1. Cucuzza ME, Marino SD, Schiavone L, Smilari P, Filosco F, Barone P. 2018; Diffuse alveolar haemorrage as initial presentation of systemic lupus erythematosus: a case report. Lupus. 27:507–510. DOI:
10.1177/0961203317713144. PMID:
28592199.

2. Al-Adhoubi NK, Bystrom J. 2020; Systemic lupus erythematosus and diffuse alveolar hemorrhage, etiology and novel treatment strategies. Lupus. 29:355–363. DOI:
10.1177/0961203320903798. PMID:
32036761. PMCID:
PMC7436451.

3. Kim D, Choi J, Cho SK, Choi CB, Kim TH, Jun JB, Yoo DH, Bae SC, Sung YK. 2017; Clinical characteristics and outcomes of diffuse alveolar hemorrhage in patients with systemic lupus erythematosus. Semin Arthritis Rheum. 46:782–787. DOI:
10.1016/j.semarthrit.2016.09.004. PMID:
27745903.

4. Ednalino C, Yip J, Carsons SE. 2015; Systematic review of diffuse alveolar hemorrhage in systemic lupus erythematosus: focus on outcome and therapy. J Clin Rheumatol. 21:305–310. DOI:
10.1097/RHU.0000000000000291. PMID:
26308350.

5. Parmar M. 2018; Towards stem cell based therapies for Parkinson's disease. Development. 145:dev156117. DOI:
10.1242/dev.156117. PMID:
29311261.

6. Senior PA, Pettus JH. 2019; Stem cell therapies for Type 1 diabetes: current status and proposed road map to guide successful clinical trials. Diabet Med. 36:297–307. DOI:
10.1111/dme.13846. PMID:
30362170.

7. Jin MC, Medress ZA, Azad TD, Doulames VM, Veeravagu A. 2019; Stem cell therapies for acute spinal cord injury in humans: a review. Neurosurg Focus. 46:E10. DOI:
10.3171/2018.12.FOCUS18602. PMID:
30835679.

8. Samsonraj RM, Raghunath M, Nurcombe V, Hui JH, van Wijnen AJ, Cool SM. 2017; Concise review: multifaceted characterization of human mesenchymal stem cells for use in regenerative medicine. Stem Cells Transl Med. 6:2173–2185. DOI:
10.1002/sctm.17-0129. PMID:
29076267. PMCID:
PMC5702523.

9. Zou Q, Wu M, Zhong L, Fan Z, Zhang B, Chen Q, Ma F. 2016; Development of a xeno-free feeder-layer system from human umbilical cord mesenchymal stem cells for prolonged expansion of human induced pluripotent stem cells in culture. PLoS One. 11:e0149023. DOI:
10.1371/journal.pone.0149023. PMID:
26882313. PMCID:
PMC4755601.

10. Can A, Celikkan FT, Cinar O. 2017; Umbilical cord mesenchymal stromal cell transplantations: a systemic analysis of clinical trials. Cytotherapy. 19:1351–1382. DOI:
10.1016/j.jcyt.2017.08.004. PMID:
28964742.

11. Liang J, Gu F, Wang H, Hua B, Hou Y, Shi S, Lu L, Sun L. 2010; Mesenchymal stem cell transplantation for diffuse alveolar hemorrhage in SLE. Nat Rev Rheumatol. 6:486–489. DOI:
10.1038/nrrheum.2010.80. PMID:
20517294.

13. Gurunathan S, Kang MH, Jeyaraj M, Qasim M, Kim JH. 2019; Review of the isolation, characterization, biological function, and multifarious therapeutic approaches of exosomes. Cells. 8:307. DOI:
10.3390/cells8040307. PMID:
30987213. PMCID:
PMC6523673.

15. Zhang R, Zhu Y, Li Y, Liu W, Yin L, Yin S, Ji C, Hu Y, Wang Q, Zhou X, Chen J, Xu W, Qian H. 2020; Human umbilical cord mesenchymal stem cell exosomes alleviate sepsis-associated acute kidney injury via regulating microRNA-146b expression. Biotechnol Lett. 42:669–679. DOI:
10.1007/s10529-020-02831-2. PMID:
32048128.

