Introduction
Materials and Methods
Mouse embryonic stem cell culture
Differentiation of mES cells with retinoic acid
Flow cytometry analysis
Treatment with MUC1-C inhibitor peptide
RT-PCR and quantitative PCR
Western blotting
Alkaline phosphatase assay
Cell proliferation assay
Cell cycle analysis
Analysis of apoptosis (Annexin V/PI staining)
Reactive oxygen species (ROS) measurement
Determination of glutathione (GSH) levels
Statistics
Results
The protein level of MUC1-C was decreased during differentiation of mES cells
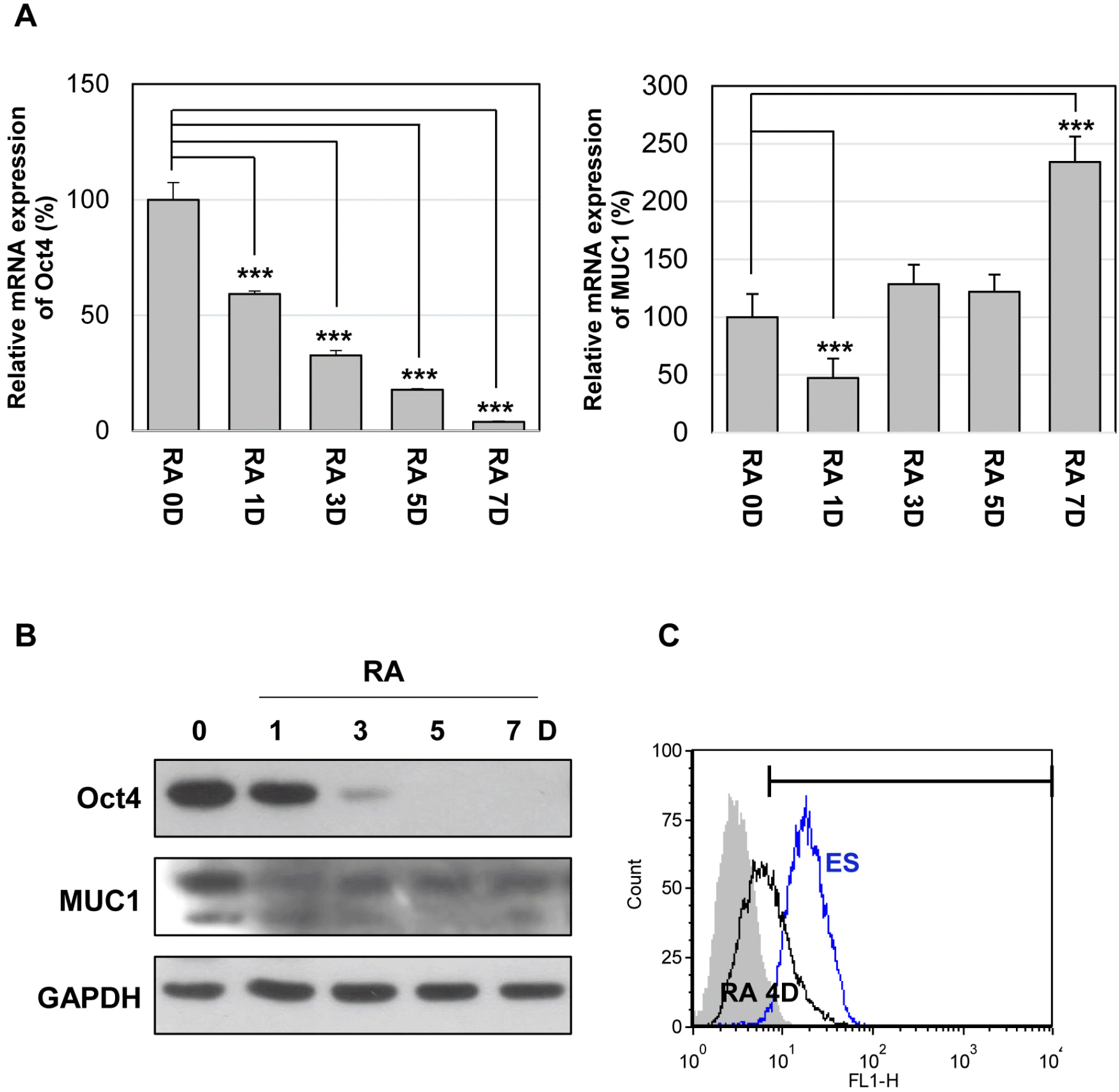 | Fig. 1Expression of MUC1 in mouse embryonic stem (mES) cells. The cells were differentiated with retinoic acid (RA, 10 μM) for the indicated period. (A) The relative expression levels of Oct4 and MUC1 mRNAs were analyzed by quantitative real-time PCR. Each bar is expressed