Developed the main idea, participated in manuscript drafting, and read the manuscript critically.
Go to : 
References
1. Siegel RL, Miller KD, Jemal A. 2016; Cancer statistics, 2016. CA Cancer J Clin. 66:7–30. DOI: 10.3322/caac.21332. PMID: 26742998.

2. Sawyers C. 2004; Targeted cancer therapy. Nature. 432:294–297. DOI: 10.1038/nature03095. PMID: 33195035. PMCID: PMC7655966.

3. Corsten MF, Shah K. 2008; Therapeutic stem-cells for cancer treatment: hopes and hurdles in tactical warfare. Lancet Oncol. 9:376–384. DOI: 10.1016/S1470-2045(08)70099-8. PMID: 18374291.

4. Teo AK, Vallier L. 2010; Emerging use of stem cells in regenerative medicine. Biochem J. 428:11–23. DOI: 10.1042/BJ20100102. PMID: 20423328.

5. Fathollahi A, Gabalou NB, Aslani S. 2018; Mesenchymal stem cell transplantation in systemic lupus erythematous, a mesenchymal stem cell disorder. Lupus. 27:1053–1064. DOI: 10.1177/0961203318768889. PMID: 29631514.

6. Zanganeh E, Soudi S, Zavaran Hosseini A, Khosrojerdi A. 2019; Repeated intravenous injection of adipose tissue derived mesenchymal stem cells enhances Th1 immune responses in Leishmania major-infected BALB/c mice. Immunol Lett. 216:97–105. DOI: 10.1016/j.imlet.2019.10.008. PMID: 31622634.

7. Khosrojerdi A, Soudi S, Hosseini AZ, Eshghi F, Shafiee A, Hashemi SM. 2021; Immunomodulatory and therapeutic effects of mesenchymal stem cells on organ dysfunction in sepsis. Shock. 55:423–440. DOI: 10.1097/SHK.0000000000001644. PMID: 32826813.

8. Jain RK, di Tomaso E, Duda DG, Loeffler JS, Sorensen AG, Batchelor TT. 2007; Angiogenesis in brain tumours. Nat Rev Neurosci. 8:610–622. DOI: 10.1038/nrn2175. PMID: 17643088.

9. Samant RS, Shevde LA. 2011; Recent advances in anti-angiogenic therapy of cancer. Oncotarget. 2:122–134. DOI: 10.18632/oncotarget.234. PMID: 21399234. PMCID: PMC3260813.

10. Javan MR, Khosrojerdi A, Moazzeni SM. 2019; New insights into implementation of mesenchymal stem cells in cancer therapy: prospects for anti-angiogenesis treatment. Front Oncol. 9:840. DOI: 10.3389/fonc.2019.00840. PMID: 31555593. PMCID: PMC6722482.

11. Friedenstein AJ, Piatetzky-Shapiro II, Petrakova KV. 1966; Osteogenesis in transplants of bone marrow cells. J Embryol Exp Morphol. 16:381–390. DOI: 10.1242/dev.16.3.381. PMID: 5336210.

12. Friedenstein AJ, Petrakova KV, Kurolesova AI, Frolova GP. 1968; Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplan-tation. 6:230–247. DOI: 10.1097/00007890-196803000-00009. PMID: 5654088.
13. Väänänen HK. 2005; Mesenchymal stem cells. Ann Med. 37:469–479. DOI: 10.1080/07853890500371957. PMID: 32807933. PMCID: PMC7704061.

14. Uccelli A, Moretta L, Pistoia V. 2008; Mesenchymal stem cells in health and disease. Nat Rev Immunol. 8:726–736. DOI: 10.1038/nri2395. PMID: 19172693.

15. Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. 1999; Multilineage potential of adult human mesenchymal stem cells. Science. 284:143–147. DOI: 10.1126/science.284.5411.143. PMID: 10102814.

16. Le Blanc K, Pittenger M. 2005; Mesenchymal stem cells: progress toward promise. Cytotherapy. 7:36–45. DOI: 10.1016/S1465-3249(05)70787-8. PMID: 16040382.

17. Fiorina P, Jurewicz M, Augello A, Vergani A, Dada S, La Rosa S, Selig M, Godwin J, Law K, Placidi C, Smith RN, Capella C, Rodig S, Adra CN, Atkinson M, Sayegh MH, Abdi R. 2009; Immunomodulatory function of bone marrow-derived mesenchymal stem cells in experimental autoimmune type 1 diabetes. J Immunol. 183:993–1004. DOI: 10.4049/jimmunol.0900803. PMID: 19561093. PMCID: PMC3895445.

18. Nauta AJ, Fibbe WE. 2007; Immunomodulatory properties of mesenchymal stromal cells. Blood. 110:3499–3506. DOI: 10.1182/blood-2007-02-069716. PMID: 30516112.

19. Loebinger MR, Janes SM. 2010; Stem cells as vectors for antitumour therapy. Thorax. 65:362–369. DOI: 10.1136/thx.2009.128025. PMID: 20388765. PMCID: PMC3401681.

20. Mishra PJ, Mishra PJ, Glod JW, Banerjee D. 2009; Mesenchymal stem cells: flip side of the coin. Cancer Res. 69:1255–1258. DOI: 10.1158/0008-5472.CAN-08-3562. PMID: 19208837.

21. Anderson DJ, Gage FH, Weissman IL. 2001; Can stem cells cross lineage boundaries? Nat Med. 7:393–395. DOI: 10.1038/86439. PMID: 11283651.

22. Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M, Du J, Aldrich S, Lisberg A, Low WC, Largaespada DA, Verfaillie CM. 2002; Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 418:41–49. DOI: 10.1038/nature00870. PMID: 12077603.

23. Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, Pickel J, McKay R, Nadal-Ginard B, Bodine DM, Leri A, Anversa P. 2001; Bone marrow cells regenerate infarcted myocardium. Nature. 410:701–705. DOI: 10.1038/35070587. PMID: 11287958.

24. Bentzon JF, Stenderup K, Hansen FD, Schroder HD, Abdallah BM, Jensen TG, Kassem M. 2005; Tissue distribution and engraftment of human mesenchymal stem cells immortalized by human telomerase reverse transcriptase gene. Biochem Biophys Res Commun. 330:633–640. DOI: 10.1016/j.bbrc.2005.03.072. PMID: 15809044.

25. Mueller SM, Glowacki J. 2001; Age-related decline in the osteogenic potential of human bone marrow cells cultured in three-dimensional collagen sponges. J Cell Biochem. 82:583–590. DOI: 10.1002/jcb.1174. PMID: 11500936.

26. Stewart MC, Stewart AA. 2011; Mesenchymal stem cells: characteristics, sources, and mechanisms of action. Vet Clin North Am Equine Pract. 27:243–261. DOI: 10.1016/j.cveq.2011.06.004. PMID: 21872757.

27. Kern S, Eichler H, Stoeve J, Klüter H, Bieback K. 2006; Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 24:1294–1301. DOI: 10.1634/stemcells.2005-0342. PMID: 16410387.

28. Prindull G, Ben-Ishay Z, Ebell W, Bergholz M, Dirk T, Prindull B. 1987; CFU-F circulating in cord blood. Blut. 54:351–359. DOI: 10.1007/BF00626017. PMID: 3496136.

29. Erices A, Conget P, Minguell JJ. 2000; Mesenchymal progenitor cells in human umbilical cord blood. Br J Haematol. 109:235–242. DOI: 10.1046/j.1365-2141.2000.01986.x. PMID: 10848804.

