2. Lancaster MA, Knoblich JA. 2014; Organogenesis in a dish: modeling development and disease using organoid technologies. Science. 345:1247125. DOI:
10.1126/science.1247125. PMID:
25035496.

3. Sasai Y. 2013; Cytosystems dynamics in self-organization of tissue architecture. Nature. 493:318–326. DOI:
10.1038/nature11859. PMID:
23325214.

4. Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, Thomson JA. 2007; Induced pluripotent stem cell lines derived from human somatic cells. Science. 318:1917–1920. DOI:
10.1126/science.1151526. PMID:
18029452.

5. Orkin RW, Gehron P, McGoodwin EB, Martin GR, Valentine T, Swarm R. 1977; A murine tumor producing a matrix of basement membrane. J Exp Med. 145:204–220. DOI:
10.1084/jem.145.1.204. PMID:
830788. PMCID:
PMC2180589.

6. Ehrmann RL, Gey GO. 1956; The growth of cells on a transparent gel of reconstituted rat-tail collagen. J Natl Cancer Inst. 16:1375–1403. PMID:
13320119.
7. Hughes CS, Postovit LM, Lajoie GA. 2010; Matrigel: a complex protein mixture required for optimal growth of cell culture. Proteomics. 10:1886–1890. DOI:
10.1002/pmic.200900758. PMID:
20162561.

8. Freytes DO, Martin J, Velankar SS, Lee AS, Badylak SF. 2008; Preparation and rheological characterization of a gel form of the porcine urinary bladder matrix. Biomaterials. 29:1630–1637. DOI:
10.1016/j.biomaterials.2007.12.014. PMID:
18201760.

9. Spence JR, Mayhew CN, Rankin SA, Kuhar MF, Vallance JE, Tolle K, Hoskins EE, Kalinichenko VV, Wells SI, Zorn AM, Shroyer NF, Wells JM. 2011; Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature. 470:105–109. DOI:
10.1038/nature09691. PMID:
21151107. PMCID:
PMC3033971.

10. Zhong X, Gutierrez C, Xue T, Hampton C, Vergara MN, Cao LH, Peters A, Park TS, Zambidis ET, Meyer JS, Gamm DM, Yau KW, Canto-Soler MV. 2014; Generation of three-dimensional retinal tissue with functional photoreceptors from human iPSCs. Nat Commun. 5:4047. DOI:
10.1038/ncomms5047. PMID:
24915161. PMCID:
PMC4370190.

11. Takebe T, Sekine K, Enomura M, Koike H, Kimura M, Ogaeri T, Zhang RR, Ueno Y, Zheng YW, Koike N, Aoyama S, Adachi Y, Taniguchi H. 2013; Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature. 499:481–484. DOI:
10.1038/nature12271. PMID:
23823721.

12. Lancaster MA, Renner M, Martin CA, Wenzel D, Bicknell LS, Hurles ME, Homfray T, Penninger JM, Jackson AP, Knoblich JA. 2013; Cerebral organoids model human brain development and microcephaly. Nature. 501:373–379. DOI:
10.1038/nature12517. PMID:
23995685. PMCID:
PMC3817409.

13. McCracken KW, Catá EM, Crawford CM, Sinagoga KL, Schumacher M, Rockich BE, Tsai YH, Mayhew CN, Spence JR, Zavros Y, Wells JM. 2014; Modelling human development and disease in pluripotent stem-cell-derived gastric organoids. Nature. 516:400–404. DOI:
10.1038/nature13863. PMID:
25363776. PMCID:
PMC4270898.

14. Miller AJ, Hill DR, Nagy MS, Aoki Y, Dye BR, Chin AM, Huang S, Zhu F, White ES, Lama V, Spence JR. 2018; In vitro induction and in vivo engraftment of lung bud tip progenitor cells derived from human pluripotent stem cells. Stem Cell Reports. 10:101–119. DOI:
10.1016/j.stemcr.2017.11.012. PMID:
29249664. PMCID:
PMC5770275.

15. Morizane R, Lam AQ, Freedman BS, Kishi S, Valerius MT, Bonventre JV. 2015; Nephron organoids derived from human pluripotent stem cells model kidney development and injury. Nat Biotechnol. 33:1193–1200. DOI:
10.1038/nbt.3392. PMID:
26458176. PMCID:
PMC4747858.

16. Hohwieler M, Illing A, Hermann PC, Mayer T, Stockmann M, Perkhofer L, Eiseler T, Antony JS, Müller M, Renz S, Kuo CC, Lin Q, Sendler M, Breunig M, Kleiderman SM, Lechel A, Zenker M, Leichsenring M, Rosendahl J, Zenke M, Sainz B Jr, Mayerle J, Costa IG, Seufferlein T, Kormann M, Wagner M, Liebau S, Kleger A. 2017; Human pluripotent stem cell-derived acinar/ductal organoids generate human pancreas upon orthotopic transplantation and allow disease modelling. Gut. 66:473–486. DOI:
10.1136/gutjnl-2016-312423. PMID:
27633923. PMCID:
PMC5534761.

17. Koehler KR, Nie J, Longworth-Mills E, Liu XP, Lee J, Holt JR, Hashino E. 2017; Generation of inner ear organoids containing functional hair cells from human pluripotent stem cells. Nat Biotechnol. 35:583–589. DOI:
10.1038/nbt.3840. PMID:
28459451. PMCID:
PMC5462862.

18. Mills RJ, Titmarsh DM, Koenig X, Parker BL, Ryall JG, Quaife-Ryan GA, Voges HK, Hodson MP, Ferguson C, Drowley L, Plowright AT, Needham EJ, Wang QD, Gregorevic P, Xin M, Thomas WG, Parton RG, Nielsen LK, Launikonis BS, James DE, Elliott DA, Porrello ER, Hudson JE. 2017; Functional screening in human cardiac organoids reveals a metabolic mechanism for cardiomyocyte cell cycle arrest. Proc Natl Acad Sci U S A. 114:E8372–E8381. DOI:
10.1073/pnas.1707316114. PMID:
28916735. PMCID:
PMC5635889.

