Introduction
Materials and Methods
Ethics statement
Cell cultures
Lentiviral vector construction, packaging, and infection
Preparation of animals and establishment of ALI models
Animal grouping
Dual-luciferase reporter assay
Evaluation of lung edema
Quantitative reverse transcription polymerase chain reaction (qRT-PCR)
Table 1
Immunoblot
Histopathology
Enzyme-linked immunosorbent assay (ELISA)
Statistical analysis
Results
MiR-23b-3p was low-expressed in the lung tissues of ALI mice
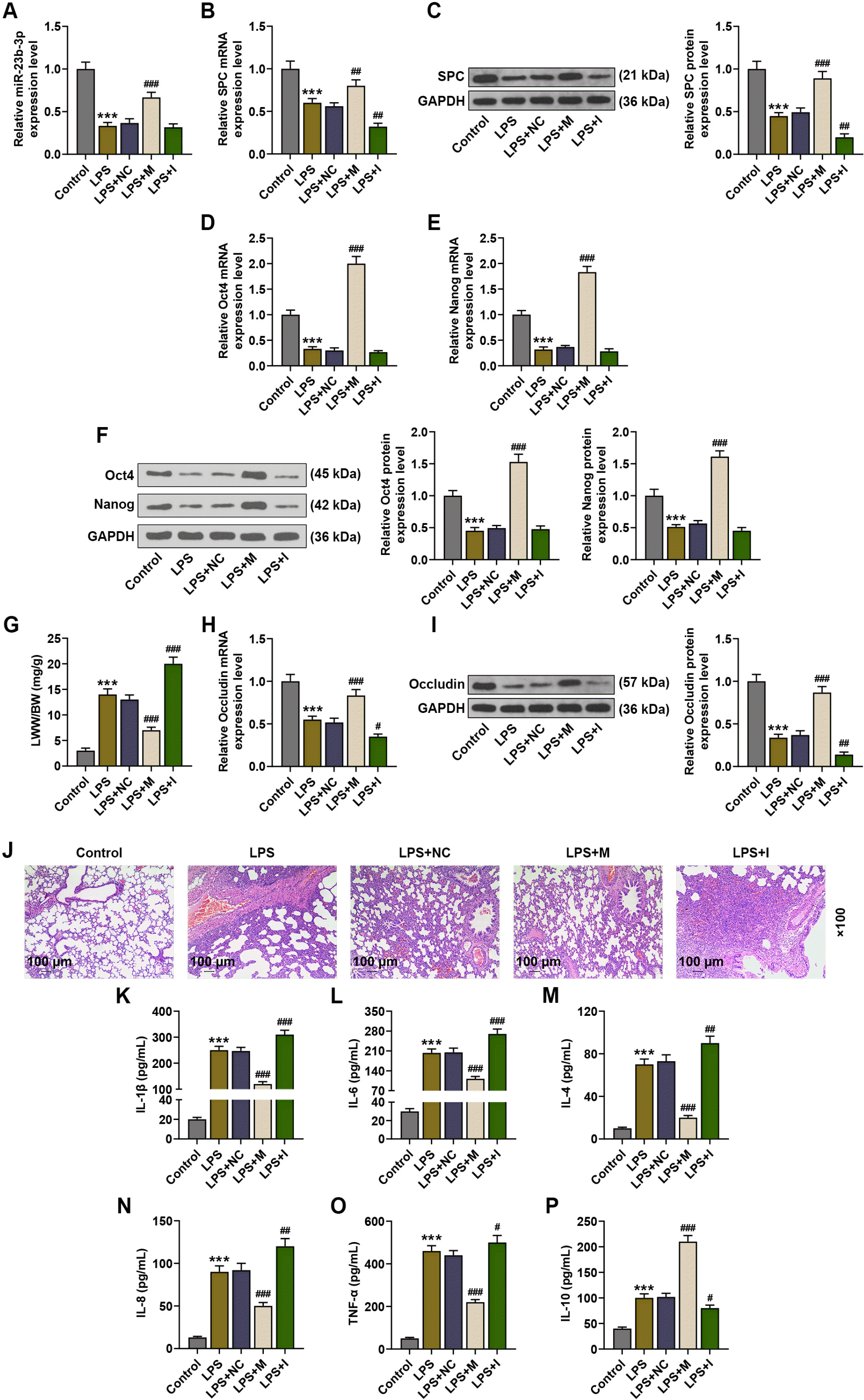 | Fig. 1MiR-23b-3p-overexpressing BMSCs promoted the differentiation of BMSCs into TypeII pneumocytes and alleviated injuries in ALI lung tissues. (A, B, D, E, H) The expressions of miR-23b-3p, SPC, Oct4, Nanog and Occludin in the lung tissues of LPS-induced ALI mice were analyzed by qRT-PCR, after injection of BMSCs with no altered gene expression or BMSCs with miR-27a-3p overexpression or underexpression. (C, F, I) The expressions of SPC, Oct4, Nanog and Occludin in the lung tissues of LPS-induced ALI mice were analyzed by immunoblot, with GAPDH serving as a reference gene, after injection of BMSCs with no altered gene expression or BMSCs with miR-27a-3p overexpression or underexpression. (G) The ratio of LWW to BW of LPS-induced ALI mice was calculated, after