30. Goodwin HS, Bicknese AR, Chien SN, Bogucki BD, Quinn CO, Wall DA. 2001; Multilineage differentiation activity by cells isolated from umbilical cord blood: expression of bone, fat, and neural markers. Biol Blood Marrow Transplant. 7:581–588. DOI: 10.1053/bbmt.2001.v7.pm11760145. PMID: 11760145.

31. Chang YJ, Shih DT, Tseng CP, Hsieh TB, Lee DC, Hwang SM. 2006; Disparate mesenchyme-lineage tendencies in mesenchymal stem cells from human bone marrow and umbilical cord blood. Stem Cells. 24:679–685. DOI: 10.1634/stemcells.2004-0308. PMID: 16179428.

32. He Q, Wan C, Li G. 2007; Concise review: multipotent mesenchymal stromal cells in blood. Stem Cells. 25:69–77. DOI: 10.1634/stemcells.2006-0335. PMID: 16973831.

33. Hong HS, Lee J, Lee E, Kwon YS, Lee E, Ahn W, Jiang MH, Kim JC, Son Y. 2009; A new role of substance P as an injury-inducible messenger for mobilization of CD29(+) stromal-like cells. Nat Med. 15:425–435. DOI: 10.1038/nm.1909.

34. Spaeth EL, Kidd S, Marini FC. 2012; Tracking inflammation-induced mobilization of mesenchymal stem cells. Methods Mol Biol. 904:173–190. DOI: 10.1007/978-1-61779-943-3_15. PMID: 22890932.

35. Spaeth E, Klopp A, Dembinski J, Andreeff M, Marini F. 2008; Inflammation and tumor microenvironments: defining the migratory itinerary of mesenchymal stem cells. Gene Ther. 15:730–738. DOI: 10.1038/gt.2008.39. PMID: 18401438.

36. Momin EN, Vela G, Zaidi HA, Quiñones-Hinojosa A. 2010; The oncogenic potential of mesenchymal stem cells in the treatment of cancer: directions for future research. Curr Immunol Rev. 6:137–148. DOI: 10.2174/157339510791111718. PMID: 20490366. PMCID: PMC2873198.

37. Imitola J, Raddassi K, Park KI, Mueller FJ, Nieto M, Teng YD, Frenkel D, Li J, Sidman RL, Walsh CA, Snyder EY, Khoury SJ. 2004; Directed migration of neural stem cells to sites of CNS injury by the stromal cell-derived factor 1alpha/CXC chemokine receptor 4 pathway. Proc Natl Acad Sci U S A. 101:18117–18122. DOI: 10.1073/pnas.0408258102. PMID: 15608062. PMCID: PMC536055.

38. Son BR, Marquez-Curtis LA, Kucia M, Wysoczynski M, Turner AR, Ratajczak J, Ratajczak MZ, Janowska-Wieczorek A. 2006; Migration of bone marrow and cord blood mesenchymal stem cells in vitro is regulated by stromal-derived factor-1-CXCR4 and hepatocyte growth factor-c-met axes and involves matrix metalloproteinases. Stem Cells. 24:1254–1264. DOI: 10.1634/stemcells.2005-0271. PMID: 16410389.

39. Nakamizo A, Marini F, Amano T, Khan A, Studeny M, Gumin J, Chen J, Hentschel S, Vecil G, Dembinski J, Andreeff M, Lang FF. 2005; Human bone marrow-derived mesenchymal stem cells in the treatment of gliomas. Cancer Res. 65:3307–3318. DOI: 10.1158/0008-5472.CAN-04-1874. PMID: 15833864.

40. Quante M, Tu SP, Tomita H, Gonda T, Wang SS, Takashi S, Baik GH, Shibata W, Diprete B, Betz KS, Friedman R, Varro A, Tycko B, Wang TC. 2011; Bone marrow-derived myofibroblasts contribute to the mesenchymal stem cell niche and promote tumor growth. Cancer Cell. 19:257–272. DOI: 10.1016/j.ccr.2011.01.020. PMID: 21316604. PMCID: PMC3060401.

41. Smith H, Whittall C, Weksler B, Middleton J. 2012; Chemokines stimulate bidirectional migration of human mesenchymal stem cells across bone marrow endothelial cells. Stem Cells Dev. 21:476–486. DOI: 10.1089/scd.2011.0025. PMID: 21513440.

42. Darash-Yahana M, Gillespie JW, Hewitt SM, Chen YY, Maeda S, Stein I, Singh SP, Bedolla RB, Peled A, Troyer DA, Pikarsky E, Karin M, Farber JM. 2009; The chemokine CXCL16 and its receptor, CXCR6, as markers and promoters of inflammation-associated cancers. PLoS One. 4:e6695. DOI: 10.1371/journal.pone.0006695. PMID: 19690611. PMCID: PMC2723911.

43. Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, Zhu Y, Wei S, Kryczek I, Daniel B, Gordon A, Myers L, Lackner A, Disis ML, Knutson KL, Chen L, Zou W. 2004; Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 10:942–949. DOI: 10.1038/nm1093. PMID: 15322536.

44. Jacobs JF, Idema AJ, Bol KF, Grotenhuis JA, de Vries IJ, Wesseling P, Adema GJ. 2010; Prognostic significance and mechanism of Treg infiltration in human brain tumors. J Neuroimmunol. 225:195–199. DOI: 10.1016/j.jneuroim.2010.05.020. PMID: 20537408.

45. De Becker A, Van Hummelen P, Bakkus M, Vande Broek I, De Wever J, De Waele M, Van Riet I. 2007; Migration of culture-expanded human mesenchymal stem cells through bone marrow endothelium is regulated by matrix metalloproteinase-2 and tissue inhibitor of metalloproteinase-3. Haematologica. 92:440–449. DOI: 10.3324/haematol.10475. PMID: 17488654.

46. Qiu Y, Marquez-Curtis LA, Janowska-Wieczorek A. 2012; Mesen-chymal stromal cells derived from umbilical cord blood migrate in response to complement C1q. Cytotherapy. 14:285–295. DOI: 10.3109/14653249.2011.651532. PMID: 22264191.

47. François S, Bensidhoum M, Mouiseddine M, Mazurier C, Allenet B, Semont A, Frick J, Saché A, Bouchet S, Thierry D, Gourmelon P, Gorin NC, Chapel A. 2006; Local irradiation not only induces homing of human mesenchymal stem cells at exposed sites but promotes their widespread engraftment to multiple organs: a study of their quantitative distribution after irradiation damage. Stem Cells. 24:1020–1029. DOI: 10.1634/stemcells.2005-0260.

48. Ramasamy R, Lam EW, Soeiro I, Tisato V, Bonnet D, Dazzi F. 2007; Mesenchymal stem cells inhibit proliferation and apoptosis of tumor cells: impact on in vivo tumor growth. Leukemia. 21:304–310. DOI: 10.1038/sj.leu.2404489. PMID: 17170725.

49. Zhu Y, Sun Z, Han Q, Liao L, Wang J, Bian C, Li J, Yan X, Liu Y, Shao C, Zhao RC. 2009; Human mesenchymal stem cells inhibit cancer cell proliferation by secreting DKK-1. Leukemia. 23:925–933. DOI: 10.1038/leu.2008.384. PMID: 19148141.

50. Lu YR, Yuan Y, Wang XJ, Wei LL, Chen YN, Cong C, Li SF, Long D, Tan WD, Mao YQ, Zhang J, Li YP, Cheng JQ. 2008; The growth inhibitory effect of mesenchymal stem cells on tumor cells in vitro and in vivo. Cancer Biol Ther. 7:245–251. DOI: 10.4161/cbt.7.2.5296. PMID: 18059192.