19. Kim Y, Park N, Rim YA, Nam Y, Jung H, Lee K, Ju JH. 2018; Establishment of a complex skin structure via layered co-culture of keratinocytes and fibroblasts derived from induced pluripotent stem cells. Stem Cell Res Ther. 9:217. DOI:
10.1186/s13287-018-0958-2. PMID:
30103800. PMCID:
PMC6090613.

20. Wimmer RA, Leopoldi A, Aichinger M, Wick N, Hantusch B, Novatchkova M, Taubenschmid J, Hämmerle M, Esk C, Bagley JA, Lindenhofer D, Chen G, Boehm M, Agu CA, Yang F, Fu B, Zuber J, Knoblich JA, Kerjaschki D, Penninger JM. 2019; Human blood vessel organoids as a model of diabetic vasculopathy. Nature. 565:505–510. DOI:
10.1038/s41586-018-0858-8. PMID:
30651639. PMCID:
PMC7116578.

21. Dekkers JF, Wiegerinck CL, de Jonge HR, Bronsveld I, Janssens HM, de Winter-de Groot KM, Brandsma AM, de Jong NW, Bijvelds MJ, Scholte BJ, Nieuwenhuis EE, van den Brink S, Clevers H, van der Ent CK, Middendorp S, Beekman JM. 2013; A functional CFTR assay using primary cystic fibrosis intestinal organoids. Nat Med. 19:939–945. DOI:
10.1038/nm.3201. PMID:
23727931.

22. Yui S, Nakamura T, Sato T, Nemoto Y, Mizutani T, Zheng X, Ichinose S, Nagaishi T, Okamoto R, Tsuchiya K, Clevers H, Watanabe M. 2012; Functional engraftment of colon epithelium expanded in vitro from a single adult Lgr5
+ stem cell. Nat Med. 18:618–623. DOI:
10.1038/nm.2695. PMID:
22406745.

23. Freedman BS, Brooks CR, Lam AQ, Fu H, Morizane R, Agrawal V, Saad AF, Li MK, Hughes MR, Werff RV, Peters DT, Lu J, Baccei A, Siedlecki AM, Valerius MT, Musunuru K, McNagny KM, Steinman TI, Zhou J, Lerou PH, Bonventre JV. 2015; Modelling kidney disease with CRISPR-mutant kidney organoids derived from human pluripotent epiblast spheroids. Nat Commun. 6:8715. DOI:
10.1038/ncomms9715. PMID:
26493500. PMCID:
PMC4620584.

24. Bartfeld S, Bayram T, van de Wetering M, Huch M, Begthel H, Kujala P, Vries R, Peters PJ, Clevers H. 2015; In vitro expansion of human gastric epithelial stem cells and their responses to bacterial infection. Gastroenterology. 148:126–136.e6. DOI:
10.1053/j.gastro.2014.09.042. PMID:
25307862. PMCID:
PMC4274199.

25. Drost J, Karthaus WR, Gao D, Driehuis E, Sawyers CL, Chen Y, Clevers H. 2016; Organoid culture systems for prostate epithelial and cancer tissue. Nat Protoc. 11:347–358. DOI:
10.1038/nprot.2016.006. PMID:
26797458. PMCID:
PMC4793718.

26. Boj SF, Hwang CI, Baker LA, Chio II, Engle DD, Corbo V, Jager M, Ponz-Sarvise M, Tiriac H, Spector MS, Gracanin A, Oni T, Yu KH, van Boxtel R, Huch M, Rivera KD, Wilson JP, Feigin ME, Öhlund D, Handly-Santana A, Ardito-Abraham CM, Ludwig M, Elyada E, Alagesan B, Biffi G, Yordanov GN, Delcuze B, Creighton B, Wright K, Park Y, Morsink FH, Molenaar IQ, Borel Rinkes IH, Cuppen E, Hao Y, Jin Y, Nijman IJ, Iacobuzio-Donahue C, Leach SD, Pappin DJ, Hammell M, Klimstra DS, Basturk O, Hruban RH, Offerhaus GJ, Vries RG, Clevers H, Tuveson DA. 2015; Organoid models of human and mouse ductal pancreatic cancer. Cell. 160:324–338. DOI:
10.1016/j.cell.2014.12.021. PMID:
25557080. PMCID:
PMC4334572.

27. Chinta MS, desJardins-Park HE, Wan DC, Longaker MT. 2020; "Tissues in a dish": a review of organoids in plastic surgery. Plast Reconstr Surg Glob Open. 8:e2787. DOI:
10.1097/GOX.0000000000002787. PMID:
32440447. PMCID:
PMC7209840.
28. Sugimoto S, Ohta Y, Fujii M, Matano M, Shimokawa M, Nanki K, Date S, Nishikori S, Nakazato Y, Nakamura T, Kanai T, Sato T. 2018; Reconstruction of the human colon epithelium in vivo. Cell Stem Cell. 22:171–176.e5. DOI:
10.1016/j.stem.2017.11.012. PMID:
29290616.

29. Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ, Clevers H. 2009; Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 459:262–265. DOI:
10.1038/nature07935. PMID:
19329995.

30. Harrison SE, Sozen B, Christodoulou N, Kyprianou C, Zernicka-Goetz M. 2017; Assembly of embryonic and extraembryonic stem cells to mimic embryogenesis in vitro. Science. 356:eaal1810. DOI:
10.1126/science.aal1810. PMID:
28254784.

31. Sato T, Stange DE, Ferrante M, Vries RG, Van Es JH, Van den Brink S, Van Houdt WJ, Pronk A, Van Gorp J, Siersema PD, Clevers H. 2011; Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett's epithelium. Gastroenterology. 141:1762–1772. DOI:
10.1053/j.gastro.2011.07.050. PMID:
21889923.