injection of BMSCs with no altered gene expression or BMSCs with miR-27a-3p overexpression or underexpression. (J) Histopathological changes in the lung tissues of LPS-induced ALI mice were observed via hematoxylin-eosin staining, after injection of BMSCs with no altered gene expression or BMSCs with miR-27a-3p overexpression or underexpression (scale: 100 μm; magnification: ×100). (K∼P) The levels of IL-1β, IL-6, IL-4, IL-8, TNF-α and IL-10 in the bronchoalveolar lavage fluid of LPS-induced ALI mice were assessed by ELISA, after injection of BMSCs with no altered gene expression or BMSCs with miR-27a-3p overexpression or underexpression. #p<0.05; ##p<0.01; ***p or ###p<0.001; * vs. Control ; # vs. LPS+NC (LPS: lipopolysaccharide, SPC: surfactant protein C, NC: negative control, M: miR-23b-3p mimic, I: miR-23b-3p inhibitor, qRT-PCR: Quantitative reverse transcription polymerase chain reaction, ELISA: Enzyme-linked immunosorbent assay, ALI: acute lung injury, LWW/BW: lung wet weight/body weight, BMSCs: bone marrow-derived mesenchymal stem cells). |
MiR-23b-3p-overexpressing BMSCs promoted the differentiation of BMSCs, increased lung epithelium permeability, and improved lung edema
MiR-23b-3p-overexpressing BMSCs inhibited inflammatory factor release in ALI lung tissues