51. Qiao L, Xu ZL, Zhao TJ, Ye LH, Zhang XD. 2008; Dkk-1 secreted by mesenchymal stem cells inhibits growth of breast cancer cells via depression of Wnt signalling. Cancer Lett. 269:67–77. DOI: 10.1016/j.canlet.2008.04.032. PMID: 18571836.

52. Qiao L, Xu Z, Zhao T, Zhao Z, Shi M, Zhao RC, Ye L, Zhang X. 2008; Suppression of tumorigenesis by human mesenchymal stem cells in a hepatoma model. Cell Res. 18:500–507. DOI: 10.1038/cr.2008.40. PMID: 18364678.

53. Sun B, Roh KH, Park JR, Lee SR, Park SB, Jung JW, Kang SK, Lee YS, Kang KS. 2009; Therapeutic potential of mesenchymal stromal cells in a mouse breast cancer metastasis model. Cytotherapy. 11:289–298. DOI: 10.1080/14653240902807026. PMID: 19308770.

54. Secchiero P, Zorzet S, Tripodo C, Corallini F, Melloni E, Caruso L, Bosco R, Ingrao S, Zavan B, Zauli G. 2010; Human bone marrow mesenchymal stem cells display anti-cancer activity in SCID mice bearing disseminated non-Hodgkin's lymphoma xenografts. PLoS One. 5:e11140. DOI: 10.1371/journal.pone.0011140. PMID: 20585401. PMCID: PMC2886845.

55. Otsu K, Das S, Houser SD, Quadri SK, Bhattacharya S, Bhattacharya J. 2009; Concentration-dependent inhibition of angiogenesis by mesenchymal stem cells. Blood. 113:4197–4205. DOI: 10.1182/blood-2008-09-176198. PMID: 19036701. PMCID: PMC2676081.

56. Hanahan D, Weinberg RA. 2011; Hallmarks of cancer: the next generation. Cell. 144:646–674. DOI: 10.1016/j.cell.2011.02.013. PMID: 21376230.

57. Rhee KJ, Lee JI, Eom YW. 2015; Mesenchymal stem cell-mediated effects of tumor support or suppression. Int J Mol Sci. 16:30015–30033. DOI: 10.3390/ijms161226215. PMID: 26694366. PMCID: PMC4691158.

58. Waterman RS, Tomchuck SL, Henkle SL, Betancourt AM. 2010; A new mesenchymal stem cell (MSC) paradigm: polarization into a pro-inflammatory MSC1 or an Immuno-suppressive MSC2 phenotype. PLoS One. 5:e10088. DOI: 10.1371/journal.pone.0010088. PMID: 20436665. PMCID: PMC2859930.

59. Waterman RS, Henkle SL, Betancourt AM. 2012; Mesenchymal stem cell 1 (MSC1)-based therapy attenuates tumor growth whereas MSC2-treatment promotes tumor growth and metastasis. PLoS One. 7:e45590. DOI: 10.1371/journal.pone.0045590. PMID: 23029122. PMCID: PMC3447765.

60. Houthuijzen JM, Daenen LG, Roodhart JM, Voest EE. 2012; The role of mesenchymal stem cells in anti-cancer drug resistance and tumour progression. Br J Cancer. 106:1901–1906. DOI: 10.1038/bjc.2012.201. PMID: 22596239. PMCID: PMC3388567.

61. Poggi A, Giuliani M. 2016; Mesenchymal stromal cells can regulate the immune response in the tumor microenvironment. Vaccines (Basel). 4:41. DOI: 10.3390/vaccines4040041. PMID: 27834810. PMCID: PMC5192361.

62. Li GC, Zhang HW, Zhao QC, Sun LI, Yang JJ, Hong L, Feng F, Cai L. 2016; Mesenchymal stem cells promote tumor angiogenesis via the action of transforming growth factor β1. Oncol Lett. 11:1089–1094. DOI: 10.3892/ol.2015.3997. PMID: 26893697. PMCID: PMC4733964.

63. Zhang T, Lee YW, Rui YF, Cheng TY, Jiang XH, Li G. 2013; Bone marrow-derived mesenchymal stem cells promote growth and angiogenesis of breast and prostate tumors. Stem Cell Res Ther. 4:70. DOI: 10.1186/scrt221. PMID: 23763837. PMCID: PMC3707041.

64. Fregni G, Quinodoz M, Möller E, Vuille J, Galland S, Fusco C, Martin P, Letovanec I, Provero P, Rivolta C, Riggi N, Stamenkovic I. 2018; Reciprocal modulation of mesenchymal stem cells and tumor cells promotes lung cancer metastasis. EBioMedicine. 29:128–145. DOI: 10.1016/j.ebiom.2018.02.017. PMID: 29503225. PMCID: PMC5925622.

65. Ridge SM, Sullivan FJ, Glynn SA. 2017; Mesenchymal stem cells: key players in cancer progression. Mol Cancer. 16:31. DOI: 10.1186/s12943-017-0597-8. PMID: 28148268. PMCID: PMC5286812.

66. Bergfeld SA, Blavier L, DeClerck YA. 2014; Bone marrow-derived mesenchymal stromal cells promote survival and drug resistance in tumor cells. Mol Cancer Ther. 13:962–975. DOI: 10.1158/1535-7163.MCT-13-0400. PMID: 24502925. PMCID: PMC4000239.

67. Naderi EH, Skah S, Ugland H, Myklebost O, Sandnes DL, Torgersen ML, Josefsen D, Ruud E, Naderi S, Blomhoff HK. 2015; Bone marrow stroma-derived PGE2 protects BCP-ALL cells from DNA damage-induced p53 accumulation and cell death. Mol Cancer. 14:14. DOI: 10.1186/s12943-014-0278-9. PMID: 25623255. PMCID: PMC4323193.

68. Bonuccelli G, Avnet S, Grisendi G, Salerno M, Granchi D, Dominici M, Kusuzaki K, Baldini N. 2014; Role of mesenchymal stem cells in osteosarcoma and metabolic reprogramming of tumor cells. Oncotarget. 5:7575–7588. DOI: 10.18632/oncotarget.2243. PMID: 25277190. PMCID: PMC4202145.

69. Huang WH, Chang MC, Tsai KS, Hung MC, Chen HL, Hung SC. 2013; Mesenchymal stem cells promote growth and angiogenesis of tumors in mice. Oncogene. 32:4343–4354. DOI: 10.1038/onc.2012.458. PMID: 23085755.

70. Lin G, Yang R, Banie L, Wang G, Ning H, Li LC, Lue TF, Lin CS. 2010; Effects of transplantation of adipose tissue-derived stem cells on prostate tumor. Prostate. 70:1066–1073. DOI: 10.1002/pros.21140. PMID: 20232361. PMCID: PMC2877148.

71. Suzuki K, Sun R, Origuchi M, Kanehira M, Takahata T, Itoh J, Umezawa A, Kijima H, Fukuda S, Saijo Y. 2011; Mesenchymal stromal cells promote tumor growth through the enhancement of neovascularization. Mol Med. 17:579–587. DOI: 10.2119/molmed.2010.00157. PMID: 21424106. PMCID: PMC3146617.

72. Li Z. 2013; CD133: a stem cell biomarker and beyond. Exp Hematol Oncol. 2:17. DOI: 10.1186/2162-3619-2-17. PMID: 23815814. PMCID: PMC3701589.

73. Liu S, Ginestier C, Ou SJ, Clouthier SG, Patel SH, Monville F, Korkaya H, Heath A, Dutcher J, Kleer CG, Jung Y, Dontu G, Taichman R, Wicha MS. 2011; Breast cancer stem cells are regulated by mesenchymal stem cells through cytokine networks. Cancer Res. 71:614–624. DOI: 10.1158/0008-5472.CAN-10-0538. PMID: 21224357. PMCID: PMC3100554.