32. Barker N, Huch M, Kujala P, van de Wetering M, Snippert HJ, van Es JH, Sato T, Stange DE, Begthel H, van den Born M, Danenberg E, van den Brink S, Korving J, Abo A, Peters PJ, Wright N, Poulsom R, Clevers H. 2010; Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell. 6:25–36. DOI:
10.1016/j.stem.2009.11.013. PMID:
20085740.

33. Jung KB, Lee H, Son YS, Lee MO, Kim YD, Oh SJ, Kwon O, Cho S, Cho HS, Kim DS, Oh JH, Zilbauer M, Min JK, Jung CR, Kim J, Son MY. 2018; Interleukin-2 induces the in vitro maturation of human pluripotent stem cell-derived intestinal organoids. Nat Commun. 9:3039. DOI:
10.1038/s41467-018-05450-8. PMID:
30072687. PMCID:
PMC6072745.

34. McCracken KW, Aihara E, Martin B, Crawford CM, Broda T, Treguier J, Zhang X, Shannon JM, Montrose MH, Wells JM. 2017; Wnt/β-catenin promotes gastric fundus specification in mice and humans. Nature. 541:182–187. DOI:
10.1038/nature21021. PMID:
28052057. PMCID:
PMC5526592.

35. Múnera JO, Sundaram N, Rankin SA, Hill D, Watson C, Mahe M, Vallance JE, Shroyer NF, Sinagoga KL, Zarzoso-Lacoste A, Hudson JR, Howell JC, Chatuvedi P, Spence JR, Shannon JM, Zorn AM, Helmrath MA, Wells JM. 2017; Differentiation of human pluripotent stem cells into colonic organoids via transient activation of BMP signaling. Cell Stem Cell. 21:51–64.e6. DOI:
10.1016/j.stem.2017.05.020. PMID:
28648364. PMCID:
PMC5531599.

36. Chichagova V, Hilgen G, Ghareeb A, Georgiou M, Carter M, Sernagor E, Lako M, Armstrong L. 2020; Human iPSC differentiation to retinal organoids in response to IGF1 and BMP4 activation is line- and method-dependent. Stem Cells. 38:195–201. DOI:
10.1002/stem.3116. PMID:
31721366. PMCID:
PMC7383896.

37. Cruz-Acuña R, Quirós M, Farkas AE, Dedhia PH, Huang S, Siuda D, García-Hernández V, Miller AJ, Spence JR, Nusrat A, García AJ. 2017; Synthetic hydrogels for human intestinal organoid generation and colonic wound repair. Nat Cell Biol. 19:1326–1335. DOI:
10.1038/ncb3632. PMID:
29058719. PMCID:
PMC5664213.

38. Sachs N, Tsukamoto Y, Kujala P, Peters PJ, Clevers H. 2017; Intestinal epithelial organoids fuse to form self-organizing tubes in floating collagen gels. Development. 144:1107–1112. DOI:
10.1242/dev.143933. PMID:
28292848.

39. Giobbe GG, Crowley C, Luni C, Campinoti S, Khedr M, Kretzschmar K, De Santis MM, Zambaiti E, Michielin F, Meran L, Hu Q, van Son G, Urbani L, Manfredi A, Giomo M, Eaton S, Cacchiarelli D, Li VSW, Clevers H, Bonfanti P, Elvassore N, De Coppi P. 2019; Extracellular matrix hydrogel derived from decellularized tissues enables endodermal organoid culture. Nat Commun. 10:5658. DOI:
10.1038/s41467-019-13605-4. PMID:
31827102. PMCID:
PMC6906306.

40. Kumar SV, Er PX, Lawlor KT, Motazedian A, Scurr M, Ghobrial I, Combes AN, Zappia L, Oshlack A, Stanley EG, Little MH. 2019; Kidney micro-organoids in suspension culture as a scalable source of human pluripotent stem cell-derived kidney cells. Development. 146:dev172361. DOI:
10.1242/dev.172361. PMID:
30846463. PMCID:
PMC6432662.

41. DiStefano T, Chen HY, Panebianco C, Kaya KD, Brooks MJ, Gieser L, Morgan NY, Pohida T, Swaroop A. 2018; Accelerated and improved differentiation of retinal organoids from pluripotent stem cells in rotating-wall vessel bioreactors. Stem Cell Reports. 10:300–313. DOI:
10.1016/j.stemcr.2017.11.001. PMID:
29233554. PMCID:
PMC5768666.

42. Muguruma K, Nishiyama A, Kawakami H, Hashimoto K, Sasai Y. 2015; Self-organization of polarized cerebellar tissue in 3D culture of human pluripotent stem cells. Cell Rep. 10:537–550. DOI:
10.1016/j.celrep.2014.12.051. PMID:
25640179.

43. Giandomenico SL, Mierau SB, Gibbons GM, Wenger LMD, Masullo L, Sit T, Sutcliffe M, Boulanger J, Tripodi M, Derivery E, Paulsen O, Lakatos A, Lancaster MA. 2019; Cerebral organoids at the air-liquid interface generate diverse nerve tracts with functional output. Nat Neurosci. 22:669–679. DOI:
10.1038/s41593-019-0350-2. PMID:
30886407. PMCID:
PMC6436729.

44. Upadhyay S, Palmberg L. 2018; Air-liquid interface: relevant in vitro models for investigating air pollutant-induced pulmonary toxicity. Toxicol Sci. 164:21–30. DOI:
10.1093/toxsci/kfy053. PMID:
29534242.

45. Takasato M, Er PX, Chiu HS, Little MH. 2016; Generation of kidney organoids from human pluripotent stem cells. Nat Protoc. 11:1681–1692. DOI:
10.1038/nprot.2016.098. PMID:
27560173. PMCID:
PMC5113819.