16. Zhang X, Liu J, Yu B, Ma F, Ren X, Li X. 2018; Effects of mesenchymal stem cells and their exosomes on the healing of large and refractory macular holes. Graefes Arch Clin Exp Ophthalmol. 256:2041–2052. DOI:
10.1007/s00417-018-4097-3. PMID:
30167916.

17. Wu Y, Qiu W, Xu X, Kang J, Wang J, Wen Y, Tang X, Yan Y, Qian H, Zhang X, Xu W, Mao F. 2018; Exosomes derived from human umbilical cord mesenchymal stem cells alleviate inflammatory bowel disease in mice through ubiquiti-nation. Am J Transl Res. 10:2026–2036. PMID:
30093940. PMCID:
PMC6079129.
18. Ding M, Shen Y, Wang P, Xie Z, Xu S, Zhu Z, Wang Y, Lyu Y, Wang D, Xu L, Bi J, Yang H. 2018; Exosomes isolated from human umbilical cord mesenchymal stem cells alleviate neuroinflammation and reduce amyloid-beta deposition by modulating microglial activation in Alzheimer's disease. Neurochem Res. 43:2165–2177. DOI:
10.1007/s11064-018-2641-5. PMID:
30259257.

19. Yaghoubi Y, Movassaghpour A, Zamani M, Talebi M, Mehdizadeh A, Yousefi M. 2019; Human umbilical cord mesenchymal stem cells derived-exosomes in diseases treatment. Life Sci. 233:116733. DOI:
10.1016/j.lfs.2019.116733. PMID:
31394127.

20. Sun G, Li G, Li D, Huang W, Zhang R, Zhang H, Duan Y, Wang B. 2018; hucMSC derived exosomes promote functional recovery in spinal cord injury mice via attenuating infla-mmation. Mater Sci Eng C Mater Biol Appl. 89:194–204. DOI:
10.1016/j.msec.2018.04.006. PMID:
29752089.

21. Fang S, Xu C, Zhang Y, Xue C, Yang C, Bi H, Qian X, Wu M, Ji K, Zhao Y, Wang Y, Liu H, Xing X. 2016; Umbilical cord-derived mesenchymal stem cell-derived exosomal microRNAs suppress myofibroblast differentiation by inhibiting the transforming growth factor-β/SMAD2 pathway during wound healing. Stem Cells Transl Med. 5:1425–1439. DOI:
10.5966/sctm.2015-0367. PMID:
27388239. PMCID:
PMC5031180.

22. Perez-Hernandez J, Redon J, Cortes R. 2017; Extracellular vesicles as therapeutic agents in systemic lupus erythematosus. Int J Mol Sci. 18:717. DOI:
10.3390/ijms18040717. PMID:
28350323. PMCID:
PMC5412303.

23. Zhou D, Huang C, Lin Z, Zhan S, Kong L, Fang C, Li J. 2014; Macrophage polarization and function with emphasis on the evolving roles of coordinated regulation of cellular signaling pathways. Cell Signal. 26:192–197. DOI:
10.1016/j.cellsig.2013.11.004. PMID:
24219909.

24. Shapouri-Moghaddam A, Mohammadian S, Vazini H, Taghadosi M, Esmaeili SA, Mardani F, Seifi B, Mohammadi A, Afshari JT, Sahebkar A. 2018; Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol. 233:6425–6440. DOI:
10.1002/jcp.26429. PMID:
29319160.

26. Barros MH, Hauck F, Dreyer JH, Kempkes B, Niedobitek G. 2013; Macrophage polarisation: an immunohistochemical approach for identifying M1 and M2 macrophages. PLoS One. 8:e80908. DOI:
10.1371/journal.pone.0080908. PMID:
24260507. PMCID:
PMC3829941.