74. Nishimura K, Semba S, Aoyagi K, Sasaki H, Yokozaki H. 2012; Mesenchymal stem cells provide an advantageous tumor microenvironment for the restoration of cancer stem cells. Pathobiology. 79:290–306. DOI: 10.1159/000337296. PMID: 22688186.

75. Corcoran KE, Trzaska KA, Fernandes H, Bryan M, Taborga M, Srinivas V, Packman K, Patel PS, Rameshwar P. 2008; Mesenchymal stem cells in early entry of breast cancer into bone marrow. PLoS One. 3:e2563. DOI: 10.1371/journal.pone.0002563. PMID: 18575622. PMCID: PMC2430536.

76. Xu WT, Bian ZY, Fan QM, Li G, Tang TT. 2009; Human mesenchymal stem cells (hMSCs) target osteosarcoma and promote its growth and pulmonary metastasis. Cancer Lett. 281:32–41. DOI: 10.1016/j.canlet.2009.02.022. PMID: 19342158.

77. Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW, Richardson AL, Polyak K, Tubo R, Weinberg RA. 2007; Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 449:557–563. DOI: 10.1038/nature06188. PMID: 17914389.

78. Tsukamoto S, Honoki K, Fujii H, Tohma Y, Kido A, Mori T, Tsujiuchi T, Tanaka Y. 2012; Mesenchymal stem cells promote tumor engraftment and metastatic colonization in rat osteosarcoma model. Int J Oncol. 40:163–169. DOI: 10.3892/ijo.2011.1220. PMID: 21971610.

79. Djouad F, Plence P, Bony C, Tropel P, Apparailly F, Sany J, Noël D, Jorgensen C. 2003; Immunosuppressive effect of mesenchymal stem cells favors tumor growth in allogeneic animals. Blood. 102:3837–3844. DOI: 10.1182/blood-2003-04-1193. PMID: 12881305.

80. Lee HY, Hong IS. 2017; Double-edged sword of mesenchymal stem cells: cancer-promoting versus therapeutic potential. Cancer Sci. 108:1939–1946. DOI: 10.1111/cas.13334. PMID: 28756624. PMCID: PMC5623746.

81. Rhodes LV, Antoon JW, Muir SE, Elliott S, Beckman BS, Burow ME. 2010; Effects of human mesenchymal stem cells on ER-positive human breast carcinoma cells mediated through ER-SDF-1/CXCR4 crosstalk. Mol Cancer. 9:295. DOI: 10.1186/1476-4598-9-295. PMID: 21087507. PMCID: PMC2998478.

82. Halpern JL, Kilbarger A, Lynch CC. 2011; Mesenchymal stem cells promote mammary cancer cell migration in vitro via the CXCR2 receptor. Cancer Lett. 308:91–99. DOI: 10.1016/j.canlet.2011.04.018. PMID: 21601983. PMCID: PMC3311035.

83. Tsai KS, Yang SH, Lei YP, Tsai CC, Chen HW, Hsu CY, Chen LL, Wang HW, Miller SA, Chiou SH, Hung MC, Hung SC. 2011; Mesenchymal stem cells promote formation of colorectal tumors in mice. Gastroenterology. 141:1046–1056. DOI: 10.1053/j.gastro.2011.05.045. PMID: 21699785.

84. Melzer C, Yang Y, Hass R. 2016; Interaction of MSC with tumor cells. Cell Commun Signal. 14:20. DOI: 10.1186/s12964-016-0143-0. PMID: 27608835. PMCID: PMC5016940.

85. Norozi F, Ahmadzadeh A, Shahrabi S, Vosoughi T, Saki N. 2016; Mesenchymal stem cells as a double-edged sword in suppression or progression of solid tumor cells. Tumour Biol. 37:11679–11689. DOI: 10.1007/s13277-016-5187-7. PMID: 27440203.

86. Raposo G, Stoorvogel W. 2013; Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 200:373–383. DOI: 10.1083/jcb.201211138. PMID: 23420871. PMCID: PMC3575529.

87. Braicu C, Tomuleasa C, Monroig P, Cucuianu A, Berindan-Neagoe I, Calin GA. 2015; Exosomes as divine messengers: are they the Hermes of modern molecular oncology? Cell Death Differ. 22:34–45. DOI: 10.1038/cdd.2014.130. PMID: 25236394. PMCID: PMC4262777.

88. Zhang L, Hao C, Yao S, Tang R, Guo W, Cong H, Li J, Bao L, Wang D, Li Y, Yu X, Duan S, Yao W. 2018; Exosomal miRNA profiling to identify nanoparticle phagocytic mechanisms. Small. 14:e1704008. DOI: 10.1002/smll.201704008. PMID: 29516679.

89. Zarredar H, Ansarin K, Baradaran B, Shekari N, Eyvazi S, Safari F, Farajnia S. 2018; Critical microRNAs in lung cancer: recent advances and potential applications. Anticancer Agents Med Chem. 18:1991–2005. DOI: 10.2174/1871520618666180808125459. PMID: 30088452.

90. Zafari V, Shanehbandi D, Bornehdeli S, Sadeghzadeh M, Zarredar H, Asadi M, Sharifi A. 2021; MicroRNA profiling in non-small cell lung cancer and its implications for the disease pathogenesis. Middle East J Cancer. 12:79–85.
91. Neviani P, Fabbri M. 2015; Exosomic microRNAs in the tumor microenvironment. Front Med (Lausanne). 2:47. DOI: 10.3389/fmed.2015.00047. PMID: 26258125. PMCID: PMC4510410.

92. Ratajczak J, Miekus K, Kucia M, Zhang J, Reca R, Dvorak P, Ratajczak MZ. 2006; Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: evidence for horizontal transfer of mRNA and protein delivery. Leukemia. 20:847–856. DOI: 10.1038/sj.leu.2404132. PMID: 16453000.

93. Burrello J, Monticone S, Gai C, Gomez Y, Kholia S, Camussi G. 2016; Stem cell-derived extracellular vesicles and immune-modulation. Front Cell Dev Biol. 4:83. DOI: 10.3389/fcell.2016.00083. PMID: 27597941. PMCID: PMC4992732.

94. Pinto A, Marangon I, Méreaux J, Nicolás-Boluda A, Lavieu G, Wilhelm C, Sarda-Mantel L, Silva AKA, Pocard M, Gazeau F. 2021; Immune reprogramming precision photodynamic therapy of peritoneal metastasis by scalable stem-cell-derived extracellular vesicles. ACS Nano. 15:3251–3263. DOI: 10.1021/acsnano.0c09938. PMID: 33481565.

95. Gowen A, Shahjin F, Chand S, Odegaard KE, Yelamanchili SV. 2020; Mesenchymal stem cell-derived extracellular vesicles: challenges in clinical applications. Front Cell Dev Biol. 8:149. DOI: 10.3389/fcell.2020.00149. PMID: 32226787. PMCID: PMC7080981.

96. Zhou Y, Xu H, Xu W, Wang B, Wu H, Tao Y, Zhang B, Wang M, Mao F, Yan Y, Gao S, Gu H, Zhu W, Qian H. 2013; Exosomes released by human umbilical cord mesenchymal stem cells protect against cisplatin-induced renal oxidative stress and apoptosis in vivo and in vitro. Stem Cell Res Ther. 4:34. DOI: 10.1186/scrt194. PMID: 23618405. PMCID: PMC3707035.