as the mean±standard deviation (SD) of three experiments. ***p<0.001 (vs RA 0D). The protein level of MUC1-C was analyzed by western blotting (B) and flow cytometry (C). |
Inhibition of MUC1-C reduces growth of mES cells
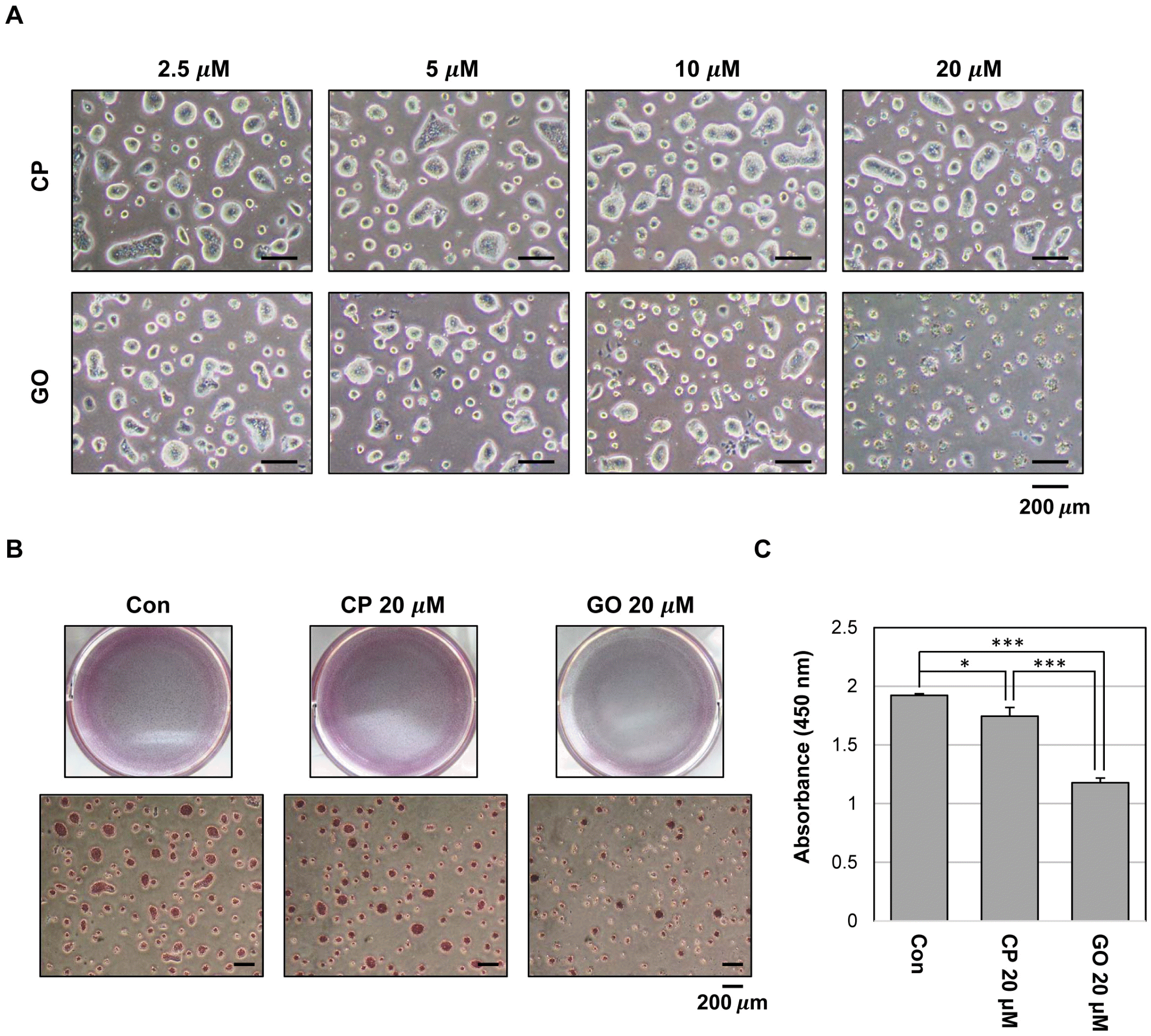 | Fig. 2Effect of MUC1-C inhibition on growth in mES cells. (A) Phase-contrast micrograph images of mES cell colonies treated with CP1 or GO201 at the indicated concentrations for 24 hr. (B) The cells were grown in the presence of CP1 or GO201 at the indicated concentration for 24 hr. Colonies were then stained with an alkaline phosphatase kit. (C) Cell proliferation assay was performed using Cell Proliferation ELISA, BrdU kit. The cells were treated with the indicated concentration of CP1 or GO201 for 24 hr. BrdU incorporation was quantified by measuring the