27. Xu R, Zhang F, Chai R, Zhou W, Hu M, Liu B, Chen X, Liu M, Xu Q, Liu N, Liu S. 2019; Exosomes derived from pro-inflammatory bone marrow-derived mesenchymal stem cells reduce inflammation and myocardial injury via mediating macrophage polarization. J Cell Mol Med. 23:7617–7631. DOI:
10.1111/jcmm.14635. PMID:
31557396. PMCID:
PMC6815833.

28. Zhao J, Li X, Hu J, Chen F, Qiao S, Sun X, Gao L, Xie J, Xu B. 2019; Mesenchymal stromal cell-derived exosomes attenuate myocardial ischaemia-reperfusion injury through miR-182-regulated macrophage polarization. Cardiovasc Res. 115:1205–1216. DOI:
10.1093/cvr/cvz040. PMID:
30753344. PMCID:
PMC6529919.

29. Liu H, Liang Z, Wang F, Zhou C, Zheng X, Hu T, He X, Wu X, Lan P. 2019; Exosomes from mesenchymal stromal cells reduce murine colonic inflammation via a macrophage-dependent mechanism. JCI Insight. 4:e131273. DOI:
10.1172/jci.insight.131273. PMID:
31689240. PMCID:
PMC6975270.

30. Zhuang H, Han S, Lee PY, Khaybullin R, Shumyak S, Lu L, Chatha A, Afaneh A, Zhang Y, Xie C, Nacionales D, Moldawer L, Qi X, Yang LJ, Reeves WH. 2017; Pathogenesis of diffuse alveolar hemorrhage in murine lupus. Arthritis Rheumatol. 69:1280–1293. DOI:
10.1002/art.40077. PMID:
28217966. PMCID:
PMC5449234.

31. Shen M, Zeng X, Tian X, Zhang F, Zeng X, Zhang X, Xu W. 2010; Diffuse alveolar hemorrhage in systemic lupus erythematosus: a retrospective study in China. Lupus. 19:1326–1330. DOI:
10.1177/0961203310373106. PMID:
20647253.

33. Morales-Nebreda L, Alakija O, Ferguson KT, Singer BD. 2018; Systemic lupus erythematosus-associated diffuse alveolar hemorrhage: a case report and review of the literature. Clin Pulm Med. 25:166–169. DOI:
10.1097/CPM.0000000000000271. PMID:
30220838. PMCID:
PMC6136257.

35. Reeves WH, Lee PY, Weinstein JS, Satoh M, Lu L. 2009; Induction of autoimmunity by pristane and other naturally occurring hydrocarbons. Trends Immunol. 30:455–464. DOI:
10.1016/j.it.2009.06.003. PMID:
19699150. PMCID:
PMC2746238.

36. Barker TT, Lee PY, Kelly-Scumpia KM, Weinstein JS, Nacionales DC, Kumagai Y, Akira S, Croker BP, Sobel ES, Reeves WH, Satoh M. 2011; Pathogenic role of B cells in the development of diffuse alveolar hemorrhage induced by pristane. Lab Invest. 91:1540–1550. DOI:
10.1038/labinvest.2011.108. PMID:
21808234. PMCID:
PMC3184182.

37. Pittenger MF, Discher DE, Péault BM, Phinney DG, Hare JM, Caplan AI. 2019; Mesenchymal stem cell perspective: cell biology to clinical progress. NPJ Regen Med. 4:22. DOI:
10.1038/s41536-019-0083-6. PMID:
31815001. PMCID:
PMC6889290.

38. Pashoutan Sarvar D, Shamsasenjan K, Akbarzadehlaleh P. 2016; Mesenchymal stem cell-derived exosomes: new opportunity in cell-free therapy. Adv Pharm Bull. 6:293–299. DOI:
10.15171/apb.2016.041. PMID:
27766213. PMCID:
PMC5071792.

39. Shi D, Wang D, Li X, Zhang H, Che N, Lu Z, Sun L. 2012; Allogeneic transplantation of umbilical cord-derived mesenchymal stem cells for diffuse alveolar hemorrhage in systemic lupus erythematosus. Clin Rheumatol. 31:841–846. DOI:
10.1007/s10067-012-1943-2. PMID:
22302582.