97. Zhu W, Huang L, Li Y, Zhang X, Gu J, Yan Y, Xu X, Wang M, Qian H, Xu W. 2012; Exosomes derived from human bone marrow mesenchymal stem cells promote tumor growth in vivo. Cancer Lett. 315:28–37. DOI: 10.1016/j.canlet.2011.10.002. PMID: 22055459.

98. Bruno S, Collino F, Deregibus MC, Grange C, Tetta C, Camussi G. 2013; Microvesicles derived from human bone marrow mesenchymal stem cells inhibit tumor growth. Stem Cells Dev. 22:758–771. DOI: 10.1089/scd.2012.0304. PMID: 23034046.

99. Lee JK, Park SR, Jung BK, Jeon YK, Lee YS, Kim MK, Kim YG, Jang JY, Kim CW. 2013; Exosomes derived from mesenchymal stem cells suppress angiogenesis by down-regulating VEGF expression in breast cancer cells. PLoS One. 8:e84256. DOI: 10.1371/journal.pone.0084256. PMID: 24391924. PMCID: PMC3877259.

100. Hua Z, Lv Q, Ye W, Wong CK, Cai G, Gu D, Ji Y, Zhao C, Wang J, Yang BB, Zhang Y. 2006; MiRNA-directed regulation of VEGF and other angiogenic factors under hypoxia. PLoS One. 1:e116. DOI: 10.1371/journal.pone.0000116. PMID: 17205120. PMCID: PMC1762435.

101. Karimi L, Zeinali T, Hosseinahli N, Mansoori B, Mohammadi A, Yousefi M, Asadi M, Sadreddini S, Baradaran B, Shanehbandi D. 2019; miRNA-143 replacement therapy harnesses the proliferation and migration of colorectal cancer cells in vitro. J Cell Physiol. 234:21359–21368. DOI: 10.1002/jcp.28745. PMID: 31032951.

102. Shimbo K, Miyaki S, Ishitobi H, Kato Y, Kubo T, Shimose S, Ochi M. 2014; Exosome-formed synthetic microRNA-143 is transferred to osteosarcoma cells and inhibits their migration. Biochem Biophys Res Commun. 445:381–387. DOI: 10.1016/j.bbrc.2014.02.007. PMID: 24525123.

103. Maestroni GJ, Hertens E, Galli P. 1999; Factor(s) from nonmacrophage bone marrow stromal cells inhibit Lewis lung carcinoma and B16 melanoma growth in mice. Cell Mol Life Sci. 55:663–667. DOI: 10.1007/s000180050322. PMID: 10357234.

104. Nakamura K, Ito Y, Kawano Y, Kurozumi K, Kobune M, Tsuda H, Bizen A, Honmou O, Niitsu Y, Hamada H. 2004; Antitumor effect of genetically engineered mesenchymal stem cells in a rat glioma model. Gene Ther. 11:1155–1164. DOI: 10.1038/sj.gt.3302276. PMID: 15141157.

105. Qiao C, Xu W, Zhu W, Hu J, Qian H, Yin Q, Jiang R, Yan Y, Mao F, Yang H, Wang X, Chen Y. 2008; Human mesenchymal stem cells isolated from the umbilical cord. Cell Biol Int. 32:8–15. DOI: 10.1016/j.cellbi.2007.08.002. PMID: 17904875.

106. Khakoo AY, Pati S, Anderson SA, Reid W, Elshal MF, Rovira II, Nguyen AT, Malide D, Combs CA, Hall G, Zhang J, Raffeld M, Rogers TB, Stetler-Stevenson W, Frank JA, Reitz M, Finkel T. 2006; Human mesenchymal stem cells exert potent antitumorigenic effects in a model of Kaposi's sarcoma. J Exp Med. 203:1235–1247. DOI: 10.1084/jem.20051921. PMCID: PMC2121206.

107. Gondi CS, Veeravalli KK, Gorantla B, Dinh DH, Fassett D, Klopfenstein JD, Gujrati M, Rao JS. 2010; Human umbilical cord blood stem cells show PDGF-D-dependent glioma cell tropism in vitro and in vivo. Neuro Oncol. 12:453–465. DOI: 10.1093/neuonc/nop049. PMID: 20406896. PMCID: PMC2940623.
108. Dasari VR, Velpula KK, Kaur K, Fassett D, Klopfenstein JD, Dinh DH, Gujrati M, Rao JS. 2010; Cord blood stem cell-mediated induction of apoptosis in glioma downregulates X-linked inhibitor of apoptosis protein (XIAP). PLoS One. 5:e11813. DOI: 10.1371/journal.pone.0011813. PMID: 20676365. PMCID: PMC2911373.

109. Akimoto K, Kimura K, Nagano M, Takano S, To'a Salazar G, Yamashita T, Ohneda O. 2013; Umbilical cord blood-derived mesenchymal stem cells inhibit, but adipose tissue-derived mesenchymal stem cells promote, glioblastoma multiforme proliferation. Stem Cells Dev. 22:1370–1386. DOI: 10.1089/scd.2012.0486. PMID: 23231075. PMCID: PMC3696928.

110. Wilting J, Christ B. 1996; Embryonic angiogenesis: a review. Naturwissenschaften. 83:153–164. DOI: 10.1007/BF01143056. PMID: 8643122.

111. Risau W, Flamme I. 1995; Vasculogenesis. Annu Rev Cell Dev Biol. 11:73–91. DOI: 10.1146/annurev.cb.11.110195.000445. PMID: 8689573.

112. Tarhriz V, Bandehpour M, Dastmalchi S, Ouladsahebmadarek E, Zarredar H, Eyvazi S. 2019; Overview of CD24 as a new molecular marker in ovarian cancer. J Cell Physiol. 234:2134–2142. DOI: 10.1002/jcp.27581. PMID: 30317611.

113. Folkman J. 1971; Tumor angiogenesis: therapeutic implications. N Engl J Med. 285:1182–1186. DOI: 10.1056/NEJM197111182852108. PMID: 4938153.

114. Ribatti D. 2004; The involvement of endothelial progenitor cells in tumor angiogenesis. J Cell Mol Med. 8:294–300. DOI: 10.1111/j.1582-4934.2004.tb00319.x. PMID: 15491505. PMCID: PMC6740146.

115. Ferrara N, Henzel WJ. 1989; Pituitary follicular cells secrete a novel heparin-binding growth factor specific for vascular endothelial cells. Biochem Biophys Res Commun. 161:851–858. DOI: 10.1016/0006-291X(89)92678-8. PMID: 22925671.

116. Khoury CC, Ziyadeh FN. 2011; Angiogenic factors. Contrib Nephrol. 170:83–92. DOI: 10.1159/000324950. PMID: 21659761.

117. Taraboletti G, Rusnati M, Ragona L, Colombo G. 2010; Targeting tumor angiogenesis with TSP-1-based compounds: rational design of antiangiogenic mimetics of endogenous inhibitors. Oncotarget. 1:662–673. DOI: 10.18632/oncotarget.200. PMID: 21317461. PMCID: PMC3248139.

118. Algire GH, Chalkley HW, Legallais FY, Park HD. 1945; Vasculae reactions of normal and malignant tissues in vivo. I. vascular reactions of mice to wounds and to normal and neoplastic transplants. J Natl Cancer Inst. 6:73–85. DOI: 10.1093/jnci/6.1.73.

119. Zarredar H, Ansarin K, Baradaran B, Ahdi Khosroshahi S, Farajnia S. 2018; Potential molecular targets in the treatment of lung cancer using siRNA technology. Cancer Invest. 36:37–58. DOI: 10.1080/07357907.2017.1416393. PMID: 29336624.