absorbance at 450 nm using an ELISA reader. Each bar is expressed as the mean±SD of three experiments. *p<0.05, ***p< 0.001 (vs control or CP1). Con, Control; CP, CP1; GO, GO201. |
Inhibition of MUC1-C does not affect pluripotency of mES cells
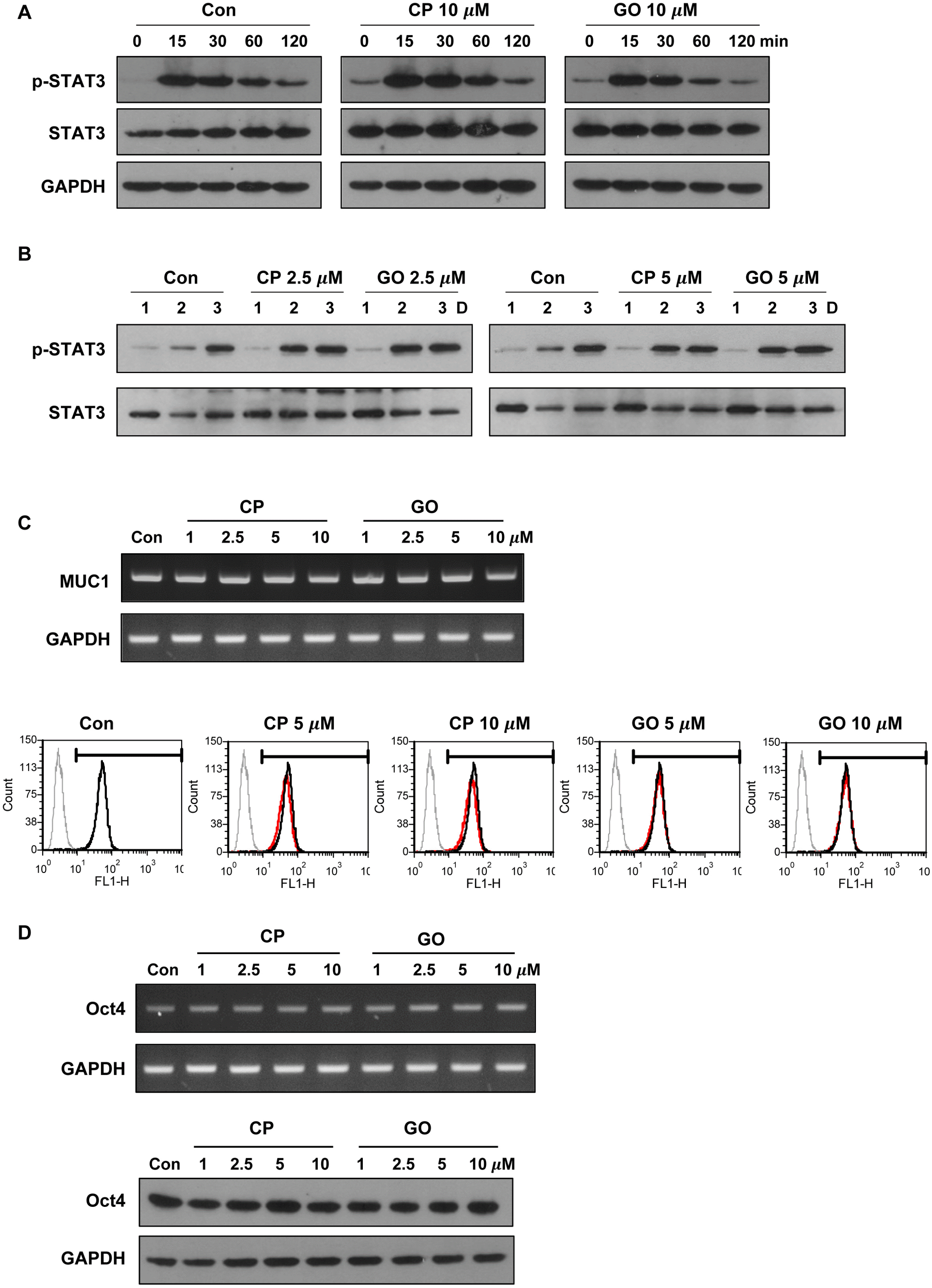 | Fig. 3Effect of MUC1-C inhibition on STAT3 signaling and expression of pluripotency marker in mES cells. (A) The cells were serum starved overnight and then preincubated for 1 hr in the presence of CP1 or GO201 (10 μM). They were then stimulated with FBS (15%) and LIF for the indicated period and analyzed by western blotting. (B) The cells were treated with CP1 or GO201 at the indicated concentrations for three days and analyzed by western blotting. (C) The cells were treated with CP1 or GO201 at the indicated concentrations for 24 hr. Expression