46. Watanabe M, Buth JE, Vishlaghi N, de la Torre-Ubieta L, Taxidis J, Khakh BS, Coppola G, Pearson CA, Yamauchi K, Gong D, Dai X, Damoiseaux R, Aliyari R, Liebscher S, Schenke-Layland K, Caneda C, Huang EJ, Zhang Y, Cheng G, Geschwind DH, Golshani P, Sun R, Novitch BG. 2017; Self-organized cerebral organoids with human-specific features predict effective drugs to combat Zika virus infection. Cell Rep. 21:517–532. DOI:
10.1016/j.celrep.2017.09.047. PMID:
29020636. PMCID:
PMC5637483.

47. Qian X, Nguyen HN, Song MM, Hadiono C, Ogden SC, Hammack C, Yao B, Hamersky GR, Jacob F, Zhong C, Yoon KJ, Jeang W, Lin L, Li Y, Thakor J, Berg DA, Zhang C, Kang E, Chickering M, Nauen D, Ho CY, Wen Z, Christian KM, Shi PY, Maher BJ, Wu H, Jin P, Tang H, Song H, Ming GL. 2016; Brain-region-specific organoids using mini-bioreactors for modeling ZIKV exposure. Cell. 165:1238–1254. DOI:
10.1016/j.cell.2016.04.032. PMID:
27118425. PMCID:
PMC4900885.

48. Kim H, Xu R, Padmashri R, Dunaevsky A, Liu Y, Dreyfus CF, Jiang P. 2019; Pluripotent stem cell-derived cerebral organoids reveal human oligodendrogenesis with dorsal and ventral origins. Stem Cell Reports. 12:890–905. DOI:
10.1016/j.stemcr.2019.04.011. PMID:
31091434. PMCID:
PMC6524754.

49. Pellegrini L, Bonfio C, Chadwick J, Begum F, Skehel M, Lancaster MA. 2020; Human CNS barrier-forming organoids with cerebrospinal fluid production. Science. 369:eaaz5626. DOI:
10.1126/science.aaz5626. PMID:
32527923. PMCID:
PMC7116154.

50. Susaimanickam PJ, Maddileti S, Pulimamidi VK, Boyinpally SR, Naik RR, Naik MN, Reddy GB, Sangwan VS, Mariappan I. 2017; Generating minicorneal organoids from human induced pluripotent stem cells. Development. 144:2338–2351. DOI:
10.1242/dev.143040. PMID:
28559289.

51. Low JH, Li P, Chew EGY, Zhou B, Suzuki K, Zhang T, Lian MM, Liu M, Aizawa E, Rodriguez Esteban C, Yong KSM, Chen Q, Campistol JM, Fang M, Khor CC, Foo JN, Izpisua Belmonte JC, Xia Y. 2019; Generation of human PSC-derived kidney organoids with patterned nephron segments and a de novo vascular network. Cell Stem Cell. 25:373–387.e9. DOI:
10.1016/j.stem.2019.06.009. PMID:
31303547. PMCID:
PMC6731150.

52. Workman MJ, Mahe MM, Trisno S, Poling HM, Watson CL, Sundaram N, Chang CF, Schiesser J, Aubert P, Stanley EG, Elefanty AG, Miyaoka Y, Mandegar MA, Conklin BR, Neunlist M, Brugmann SA, Helmrath MA, Wells JM. 2017; Engineered human pluripotent-stem-cell-derived intestinal tissues with a functional enteric nervous system. Nat Med. 23:49–59. DOI:
10.1038/nm.4233. PMID:
27869805. PMCID:
PMC5562951.

53. Jung KB, Lee H, Son YS, Lee JH, Cho HS, Lee MO, Oh JH, Lee J, Kim S, Jung CR, Kim J, Son MY. 2018; In vitro and in vivo imaging and tracking of intestinal organoids from human induced pluripotent stem cells. FASEB J. 32:111–122. DOI:
10.1096/fj.201700504r. PMID:
28855280.

54. Mithal A, Capilla A, Heinze D, Berical A, Villacorta- Martin C, Vedaie M, Jacob A, Abo K, Szymaniak A, Peasley M, Stuffer A, Mahoney J, Kotton DN, Hawkins F, Mostoslavsky G. 2020; Generation of mesenchyme free intestinal organoids from human induced pluripotent stem cells. Nat Commun. 11:215. DOI:
10.1038/s41467-019-13916-6. PMID:
31924806. PMCID:
PMC6954238.

55. Tao T, Wang Y, Chen W, Li Z, Su W, Guo Y, Deng P, Qin J. 2019; Engineering human islet organoids from iPSCs using an organ-on-chip platform. Lab Chip. 19:948–958. DOI:
10.1039/C8LC01298A. PMID:
30719525.

56. Asai A, Aihara E, Watson C, Mourya R, Mizuochi T, Shivakumar P, Phelan K, Mayhew C, Helmrath M, Takebe T, Wells J, Bezerra JA. 2017; Paracrine signals regulate human liver organoid maturation from induced pluripotent stem cells. Development. 144:1056–1064. DOI:
10.1242/dev.142794. PMID:
28275009. PMCID:
PMC5358109.
57. Miller AJ, Dye BR, Ferrer-Torres D, Hill DR, Overeem AW, Shea LD, Spence JR. 2019; Generation of lung organoids from human pluripotent stem cells in vitro. Nat Protoc. 14:518–540. DOI:
10.1038/s41596-018-0104-8. PMID:
30664680. PMCID:
PMC6531049.

58. Koike H, Iwasawa K, Ouchi R, Maezawa M, Giesbrecht K, Saiki N, Ferguson A, Kimura M, Thompson WL, Wells JM, Zorn AM, Takebe T. 2019; Modelling human hepato-biliary-pancreatic organogenesis from the foregut-midgut boundary. Nature. 574:112–116. DOI:
10.1038/s41586-019-1598-0. PMID:
31554966. PMCID:
PMC7643931.