MiR-23b-3p directly targeted FGF2
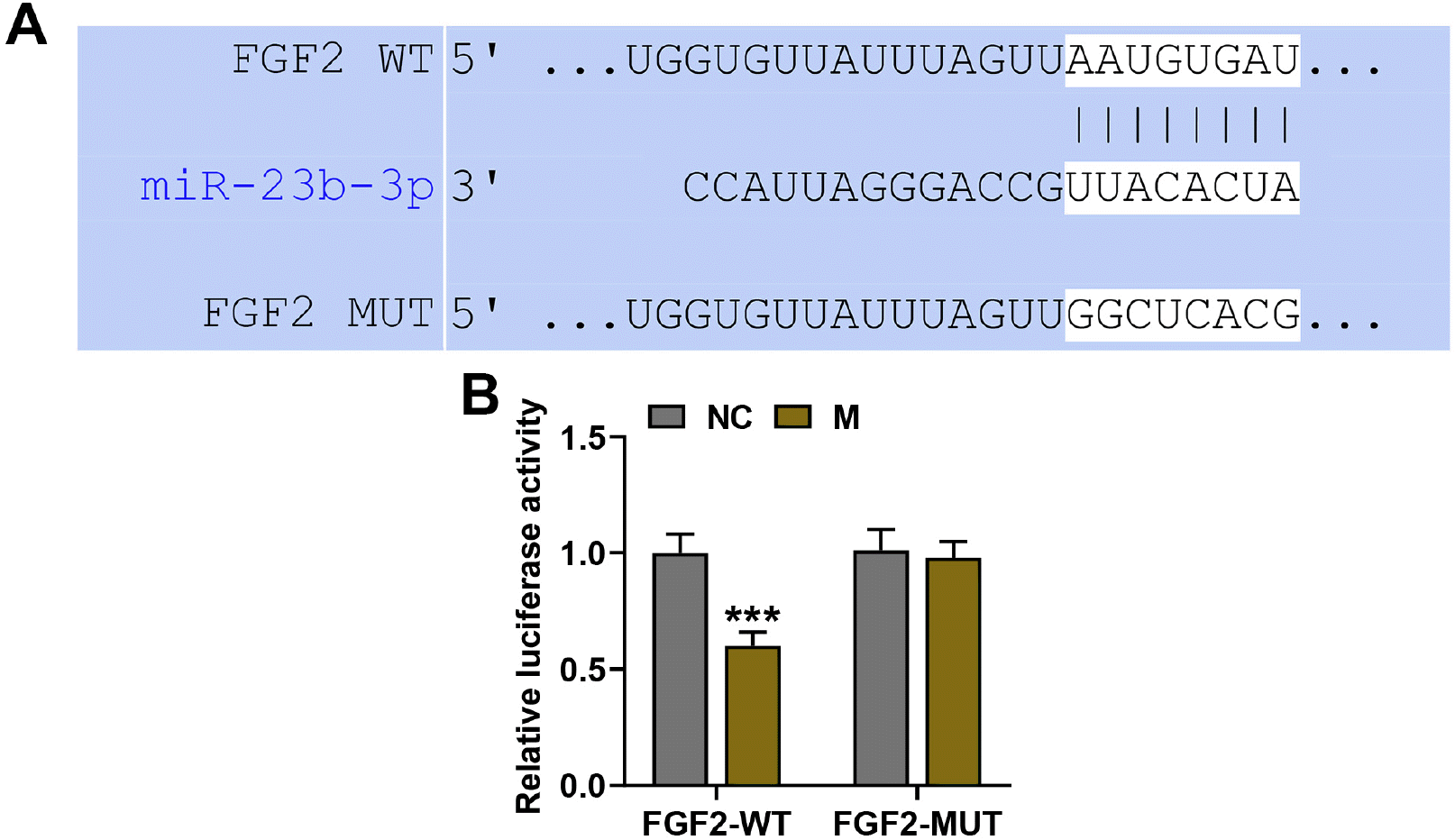 | Fig. 2MiR-23b-3p directly targeted FGF2. (A) The putative binding sites of miR-23b-3p on FGF2 were predicted by TargetScan V7.2. (B) The interaction between miR-23b-3p and FGF2 was validated by dual-luciferase reporter assay. ***p<0.001; * vs. NC (NC: negative control, M: miR-23b-3p mimic, WT: wild type, MUT: mutant type, FGF2: fibroblast growth factor 2). |
FGF2 expression was inhibited by miR-23b-3p overexpressing BMSCs, and it inhibited the differentiation of BMSCs into TypeII pneumocytes
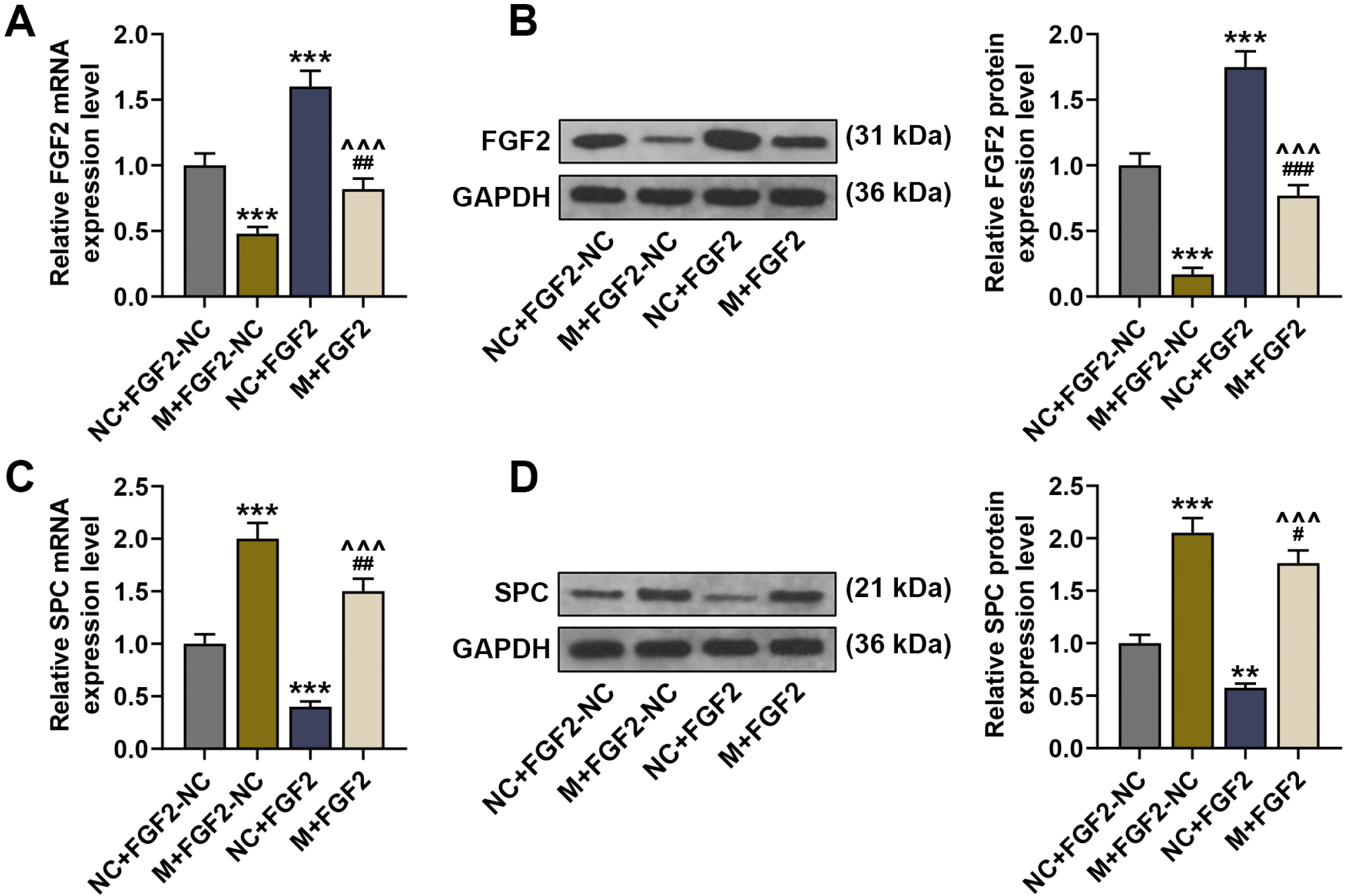 | Fig. 3FGF2 expression was inhibited by miR-23b-3p overexpressing BMSCs and it inhibited the differentiation of BMSCs into TypeII pneumocytes. (A, C) The expressions of FGF2 and SPC in the lung tissues of LPS-induced ALI mice were analyzed by qRT-PCR, after injection of BMSCs with no altered gene expression, BMSCs with overexpression of miR-27a-3p or FGF2 alone, or BMSCs with co-overexpression of miR-27a-3p and FGF2. (B, D) The expressions of FGF2 and SPC in the lung tissues of LPS-induced ALI mice were analyzed by immunoblot, with GAPDH serving as a reference gene, after injection of BMSCs with no altered gene expression, BMSCs with overexpression of miR-27a-3p or FGF2 alone, or BMSCs with