levels of MUC1 mRNA and protein were analyzed by RT-PCR (upper) and flow cytometry (lower). The histogram peak represents the expression of MUC1-C: Grey line, negative control; Black line, untreated control; Red line, CP1- or GO201-treated samples. (D) The cells were treated with CP1 or GO201 at the indicated concentrations for 24 hr. Expression levels of Oct4 mRNA and protein were analyzed by RT-PCR (upper) and western blotting (lower). Con, Control; CP, CP1; GO, GO201. |
Inhibition of MUC1-C induces cell cycle arrest and promotes cell death in mES cells
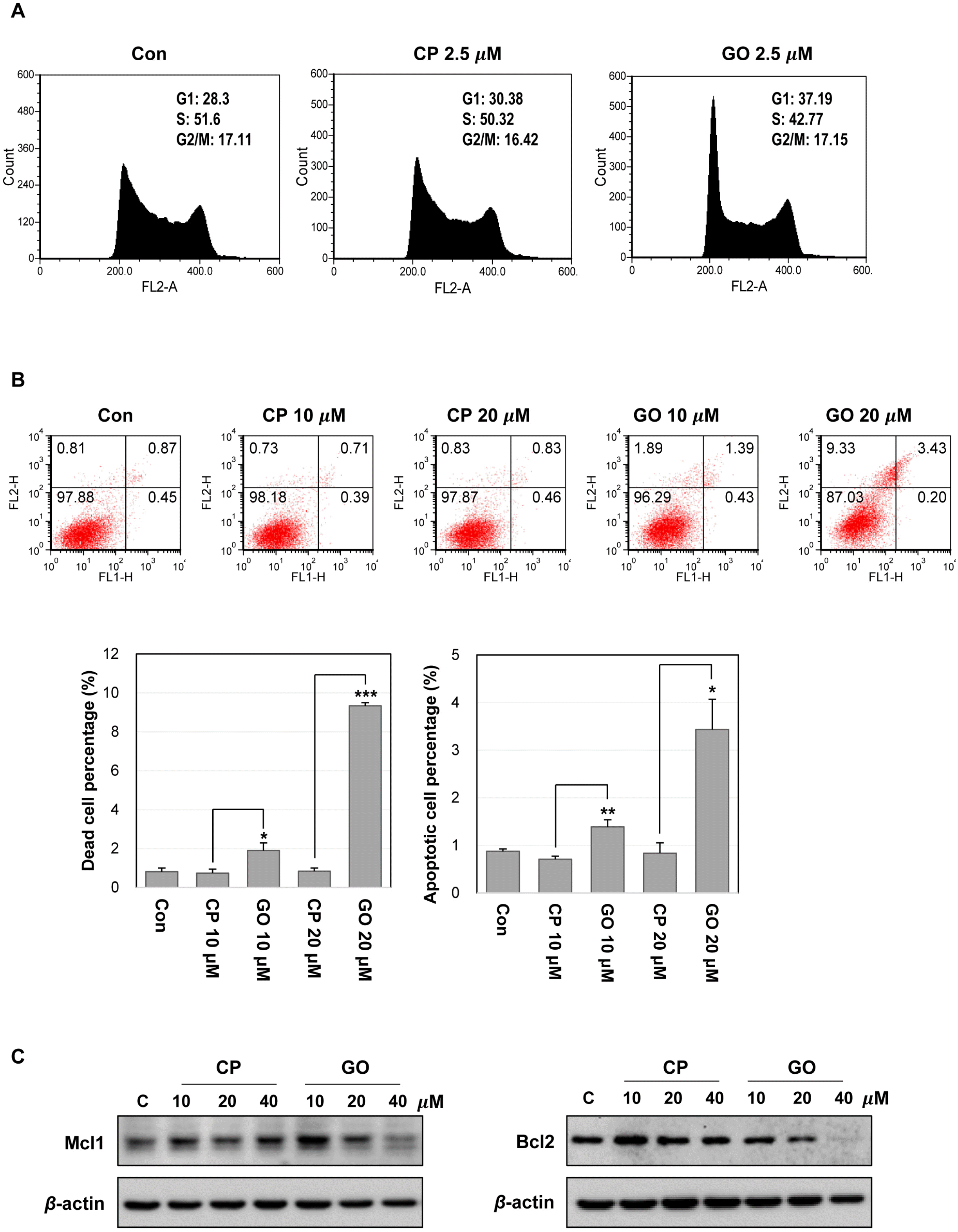 | Fig. 4Inhibition of MUC1-C induces cell cycle changes and cell death in mES cells. (A) Cell cycle analysis of mES cells by flow cyto-metry. The cells were treated with the indicated concentration of CP1 or GO201 for 24 hr. (B) Apoptosis