40. Monsel A, Zhu YG, Gudapati V, Lim H, Lee JW. 2016; Mesenchymal stem cell derived secretome and extracellular vesicles for acute lung injury and other inflammatory lung diseases. Expert Opin Biol Ther. 16:859–871. DOI:
10.1517/14712598.2016.1170804. PMID:
27011289. PMCID:
PMC5280876.

41. Teng X, Chen L, Chen W, Yang J, Yang Z, Shen Z. 2015; Mesenchymal stem cell-derived exosomes improve the microenvironment of infarcted myocardium contributing to angiogenesis and anti-inflammation. Cell Physiol Biochem. 37:2415–2424. DOI:
10.1159/000438594. PMID:
26646808.

42. Li X, Liu L, Yang J, Yu Y, Chai J, Wang L, Ma L, Yin H. 2016; Exosome derived from human umbilical cord mesenchymal stem cell mediates miR-181c attenuating burn-induced excessive inflammation. EBioMedicine. 8:72–82. DOI:
10.1016/j.ebiom.2016.04.030. PMID:
27428420. PMCID:
PMC4919539.

43. Huang JH, Yin XM, Xu Y, Xu CC, Lin X, Ye FB, Cao Y, Lin FY. 2017; Systemic administration of exosomes released from mesenchymal stromal cells attenuates apoptosis, inflammation, and promotes angiogenesis after spinal cord injury in rats. J Neurotrauma. 34:3388–3396. DOI:
10.1089/neu.2017.5063. PMID:
28665182.

44. Viola A, Munari F, Sánchez-Rodríguez R, Scolaro T, Castegna A. 2019; The metabolic signature of macrophage responses. Front Immunol. 10:1462. DOI:
10.3389/fimmu.2019.01462. PMID:
31333642. PMCID:
PMC6618143.

45. Han S, Zhuang H, Shumyak S, Wu J, Xie C, Li H, Yang LJ, Reeves WH. 2018; Liver X receptor agonist therapy prevents diffuse alveolar hemorrhage in murine lupus by repolarizing macrophages. Front Immunol. 9:135. DOI:
10.3389/fimmu.2018.00135. PMID:
29456535. PMCID:
PMC5801423.

46. Willis GR, Fernandez-Gonzalez A, Anastas J, Vitali SH, Liu X, Ericsson M, Kwong A, Mitsialis SA, Kourembanas S. 2018; Mesenchymal stromal cell exosomes ameliorate experimental bronchopulmonary dysplasia and restore lung function through macrophage immunomodulation. Am J Respir Crit Care Med. 197:104–116. DOI:
10.1164/rccm.201705-0925OC. PMID:
28853608. PMCID:
PMC5765387.

47. Ti D, Hao H, Tong C, Liu J, Dong L, Zheng J, Zhao Y, Liu H, Fu X, Han W. 2015; LPS-preconditioned mesenchymal stromal cells modify macrophage polarization for resolution of chronic inflammation via exosome-shuttled let-7b. J Transl Med. 13:308. DOI:
10.1186/s12967-015-0642-6. PMID:
26386558. PMCID:
PMC4575470.

48. Biswas S, Mandal G, Roy Chowdhury S, Purohit S, Payne KK, Anadon C, Gupta A, Swanson P, Yu X, Conejo-Garcia JR, Bhattacharyya A. 2019; Exosomes produced by mesenchymal stem cells drive differentiation of myeloid cells into immunosuppressive m2-polarized macrophages in breast cancer. J Immunol. 203:3447–3460. DOI:
10.4049/jimmunol.1900692. PMID:
31704881. PMCID:
PMC6994919.

49. Deng S, Zhou X, Ge Z, Song Y, Wang H, Liu X, Zhang D. 2019; Exosomes from adipose-derived mesenchymal stem cells ameliorate cardiac damage after myocardial infarction by activating S1P/SK1/S1PR1 signaling and promoting macrophage M2 polarization. Int J Biochem Cell Biol. 114:105564. DOI:
10.1016/j.biocel.2019.105564. PMID:
31276786.