co-overexpression of miR-27a-3p and FGF2. #p<0.05; **p or ##p<0.01; ***p or ###p or ^^^p<0.001; * vs. NC+FGF2-NC ; # vs. M+FGF2-NC; ^ vs. NC+FGF2 (LPS: lipopolysaccharide, SPC: surfactant protein C, FGF2: fibroblast growth factor 2, NC: negative control, M: miR-23b-3p mimic, qRT-PCR: Quantitative reverse transcription polymerase chain reaction, BMSCs: bone marrow-derived mesenchymal stem cells). |
FGF2 reversed the effect of the implantation of BMSCs overexpressing miR-23b-3p on ALI lung tissues
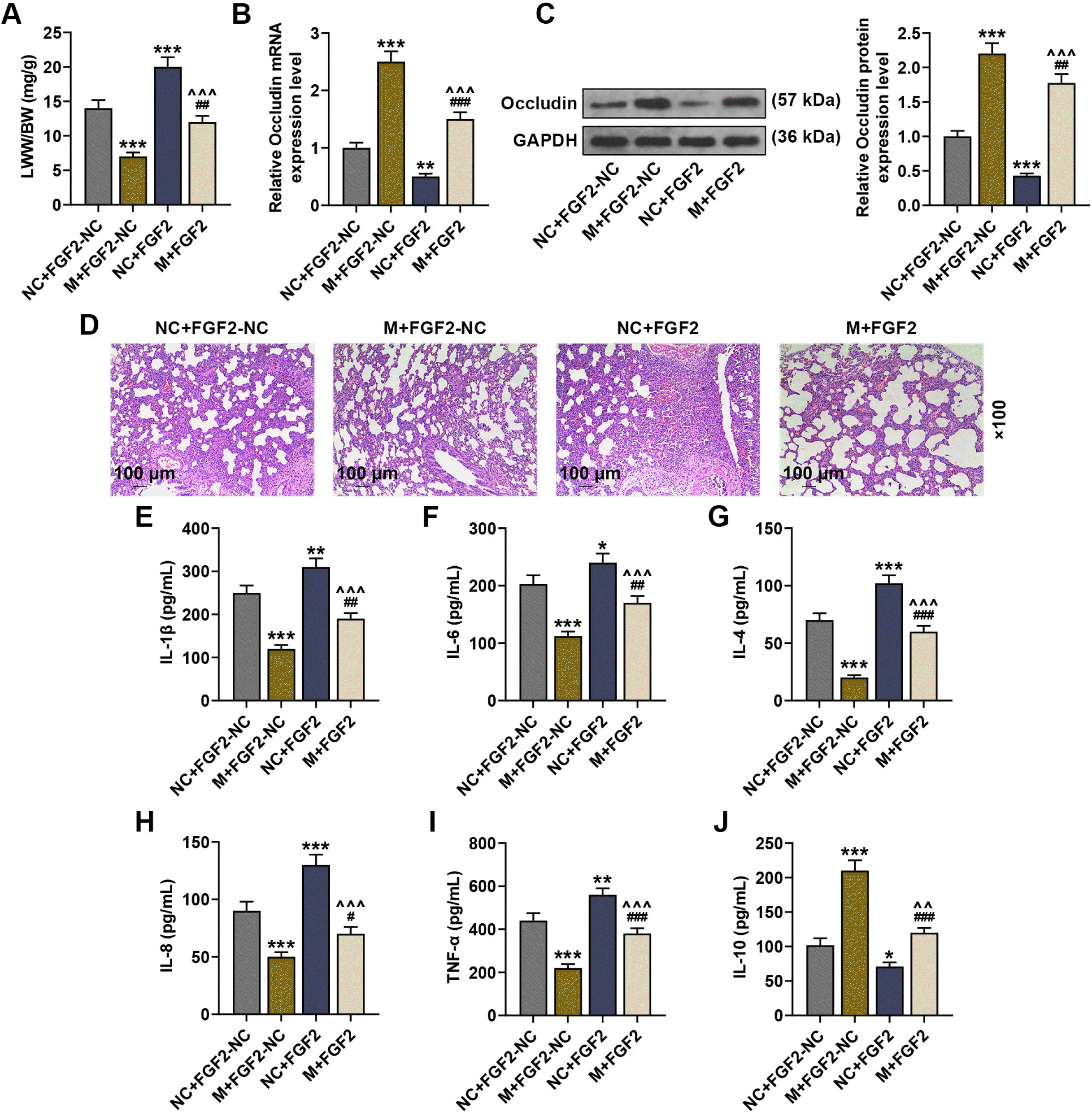 | Fig. 4FGF2 reversed the effect of the implantation of BMSCs overexpressing miR-23b-3p on ALI lung tissues. (A) The ratio of LWW to BW of LPS-induced ALI mice was calculated, after injection of BMSCs with no altered gene expression, BMSCs with overexpression of miR-27a-3p or FGF2 alone, or BMSCs with co-overexpression of miR-27a-3p and FGF2. (B) The expression of Occludin in the lung tissues was analyzed by qRT-PCR, after injection of BMSCs with no altered gene expression, BMSCs with overexpression of miR-27a-3p or FGF2 alone, or BMSCs with co-overexpression of miR-27a-3p and FGF2. (C) The expression of Occludin in the lung tissues of LPS-induced ALI mice was analyzed by immunoblot, with GAPDH serving as a reference gene, after injection of BMSCs