of mES cells treated with CP1 or GO201 for 24 hr was assessed by Annexin V/PI staining. Each bar is expressed as the mean±SD of three experiments. *p<0.05, **p<0.01, ***p<0.001 (vs CP1). The results at the bottom are shown as percentages of dead cell and apoptotic cell population. (C) Expression levels of Mcl1 and Bcl2 were analyzed by western blotting, after treatment with CP1 or GO201 at the indicated concentrations for 24 hr. Con, Control; CP, CP1; GO, GO201. |
Inhibition of MUC1-C disrupts ROS balance in mES cells
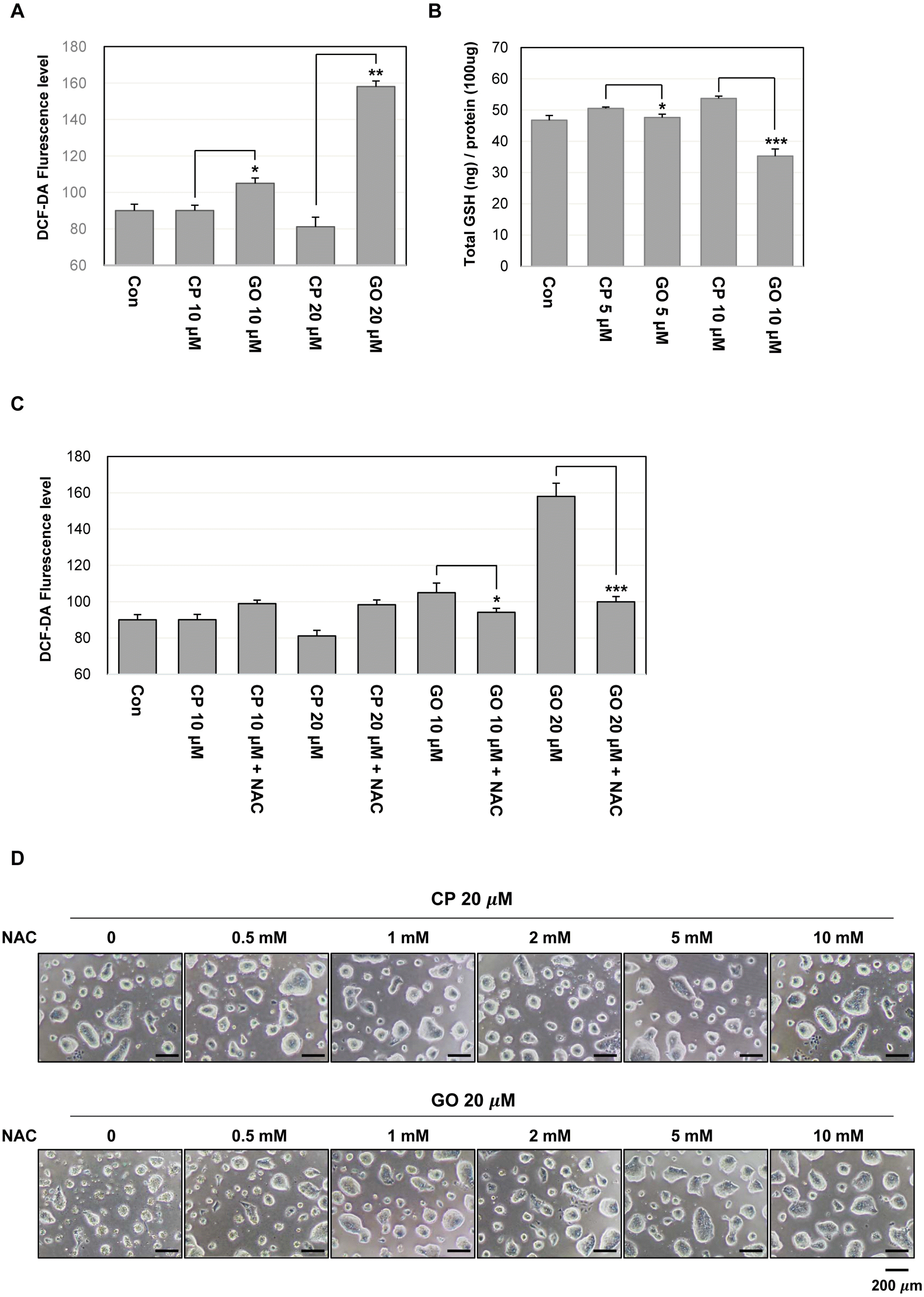 | Fig. 5MUC1-C inhibition increases ROS level in mES cells. (A) ROS level measured by flow cytometry using DCF-DA. The cells were treated with the indicated concentrations of GO201 or CP1 for 24 hr, incubated with 10 μM of DCF-DA for 30 min, and analyzed by flow cytometry. (B) The cells were treated with the indicated concentrations of GO201 or CP1 for 24 hr. The cell lysates were incubated with glutathione reductase and the GSH substrate, and the absorbance values were measured at a wavelength of 405 nm. (C) The cells were untreated or treated with 10 μM of NAC and were treated with the indicated concentrations of GO201 or CP1 for 24 hr. The cells were then incubated with 10 μM of DCF-DA for 30 min and analyzed by flow cytometry. Each bar is expressed as the mean±SD of three experiments. *p<0.05, **p<0.01, ***p<0.001 (vs CP1). (D) Images of mES cell colonies. The cells were untreated or treated with the indicated concentrations of NAC and were treated with 20 μM of GO201 or CP1 for 24 hr. Con, Control; CP, CP1; GO, GO201. |




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download