59. Lee H, Son YS, Lee MO, Ryu JW, Park K, Kwon O, Jung KB, Kim K, Ryu TY, Baek A, Kim J, Jung CR, Ryu CM, Park YJ, Han TS, Kim DS, Cho HS, Son MY. 2020; Low-dose interleukin-2 alleviates dextran sodium sulfate-induced colitis in mice by recovering intestinal integrity and inhibiting AKT-dependent pathways. Theranostics. 10:5048–5063. DOI:
10.7150/thno.41534. PMID:
32308767. PMCID:
PMC7163458.

61. Watson CL, Mahe MM, Múnera J, Howell JC, Sundaram N, Poling HM, Schweitzer JI, Vallance JE, Mayhew CN, Sun Y, Grabowski G, Finkbeiner SR, Spence JR, Shroyer NF, Wells JM, Helmrath MA. 2014; An in vivo model of human small intestine using pluripotent stem cells. Nat Med. 20:1310–1314. DOI:
10.1038/nm.3737. PMID:
25326803. PMCID:
PMC4408376.

62. Cortez AR, Poling HM, Brown NE, Singh A, Mahe MM, Helmrath MA. 2018; Transplantation of human intestinal organoids into the mouse mesentery: a more physiologic and anatomic engraftment site. Surgery. 164:643–650. DOI:
10.1016/j.surg.2018.04.048. PMID:
30072255. PMCID:
PMC6326178.

63. Mansour AA, Gonçalves JT, Bloyd CW, Li H, Fernandes S, Quang D, Johnston S, Parylak SL, Jin X, Gage FH. 2018; An in vivo model of functional and vascularized human brain organoids. Nat Biotechnol. 36:432–441. DOI:
10.1038/nbt.4127. PMID:
29658944. PMCID:
PMC6331203.

66. Czerniecki SM, Cruz NM, Harder JL, Menon R, Annis J, Otto EA, Gulieva RE, Islas LV, Kim YK, Tran LM, Martins TJ, Pippin JW, Fu H, Kretzler M, Shankland SJ, Himmelfarb J, Moon RT, Paragas N, Freedman BS. 2018; High- throughput screening enhances kidney organoid differentiation from human pluripotent stem cells and enables automated multidimensional phenotyping. Cell Stem Cell. 22:929–940.e4. DOI:
10.1016/j.stem.2018.04.022. PMID:
29779890. PMCID:
PMC5984728.

67. Crespo M, Vilar E, Tsai SY, Chang K, Amin S, Srinivasan T, Zhang T, Pipalia NH, Chen HJ, Witherspoon M, Gordillo M, Xiang JZ, Maxfield FR, Lipkin S, Evans T, Chen S. 2017; Colonic organoids derived from human induced pluripotent stem cells for modeling colorectal cancer and drug testing. Nat Med. 23:878–884. DOI:
10.1038/nm.4355. PMID:
28628110. PMCID:
PMC6055224.

68. Tocchio A, Tamplenizza M, Martello F, Gerges I, Rossi E, Argentiere S, Rodighiero S, Zhao W, Milani P, Lenardi C. 2015; Versatile fabrication of vascularizable scaffolds for large tissue engineering in bioreactor. Biomaterials. 45:124–131. DOI:
10.1016/j.biomaterials.2014.12.031. PMID:
25662502.

69. Wang Y, Gunasekara DB, Reed MI, DiSalvo M, Bultman SJ, Sims CE, Magness ST, Allbritton NL. 2017; A microen-gineered collagen scaffold for generating a polarized crypt- villus architecture of human small intestinal epithelium. Biomaterials. 128:44–55. DOI:
10.1016/j.biomaterials.2017.03.005. PMID:
28288348. PMCID:
PMC5392043.

70. Nikolaev M, Mitrofanova O, Broguiere N, Geraldo S, Dutta D, Tabata Y, Elci B, Brandenberg N, Kolotuev I, Gjorevski N, Clevers H, Lutolf MP. 2020; Homeostatic mini-intestines through scaffold-guided organoid morphogenesis. Nature. 585:574–578. DOI:
10.1038/s41586-020-2724-8. PMID:
32939089.

71. McKinnon DD, Brown TE, Kyburz KA, Kiyotake E, Anseth KS. 2014; Design and characterization of a synthetically accessible, photodegradable hydrogel for user-directed formation of neural networks. Biomacromolecules. 15:2808–2816. DOI:
10.1021/bm500731b. PMID:
24932668. PMCID:
PMC4592536.

72. Sarig-Nadir O, Livnat N, Zajdman R, Shoham S, Seliktar D. 2009; Laser photoablation of guidance microchannels into hydrogels directs cell growth in three dimensions. Biophys J. 96:4743–4752. DOI:
10.1016/j.bpj.2009.03.019. PMID:
19486697. PMCID:
PMC2894558.

73. Zhu W, Qu X, Zhu J, Ma X, Patel S, Liu J, Wang P, Lai CS, Gou M, Xu Y, Zhang K, Chen S. 2017; Direct 3D bioprinting of prevascularized tissue constructs with complex microarchitecture. Biomaterials. 124:106–115. DOI:
10.1016/j.biomaterials.2017.01.042. PMID:
28192772. PMCID:
PMC5330288.

74. Demers CJ, Soundararajan P, Chennampally P, Cox GA, Briscoe J, Collins SD, Smith RL. 2016; Development-on-chip: in vitro neural tube patterning with a microfluidic device. Development. 143:1884–1892. DOI:
10.1242/dev.126847. PMID:
27246712. PMCID:
PMC4920155.

75. Fabre KM, Livingston C, Tagle DA. 2014; Organs-on-chips (microphysiological systems): tools to expedite efficacy and toxicity testing in human tissue. Exp Biol Med (Maywood). 239:1073–1077. DOI:
10.1177/1535370214538916. PMID:
24962171.