with no altered gene expression, BMSCs with overexpression of miR-27a-3p or FGF2 alone, or BMSCs with co-overexpression of miR-27a-3p and FGF2. (D) Histopathological changes in the lung tissues of LPS-induced ALI mice were observed via hematoxylin-eosin staining, after injection of BMSCs with no altered gene expression, BMSCs with overexpression of miR-27a-3p or FGF2 alone, or BMSCs with co-overexpression of miR-27a-3p and FGF2 (scale: 100 μm; magnification: ×100). (E∼J) The levels of IL-1β, IL-6, IL-4, IL-8, TNF-α and IL-10 in the bronchoalveolar lavage fluid of LPS-induced ALI mice were assessed by ELISA, after injection of BMSCs with no altered gene expression, BMSCs with overexpression of miR-27a-3p or FGF2 alone, or BMSCs with co-overexpression of miR-27a-3p and FGF2. *p or #p<0.05; **p or ##p or ^^p<0.01; ***p or ###p or ^^^p<0.001; * vs. NC+FGF2-NC ; # vs. mimic+FGF2-NC; ^ vs. NC+FGF2 (LPS: lipopolysaccharide, FGF2: fibroblast growth factor 2, NC: negative control, M: miR-23b-3p mimic, qRT-PCR: Quantitative reverse transcription polymerase chain reaction, ELISA: Enzyme-linked immunosorbent assay, ALI: acute lung injury, LWW/BW: lung wet weight/body weight, BMSCs: bone marrow-derived mesenchymal stem cells). |




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download