120. Ferrara N, Gerber HP, LeCouter J. 2003; The biology of VEGF and its receptors. Nat Med. 9:669–676. DOI: 10.1038/nm0603-669. PMID: 12778165.

121. Wang GL, Semenza GL. 1995; Purification and characterization of hypoxia-inducible factor 1. J Biol Chem. 270:1230–1237. DOI: 10.1074/jbc.270.3.1230. PMID: 7836384.

122. Brahimi-Horn C, Pouysségur J. 2006; The role of the hypoxia-inducible factor in tumor metabolism growth and invasion. Bull Cancer. 93:E73–E80. DOI: 10.1016/j.bcp.2006.10.013. PMID: 16935775.
123. Ellis LM, Hicklin DJ. 2008; VEGF-targeted therapy: mechanisms of anti-tumour activity. Nat Rev Cancer. 8:579–591. DOI: 10.1038/nrc2403. PMID: 18596824.

124. Petrova TV, Bono P, Holnthoner W, Chesnes J, Pytowski B, Sihto H, Laakkonen P, Heikkilä P, Joensuu H, Alitalo K. 2008; VEGFR-3 expression is restricted to blood and lymphatic vessels in solid tumors. Cancer Cell. 13:554–556. DOI: 10.1016/j.ccr.2008.04.022. PMID: 18538738.

125. Zarredar H, Pashapour S, Ansarin K, Khalili M, Baghban R, Farajnia S. 2019; Combination therapy with KRAS siRNA and EGFR inhibitor AZD8931 suppresses lung cancer cell growth in vitro. J Cell Physiol. 234:1560–1566. DOI: 10.1002/jcp.27021. PMID: 30132854.

126. Kim KJ, Li B, Winer J, Armanini M, Gillett N, Phillips HS, Ferrara N. 1993; Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature. 362:841–844. DOI: 10.1038/362841a0. PMID: 7683111.

127. Presta LG, Chen H, O'Connor SJ, Chisholm V, Meng YG, Krummen L, Winkler M, Ferrara N. 1997; Humanization of an anti-vascular endothelial growth factor monoclonal antibody for the therapy of solid tumors and other disorders. Cancer Res. 57:4593–4599. PMID: 9377574.
128. Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, Ferrara N, Fyfe G, Rogers B, Ross R, Kabbinavar F. 2004; Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 350:2335–2342. DOI: 10.1056/NEJMoa032691. PMID: 15175435.

129. Asadi M, Shanehbandi D, Zarintan A, Pedram N, Baradaran B, Zafari V, Shirmohamadi M, Hashemzadeh S. 2017; TP53 gene Pro72Arg (rs1042522) single nucleotide polymorphism as not a risk factor for colorectal cancer in the Iranian Azari Population. Asian Pac J Cancer Prev. 18:3423–3427.
130. Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, Lilenbaum R, Johnson DH. 2006; Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 355:2542–2550. DOI: 10.1056/NEJMoa061884. PMID: 17167137.

131. Miller K, Wang M, Gralow J, Dickler M, Cobleigh M, Perez EA, Shenkier T, Cella D, Davidson NE. 2007; Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 357:2666–2676. DOI: 10.1056/NEJMoa072113. PMID: 18160686.

132. Holash J, Davis S, Papadopoulos N, Croll SD, Ho L, Russell M, Boland P, Leidich R, Hylton D, Burova E, Ioffe E, Huang T, Radziejewski C, Bailey K, Fandl JP, Daly T, Wiegand SJ, Yancopoulos GD, Rudge JS. 2002; VEGF-Trap: a VEGF blocker with potent antitumor effects. Proc Natl Acad Sci U S A. 99:11393–11398. DOI: 10.1073/pnas.172398299. PMID: 12177445. PMCID: PMC123267.

133. Ellis LM, Hicklin DJ. 2008; Pathways mediating resistance to vascular endothelial growth factor-targeted therapy. Clin Cancer Res. 14:6371–6375. DOI: 10.1158/1078-0432.CCR-07-5287. PMID: 18927275.

134. Kerbel RS. 2008; Tumor angiogenesis. N Engl J Med. 358:2039–2049. DOI: 10.1056/NEJMra0706596. PMID: 4938153.

135. Jiang F, Albert DH, Luo Y, Tapang P, Zhang K, Davidsen SK, Fox GB, Lesniewski R, McKeegan EM. 2011; ABT-869, a multitargeted receptor tyrosine kinase inhibitor, reduces tumor microvascularity and improves vascular wall integrity in preclinical tumor models. J Pharmacol Exp Ther. 338:134–142. DOI: 10.1124/jpet.110.178061. PMID: 21505059.

136. Tan EH, Goss GD, Salgia R, Besse B, Gandara DR, Hanna NH, Yang JC, Thertulien R, Wertheim M, Mazieres J, Hensing T, Lee C, Gupta N, Pradhan R, Qian J, Qin Q, Scappaticci FA, Ricker JL, Carlson DM, Soo RA. 2011; Phase 2 trial of linifanib (ABT-869) in patients with advanced non-small cell lung cancer. J Thorac Oncol. 6:1418–1425. DOI: 10.1097/JTO.0b013e318220c93e. PMID: 21597387.

137. You WK, Sennino B, Williamson CW, Falcón B, Hashizume H, Yao LC, Aftab DT, McDonald DM. 2011; VEGF and c-Met blockade amplify angiogenesis inhibition in pancreatic islet cancer. Cancer Res. 71:4758–4768. DOI: 10.1158/0008-5472.CAN-10-2527. PMID: 21613405. PMCID: PMC3138890.

138. Ho TH, Jonasch E. 2011; Axitinib in the treatment of metastatic renal cell carcinoma. Future Oncol. 7:1247–1253. DOI: 10.2217/fon.11.107. PMID: 22044199.

139. Eskens FA, de Jonge MJ, Bhargava P, Isoe T, Cotreau MM, Esteves B, Hayashi K, Burger H, Thomeer M, van Doorn L, Verweij J. 2011; Biologic and clinical activity of tivozanib (AV-951, KRN-951), a selective inhibitor of VEGF receptor-1, -2, and -3 tyrosine kinases, in a 4-week-on, 2-week-off schedule in patients with advanced solid tumors. Clin Cancer Res. 17:7156–7163. DOI: 10.1158/1078-0432.CCR-11-0411. PMID: 21976547.

140. Langmuir PB, Yver A. 2012; Vandetanib for the treatment of thyroid cancer. Clin Pharmacol Ther. 91:71–80. DOI: 10.1038/clpt.2011.272. PMID: 22158569.

141. O'Farrell AM, Abrams TJ, Yuen HA, Ngai TJ, Louie SG, Yee KW, Wong LM, Hong W, Lee LB, Town A, Smolich BD, Manning WC, Murray LJ, Heinrich MC, Cherrington JM. 2003; SU11248 is a novel FLT3 tyrosine kinase inhibitor with potent activity in vitro and in vivo. Blood. 101:3597–3605. DOI: 10.1182/blood-2002-07-2307. PMID: 12531805.
142. Sleijfer S, Ray-Coquard I, Papai Z, Le Cesne A, Scurr M, Schöffski P, Collin F, Pandite L, Marreaud S, De Brauwer A, van Glabbeke M, Verweij J, Blay JY. 2009; Pazopanib, a multikinase angiogenesis inhibitor, in patients with relapsed or refractory advanced soft tissue sarcoma: a phase II study from the European organisation for research and treatment of cancer-soft tissue and bone sarcoma group (EORTC study 62043). J Clin Oncol. 27:3126–3132. DOI: 10.1200/JCO.2008.21.3223. PMID: 19451427.