50. Li J, Xue H, Li T, Chu X, Xin D, Xiong Y, Qiu W, Gao X, Qian M, Xu J, Wang Z, Li G. 2019; Exosomes derived from mesenchymal stem cells attenuate the progression of atherosclerosis in ApoE
-/
- mice via miR-let7 mediated infiltration and polarization of M2 macrophage. Biochem Biophys Res Commun. 510:565–572. DOI:
10.1016/j.bbrc.2019.02.005. PMID:
30739785.

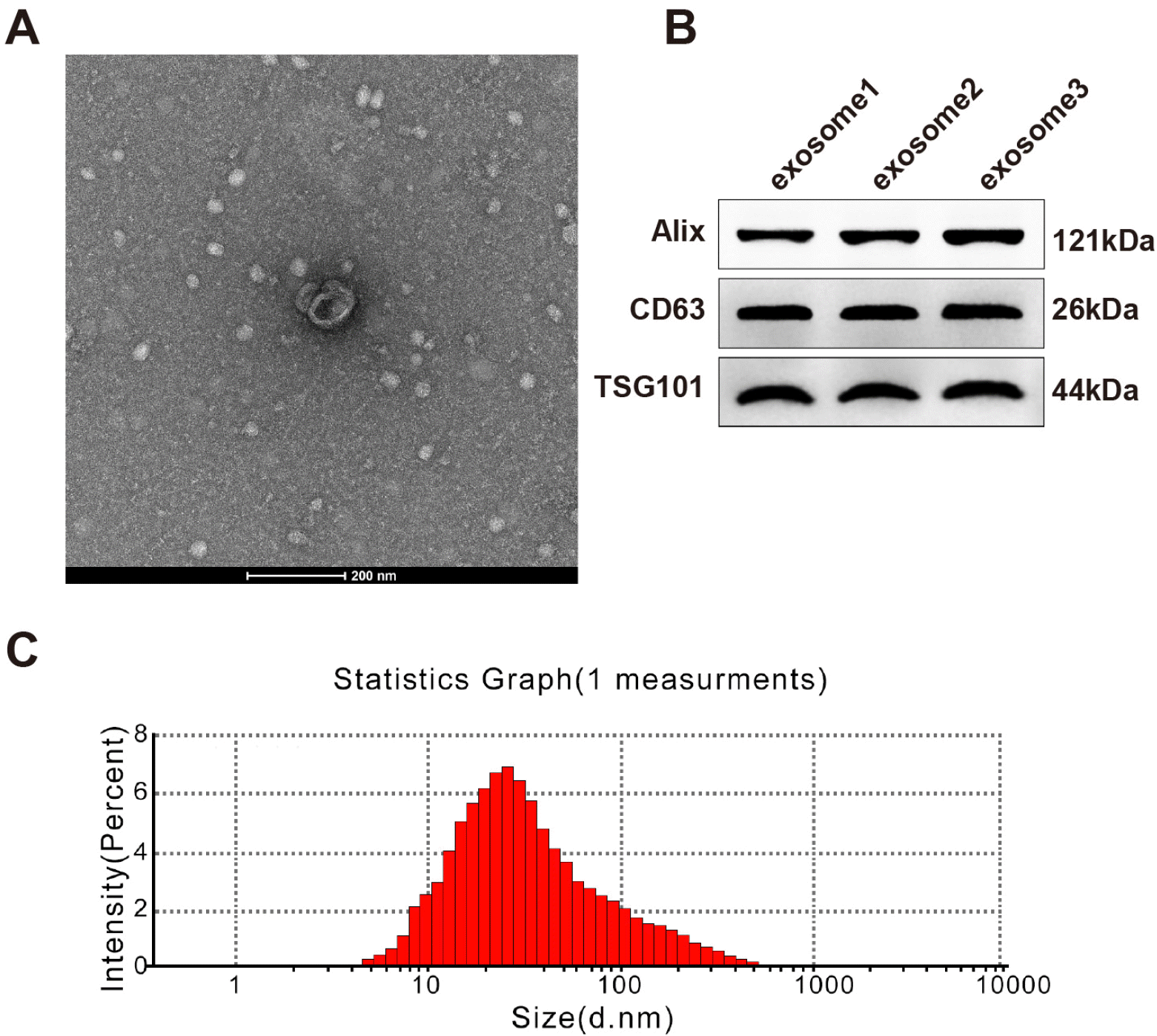
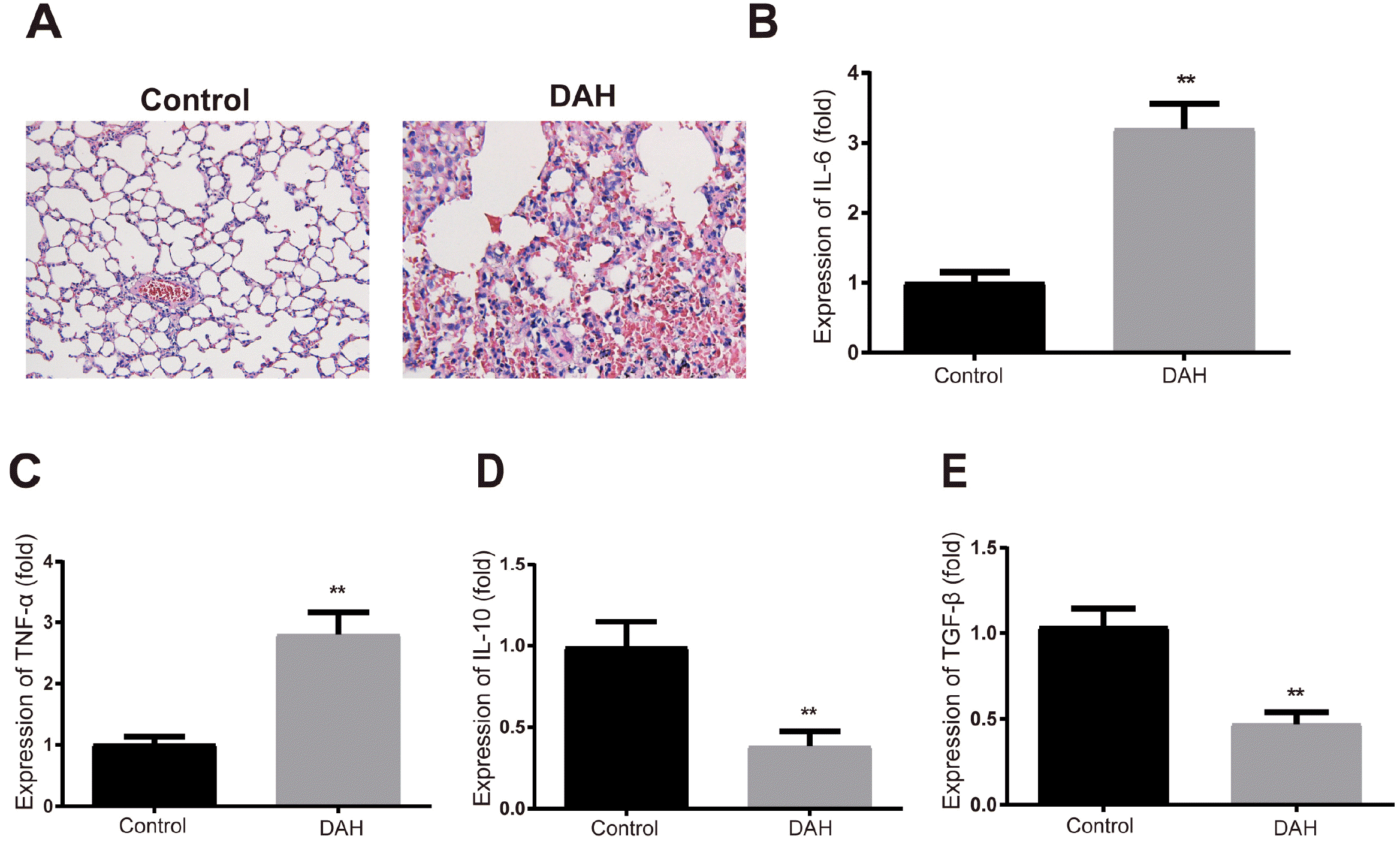