76. Zhang C, Zhao Z, Abdul Rahim NA, van Noort D, Yu H. 2009; Towards a human-on-chip: culturing multiple cell types on a chip with compartmentalized microenvironments. Lab Chip. 9:3185–3192. DOI:
10.1039/b915147h. PMID:
19865724.

77. Ronaldson-Bouchard K, Vunjak-Novakovic G. 2018; Organs-on-a- chip: a fast track for engineered human tissues in drug development. Cell Stem Cell. 22:310–324. DOI:
10.1016/j.stem.2018.02.011. PMID:
29499151. PMCID:
PMC5837068.

78. Peterson LW, Artis D. 2014; Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat Rev Immunol. 14:141–153. DOI:
10.1038/nri3608. PMID:
24566914.

79. Erkosar B, Storelli G, Mitchell M, Bozonnet L, Bozonnet N, Leulier F. 2015; Pathogen virulence impedes mutualist-mediated enhancement of host juvenile growth via inhibition of protein digestion. Cell Host Microbe. 18:445–455. DOI:
10.1016/j.chom.2015.09.001. PMID:
26439865. PMCID:
PMC4617634.

80. Cho I, Yamanishi S, Cox L, Methé BA, Zavadil J, Li K, Gao Z, Mahana D, Raju K, Teitler I, Li H, Alekseyenko AV, Blaser MJ. 2012; Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature. 488:621–626. DOI:
10.1038/nature11400. PMID:
22914093. PMCID:
PMC3553221.

81. Hviid A, Svanström H, Frisch M. 2011; Antibiotic use and inflammatory bowel diseases in childhood. Gut. 60:49–54. DOI:
10.1136/gut.2010.219683. PMID:
20966024.

82. Diaz Heijtz R, Wang S, Anuar F, Qian Y, Björkholm B, Samuelsson A, Hibberd ML, Forssberg H, Pettersson S. 2011; Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci U S A. 108:3047–3052. DOI:
10.1073/pnas.1010529108. PMID:
21282636. PMCID:
PMC3041077.

83. Leslie JL, Huang S, Opp JS, Nagy MS, Kobayashi M, Young VB, Spence JR. 2015; Persistence and toxin production by Clostridium difficile within human intestinal organoids result in disruption of epithelial paracellular barrier function. Infect Immun. 83:138–145. DOI:
10.1128/IAI.02561-14. PMID:
25312952. PMCID:
PMC4288864.

84. Son YS, Ki SJ, Thanavel R, Kim JJ, Lee MO, Kim J, Jung CR, Han TS, Cho HS, Ryu CM, Kim SH, Park DS, Son MY. 2020; Maturation of human intestinal organoids in vitro facilitates colonization by commensal lactobacilli by reinforcing the mucus layer. FASEB J. [Epub ahead of print]. DOI:
10.1096/fj.202000063R. PMID:
32602623.

85. Vatanen T, Plichta DR, Somani J, Münch PC, Arthur TD, Hall AB, Rudolf S, Oakeley EJ, Ke X, Young RA, Haiser HJ, Kolde R, Yassour M, Luopajärvi K, Siljander H, Virtanen SM, Ilonen J, Uibo R, Tillmann V, Mokurov S, Dorshakova N, Porter JA, McHardy AC, Lähdesmäki H, Vlamakis H, Huttenhower C, Knip M, Xavier RJ. 2019; Genomic variation and strain-specific functional adaptation in the human gut microbiome during early life. Nat Microbiol. 4:470–479. DOI:
10.1038/s41564-018-0321-5. PMID:
30559407. PMCID:
PMC6384140.

86. Forbester JL, Goulding D, Vallier L, Hannan N, Hale C, Pickard D, Mukhopadhyay S, Dougan G. 2015; Interaction of Salmonella enterica serovar Typhimurium with intestinal organoids derived from human induced pluripotent stem cells. Infect Immun. 83:2926–2934. DOI:
10.1128/IAI.00161-15. PMID:
25964470. PMCID:
PMC4468523.

87. Taguchi A, Kaku Y, Ohmori T, Sharmin S, Ogawa M, Sasaki H, Nishinakamura R. 2014; Redefining the in vivo origin of metanephric nephron progenitors enables generation of complex kidney structures from pluripotent stem cells. Cell Stem Cell. 14:53–67. DOI:
10.1016/j.stem.2013.11.010. PMID:
24332837.

88. Mandai M, Fujii M, Hashiguchi T, Sunagawa GA, Ito SI, Sun J, Kaneko J, Sho J, Yamada C, Takahashi M. 2017; iPSC-derived retina transplants improve vision in rd1 end-stage retinal-degeneration mice. Stem Cell Reports. 8:69–83. DOI:
10.1016/j.stemcr.2016.12.008. PMID:
28076757. PMCID:
PMC5233464.

89. Mariani J, Coppola G, Zhang P, Abyzov A, Provini L, Tomasini L, Amenduni M, Szekely A, Palejev D, Wilson M, Gerstein M, Grigorenko EL, Chawarska K, Pelphrey KA, Howe JR, Vaccarino FM. 2015; FOXG1-dependent dysregulation of GABA/glutamate neuron differentiation in autism spectrum disorders. Cell. 162:375–390. DOI:
10.1016/j.cell.2015.06.034. PMID:
26186191. PMCID:
PMC4519016.

90. Lindeboom RG, van Voorthuijsen L, Oost KC, Rodríguez- Colman MJ, Luna-Velez MV, Furlan C, Baraille F, Jansen PW, Ribeiro A, Burgering BM, Snippert HJ, Vermeulen M. 2018; Integrative multi-omics analysis of intestinal organoid differentiation. Mol Syst Biol. 14:e8227. DOI:
10.15252/msb.20188227. PMID:
29945941. PMCID:
PMC6018986.