143. Richly H, Henning BF, Kupsch P, Passarge K, Grubert M, Hilger RA, Christensen O, Brendel E, Schwartz B, Ludwig M, Flashar C, Voigtmann R, Scheulen ME, Seeber S, Strumberg D. 2006; Results of a Phase I trial of sorafenib (BAY 43-9006) in combination with doxorubicin in patients with refractory solid tumors. Ann Oncol. 17:866–873. DOI: 10.1093/annonc/mdl017. PMID: 16500908.

144. Jain RK. 2005; Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 307:58–62. DOI: 10.1126/science.1104819. PMID: 15637262.

145. Cooney MM, van Heeckeren W, Bhakta S, Ortiz J, Remick SC. 2006; Drug insight: vascular disrupting agents and angiogenesis--novel approaches for drug delivery. Nat Clin Pract Oncol. 3:682–692. DOI: 10.1038/ncponc0663. PMID: 17139319.

146. McKeage MJ, Von Pawel J, Reck M, Jameson MB, Rosenthal MA, Sullivan R, Gibbs D, Mainwaring PN, Serke M, Lafitte JJ, Chouaid C, Freitag L, Quoix E. 2008; Randomised phase II study of ASA404 combined with carboplatin and paclitaxel in previously untreated advanced non-small cell lung cancer. Br J Cancer. 99:2006–2012. DOI: 10.1038/sj.bjc.6604808. PMID: 19078952. PMCID: PMC2607218.

147. Ghaedi M, Soleimani M, Taghvaie NM, Sheikhfatollahi M, Azadmanesh K, Lotfi AS, Wu J. 2011; Mesenchymal stem cells as vehicles for targeted delivery of anti-angiogenic protein to solid tumors. J Gene Med. 13:171–180. DOI: 10.1002/jgm.1552. PMID: 21449040.

148. Toi M, Hoshina S, Takayanagi T, Tominaga T. 1994; Association of vascular endothelial growth factor expression with tumor angiogenesis and with early relapse in primary breast cancer. Jpn J Cancer Res. 85:1045–1049. DOI: 10.1111/j.1349-7006.1994.tb02904.x. PMID: 7525523. PMCID: PMC5919354.

149. Folkman J, Watson K, Ingber D, Hanahan D. 1989; Induction of angiogenesis during the transition from hyperplasia to neoplasia. Nature. 339:58–61. DOI: 10.1038/339058a0. PMID: 2469964.

150. Zheng L, Zhang D, Chen X, Yang L, Wei Y, Zhao X. 2012; Antitumor activities of human placenta-derived mesenchymal stem cells expressing endostatin on ovarian cancer. PLoS One. 7:e39119. DOI: 10.1371/journal.pone.0039119. PMID: 22911684. PMCID: PMC3404061.

151. Batchelor TT, Sorensen AG, di Tomaso E, Zhang WT, Duda DG, Cohen KS, Kozak KR, Cahill DP, Chen PJ, Zhu M, Ancukiewicz M, Mrugala MM, Plotkin S, Drappatz J, Louis DN, Ivy P, Scadden DT, Benner T, Loeffler JS, Wen PY, Jain RK. 2007; AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell. 11:83–95. DOI: 10.1016/j.ccr.2006.11.021. PMID: 17222792. PMCID: PMC2748664.

152. Kadambi A, Mouta Carreira C, Yun CO, Padera TP, Dolmans DE, Carmeliet P, Fukumura D, Jain RK. 2001; Vascular endothelial growth factor (VEGF)-C differentially affects tumor vascular function and leukocyte recruitment: role of VEGF-receptor 2 and host VEGF-A. Cancer Res. 61:2404–2408. PMID: 11289105.
153. Tong RT, Boucher Y, Kozin SV, Winkler F, Hicklin DJ, Jain RK. 2004; Vascular normalization by vascular endothelial growth factor receptor 2 blockade induces a pressure gradient across the vasculature and improves drug penetration in tumors. Cancer Res. 64:3731–3736. DOI: 10.1158/0008-5472.CAN-04-0074. PMID: 15172975.

154. Bexell D, Gunnarsson S, Tormin A, Darabi A, Gisselsson D, Roybon L, Scheding S, Bengzon J. 2009; Bone marrow multipotent mesenchymal stroma cells act as pericyte-like migratory vehicles in experimental gliomas. Mol Ther. 17:183–190. DOI: 10.1038/mt.2008.229. PMID: 18985030. PMCID: PMC2834971.

155. Mirzaei H, Salehi H, Oskuee RK, Mohammadpour A, Mirzaei HR, Sharifi MR, Salarinia R, Darani HY, Mokhtari M, Masoudifar A, Sahebkar A, Salehi R, Jaafari MR. 2018; The therapeutic potential of human adipose-derived mesenchymal stem cells producing CXCL10 in a mouse melanoma lung metastasis model. Cancer Lett. 419:30–39. DOI: 10.1016/j.canlet.2018.01.029. PMID: 29331419.

156. Hyder SM, Stancel GM. 1999; Regulation of angiogenic growth factors in the female reproductive tract by estrogens and progestins. Mol Endocrinol. 13:806–811. DOI: 10.1210/mend.13.6.0308. PMID: 10379879.

157. Zygmunt M, Herr F, Münstedt K, Lang U, Liang OD. 2003; Angiogenesis and vasculogenesis in pregnancy. Eur J Obstet Gynecol Reprod Biol. 110 Suppl 1:S10–S18. DOI: 10.1016/S0301-2115(03)00168-4. PMID: 12965086.

158. Jensen EV, Jordan VC. 2003; The estrogen receptor: a model for molecular medicine. Clin Cancer Res. 9:1980–1989. PMID: 12796359.
159. Horwitz KB. 1988; The central role of progesterone receptors and progestational agents in the management and treatment of breast cancer. Semin Oncol. 15(2 Suppl 1):14–19. PMID: 3285481.
160. Kuiper GG, Carlsson B, Grandien K, Enmark E, Häggblad J, Nilsson S, Gustafsson JA. 1997; Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology. 138:863–870. DOI: 10.1210/endo.138.3.4979. PMID: 9048584.

161. Chang WY, Prins GS. 1999; Estrogen receptor-beta: implications for the prostate gland. Prostate. 40:115–124. DOI: 10.1002/(SICI)1097-0045(19990701)40:2<115::AID-PROS7>3.0.CO;2-3. PMID: 10386472.

162. Makar AP. 2000; Hormone therapy in epithelial ovarian cancer. Endocr Relat Cancer. 7:85–93. DOI: 10.1677/erc.0.0070085. PMID: 10903526.

163. Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J. Writing Group for the Women's Health Initiative Investigators. 2002; Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. JAMA. 288:321–333. DOI: 10.1001/jama.288.3.321. PMID: 12117397.

164. Vázquez F, Rodríguez-Manzaneque JC, Lydon JP, Edwards DP, O'Malley BW, Iruela-Arispe ML. 1999; Progesterone regulates proliferation of endothelial cells. J Biol Chem. 274:2185–2192. DOI: 10.1074/jbc.274.4.2185. PMID: 9890981.

165. Iruela-Arispe ML, Rodriguez-Manzaneque JC, Abu-Jawdeh G. 1999; Endometrial endothelial cells express estrogen and progesterone receptors and exhibit a tissue specific response to angiogenic growth factors. Microcirculation. 6:127–140. DOI: 10.1080/713773947. PMID: 10466115.

166. Reynolds LP, Redmer DA. 1999; Growth and development of the corpus luteum. J Reprod Fertil Suppl. 54:181–191. PMID: 10692854.