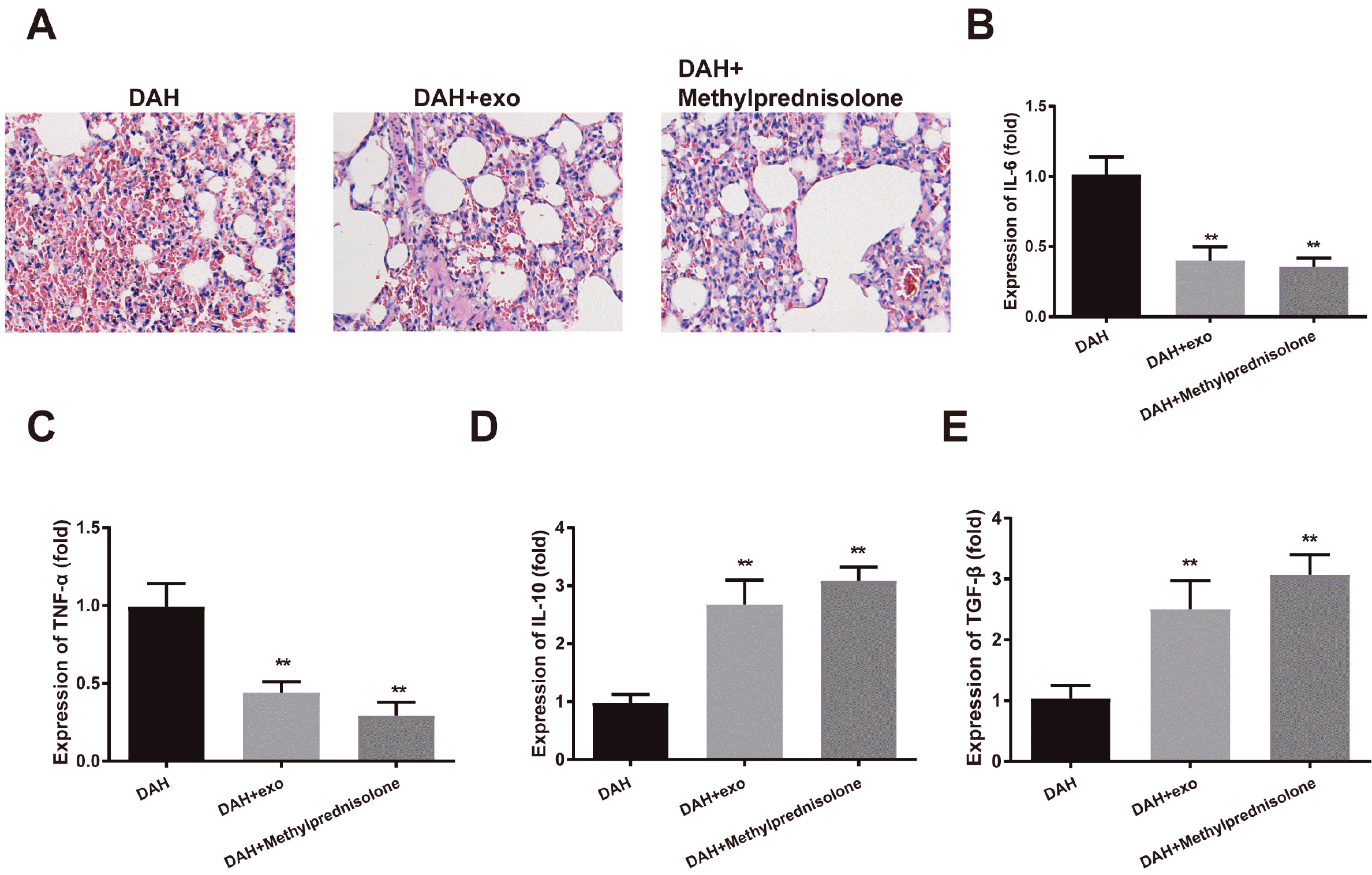
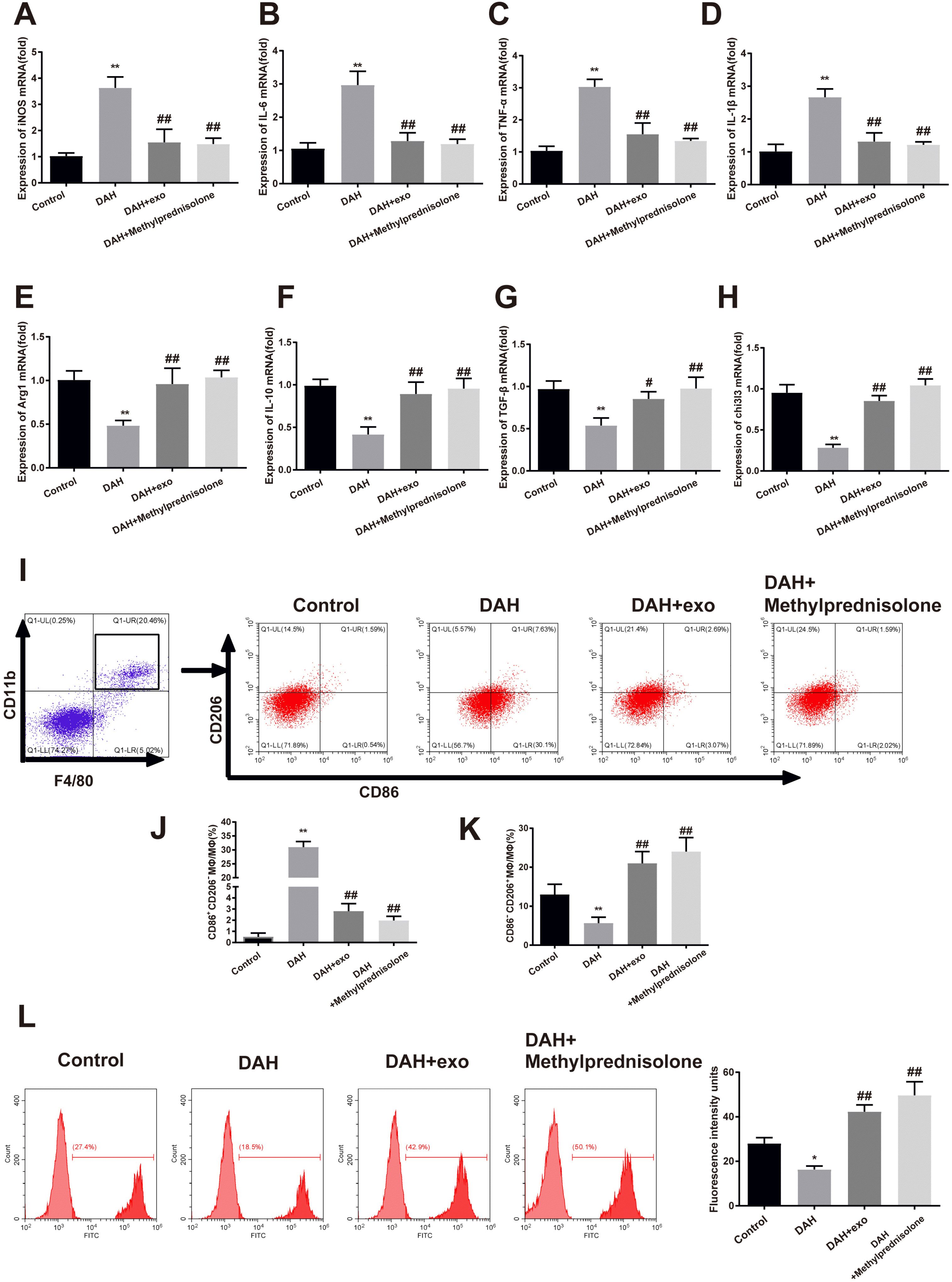




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download