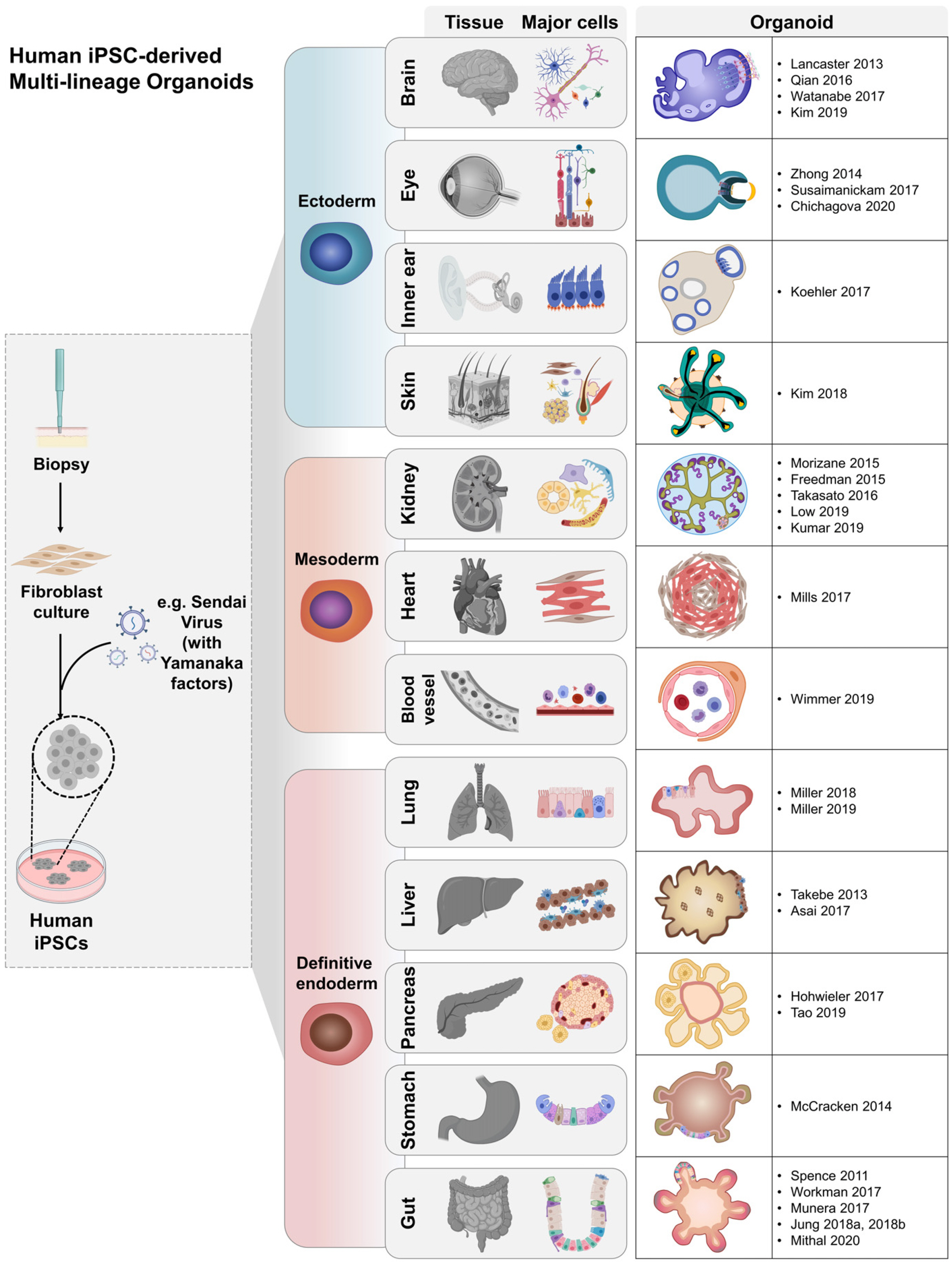
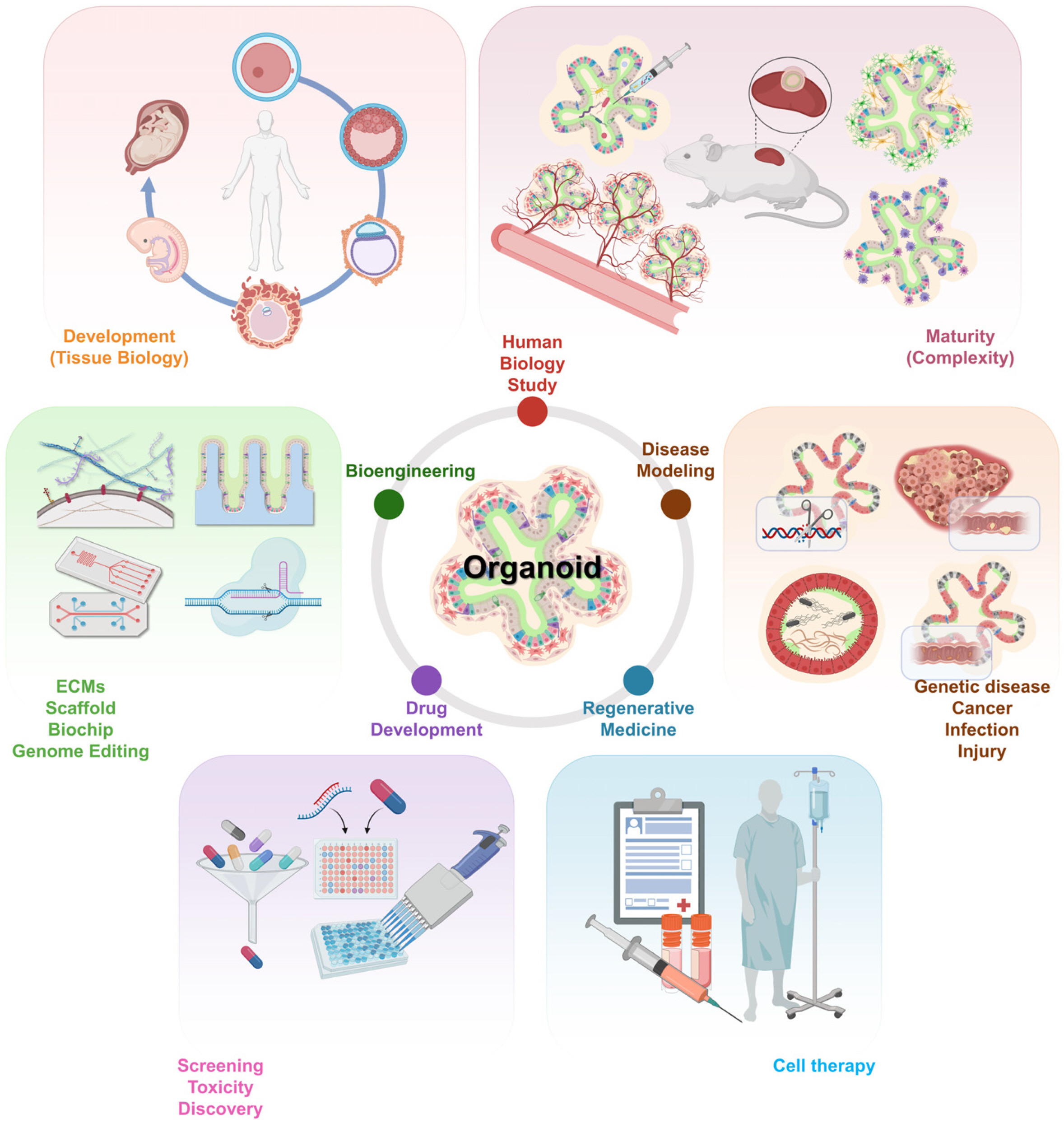