167. Chuderland D, Ben-Ami I, Kaplan-Kraicer R, Grossman H, Komsky A, Satchi-Fainaro R, Eldar-Boock A, Ron-El R, Shalgi R. 2013; Hormonal regulation of pigment epithelium-derived factor (PEDF) in granulosa cells. Mol Hum Reprod. 19:72–81. DOI: 10.1093/molehr/gas046. PMID: 23075882.

168. Shah K. 2012; Mesenchymal stem cells engineered for cancer therapy. Adv Drug Deliv Rev. 64:739–748. DOI: 10.1016/j.addr.2011.06.010. PMID: 21740940. PMCID: PMC3395998.

169. Levy O, Zhao W, Mortensen LJ, Leblanc S, Tsang K, Fu M, Phillips JA, Sagar V, Anandakumaran P, Ngai J, Cui CH, Eimon P, Angel M, Lin CP, Yanik MF, Karp JM. 2013; mRNA-engineered mesenchymal stem cells for targeted delivery of interleukin-10 to sites of inflammation. Blood. 122:e23–e32. DOI: 10.1182/blood-2013-04-495119. PMID: 23980067. PMCID: PMC3790516.

170. Vartanian A, Karshieva S, Dombrovsky V, Belyavsky A. 2016; Melanoma educates mesenchymal stromal cells towards vasculogenic mimicry. Oncol Lett. 11:4264–4268. DOI: 10.3892/ol.2016.4523. PMID: 27313776. PMCID: PMC4888129.

171. Lalu MM, McIntyre L, Pugliese C, Fergusson D, Winston BW, Marshall JC, Granton J, Stewart DJ. 2012; Safety of cell therapy with mesenchymal stromal cells (SafeCell): a systematic review and meta-analysis of clinical trials. PLoS One. 7:e47559. DOI: 10.1371/journal.pone.0047559. PMID: 23133515. PMCID: PMC3485008.

172. Hong S, Tan M, Wang S, Luo S, Chen Y, Zhang L. 2015; Efficacy and safety of angiogenesis inhibitors in advanced non-small cell lung cancer: a systematic review and meta-analysis. J Cancer Res Clin Oncol. 141:909–921. DOI: 10.1007/s00432-014-1862-5. PMID: 25373315.

173. Li X, Zhu S, Hong C, Cai H. 2016; Angiogenesis inhibitors for patients with ovarian cancer: a meta-analysis of 12 randomized controlled trials. Curr Med Res Opin. 32:555–562. DOI: 10.1185/03007995.2015.1131152. PMID: 26652645.

174. Cascone T, Herynk MH, Xu L, Du Z, Kadara H, Nilsson MB, Oborn CJ, Park YY, Erez B, Jacoby JJ, Lee JS, Lin HY, Ciardiello F, Herbst RS, Langley RR, Heymach JV. 2011; Upregulated stromal EGFR and vascular remodeling in mouse xenograft models of angiogenesis inhibitor-resistant human lung adenocarcinoma. J Clin Invest. 121:1313–1328. DOI: 10.1172/JCI42405. PMID: 21436589. PMCID: PMC3070607.

175. Semenza GL. 2014; Oxygen sensing, hypoxia-inducible factors, and disease pathophysiology. Annu Rev Pathol. 9:47–71. DOI: 10.1146/annurev-pathol-012513-104720. PMID: 23937437.

176. Pàez-Ribes M, Allen E, Hudock J, Takeda T, Okuyama H, Viñals F, Inoue M, Bergers G, Hanahan D, Casanovas O. 2009; Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell. 15:220–231. DOI: 10.1016/j.ccr.2009.01.027. PMID: 19249680. PMCID: PMC2874829.

Go to : 




 PDF
PDF Citation
Citation Print
Print


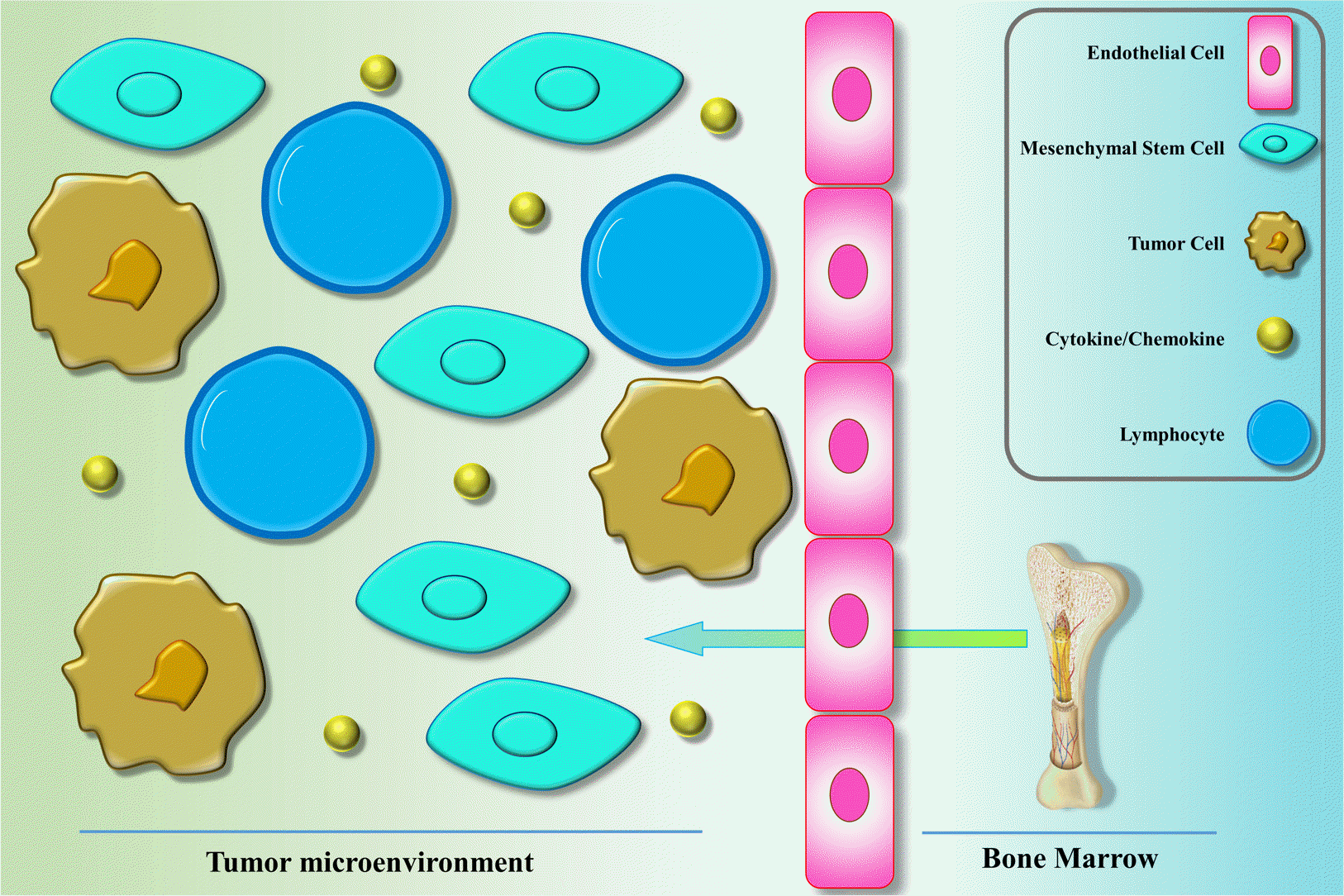
 XML Download
XML Download