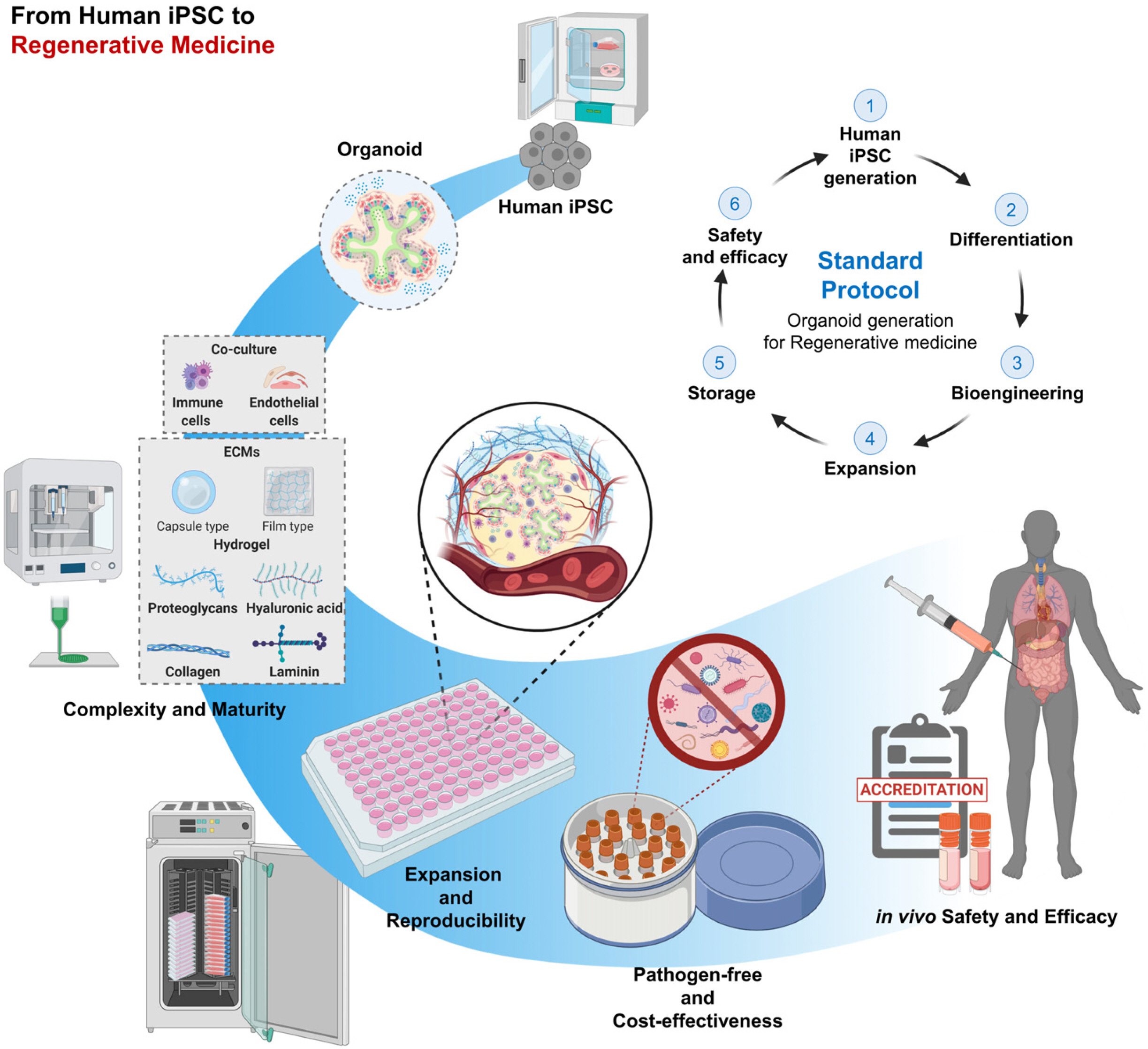




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download